Mesenchymal Stem Cells for the Treatment of Patients with COVID-19 Severe/Critical Category: A Review
Abstract
COVID-19 has developed into a public health emergency of international concern and is a major threat to global health. Several studies have been conducted with the aim of dampening the inflammatory response in COVID-19 patients. To dampen the cytokine storm, some therapies immunotargeting IL-1, IL-2, IL-6, and TNFα are being evaluated and one of the promising immune modulators is mesenchymal stem cells (MSCs). MSCs have the beneficial effect of preventing or attenuating cytokine storms by secreting potent anti-inflammatory factors and addressing the severity of the COVID-19 infection.
This study examines the mesenchymal stem cells for the treatment of patients with COVID-19 severe/critical category. This literature review outlines the therapeutic effectiveness of MSCs in the treatment of COVID-19 in the critically ill patient category.
Several research showed that the administration of MSC therapy in COVID-19 patients has significant clinical improvement results, normalized the vital signs of patients, reduced the severity and reduced mortality. In addition, overall treatment with MCS did not cause side effects.
1. INTRODUCTION
In December 2019, a new infectious disease was reported in Wuhan, China, for the first time. The disease is contagious and is transmitted through aerosols and has been named by the World Health Organization (WHO) as coronavirus disease 2019 (COVID-19) [1, 2]. COVID-19 causes morbidity and mortality rates to increase significantly worldwide [3]. Due to the high prevalence and infection rates worldwide, on March 11, 2020, WHO declared COVID-19 as a pandemic [4]. In addition, COVID-19 has developed into a public health emergency of international concern and is a major threat to global health. COVID-19 differs from common pneumonia in that it can develop very seriously even in young patients with or without co-morbidities [5-7]
The appropriate and effective COVID-19 treatment is still ongoing development up until now [7, 8]. The main management of COVID-19 is symptomatic treatment and supportive care such as oxygen therapy (mechanical ventilation) in critically ill patients and medications including antivirals (redeliver, lopinavir/ritonavir, oseltamivir, favipiravir), antibiotics (azithromycin), and immuno- modulatory drugs (tocilizumab, hydroxychloroquine, convalescent plasma, anakinra [9, 10].
Patients infected with SARS-CoV-2 in the early stages will experience mild symptoms such as fever, cough, and headache due to strong viral replication. Later in the second stage, it will show a high level of fever, difficulty breathing, and pneumonia-like symptoms. The progression to the third stage is mediated by inflammatory cytokines (IL-2, IL-6, IL-8, TNF-, G-CSF, GM-CSF), chemokines (MCP-1, MIP1α, IP10), and massive infiltration of cells inflammation. The progression indicates the occurrence of a cytokine storm [11, 12]. A persistent cytokine storm will lead to severe organ injury and virus-induced death, with severe lung injury, shock, acute respiratory disease syndrome (ARDS), and multiple organ dysfunction syndromes (MODS) [13, 14].
Currently, corticosteroids are the only drugs shown to reduce mortality in patients with severe and critical illnesses [15]. The corticosteroid drug given, namely dexamethasone, is given with a dosage rule 0f 1 x 6 mg/iv/day, hydrocortisone is given with a dosage rule of 3 x 100 mg/iv, and methylprednisolone is given with a dosage rule of 2 x 62,5 mg/iv. Administration of steroids can accelerate the reduction in the number of patients who experience it cough, and corticosteroids are potent inhibitors of suppression of inflammation [14]. Therefore, identifying safe and effective therapies is very important, especially in reducing morbidity rates. Several studies have been conducted aimed at dampening the inflammatory response in COVID-19 patients. One of them is Mesenchymal Stem Cells (MSC).
Mesenchymal stem cells (MSCs) have the beneficial effect of preventing or attenuating cytokine storms by secreting potent anti-inflammatory factors. In addition, MSCs injected into the body will partially accumulate in the lungs, which potentially protect alveolar epithelial cells, prevent pulmonary fibrosis, and improve lung function [15, 16]. The very important mechanism of MSCs is releasing many paracrine factors, such as micro-RNA, that will interact with the immune response to provide immunoregulatory and anti-inflammatory effects [17, 18]. In addition, MSCs have immunomodulatory characteristics such as the secretion of anti-inflammatory cytokines/chemokines, antiapoptotic effects, promoting lung repair and repair of other damaged tissues (epithelial cells), and can prevent morbidity [18, 19].
MSCs therapy could theoretically inhibit the over-activation of the immune system and promote endogenous repair by enhancing the microenvironment. MSCs have also been shown to improve cardiovascular, renal, hepatic, and several other disorders [16, 15, 20]. MCSs have several mechanisms of action for COVID-19 therapy. First, MCSs significantly inhibit T-cells in the G1 phase through Transforming Growth Factor beta (TGF-β) and Hepatocyte growth factor (HGF). Furthermore, MSCs modulate T cell activation and differentiation by inducing the production of Interleukin -10 (IL-10) and inhibiting the production of Interferon (IFN)-γ and IL-17, so that they reduce the production of regulatory T cells [21, 22]. Second, MSCs, as an anti-inflammatory agent, are able to induce the production of IL-1Ra and IL-1β, which anticipate anti-inflammatory effects and continue to heal damaged tissues [23]. Third, MSCs can modulate T-cell activation and differentiation. MSCs induce IL-10 production and inhibit IFN-γ and IL-17 production. In addition, MSCs also regulate dendritic cells and natural killer cells [24]. Fourth, MSCs act as an Immunomodulator. The immunomodulatory potential of MSCs is triggered when MSCs are stimulated by inflammatory cytokines such as IFN-γ and tumor necrosis factor (TNF)-α, IL-1α, or IL-1β, leading to the production of Nitrous oxide (NO) and Prostaglandin E2 (PGE2) through upregulation of Cyclooxygenase– 2 (COX-2) [25].
Based on this, further efforts are needed to comprehensively support the effectiveness of MSC therapy in severe or critical COVID-19 patients. The advantages of a literature review are that it can provide accurate conclusions and can, improve the utilization of research results can provide information to health service providers, researchers, and policymakers. While the disadvantage is the possibility that the data obtained are heterogeneous, the location and selection of articles are not homogeneous [26]. This review article provides information about the therapeutic effectiveness of MSCs in the treatment of severe or critical COVID-19. By focusing results on clinical measures (increased O2, CD3+ T cells, CD4+ T cells, and CD8+ T cells), inflammatory cytokine response outcomes (IL-2, IL-6, IL-8, TNF-, G-CSF, GM-CSF), mortality, and cure rates based on a literature review of various research articles that have been carried out.
2. METHODS
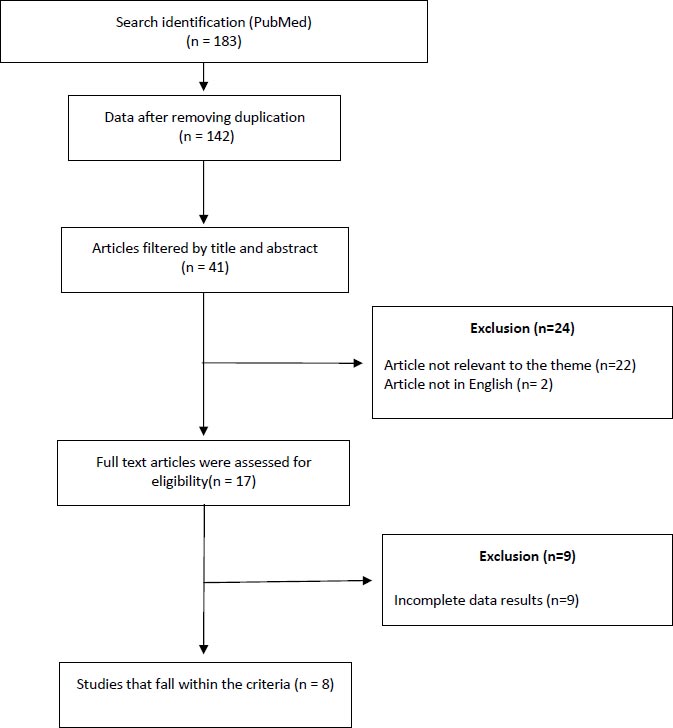
This literature review summarizes studies published in the last 10 years using the keywords “Mesenchymal Stem Cells with COVID-19”, “Mesenchymal Stem Cells for the treatment of COVID-19”, “Mesenchymal Stem Cells on COVID-19 mortality”, “Mesenchymal Stem Cells s for COVID-19” from PubMed. A total of 189 articles were obtained in the initial search, then extracted with inclusion and exclusion criteria. The inclusion criteria were literature with samples of patients whose primary diagnosis was COVID-19, categories of critically ill patients with COVID-19, and samples of patients diagnosed with COVID-19 using mesenchymal stem cells during the treatment of COVID-19. The exclusion criteria were literature with samples of patients not receiving mesenchymal stem cell therapy before and during COVID-19 treatment and literature with incomplete data. The results of the review concluded with clinical improvement results. Non-English studies and unrelated studies, such as non-human studies, were excluded. The systematic search results of the database generated 183 studies in total. Furthermore, the researcher removed the duplicates and got 142 studies. The researcher extracted the inclusion and exclusion criteria that were reviewed from the abstract and titles and obtained 17 studies. Of 17 studies, none meet the criteria. Therefore, only eight studies met the criteria. In addition, this article also contains references from related data that can be accessed on the WHO official website (Fig. 1).
The inclusion criteria used were:
1. Literature that only examines the mesenchymal stem cells for the treatment of patients with COVID-19 severe/critical category.
2. Literature with a sample of patients with COVID-19 severe/critical category.
3. Literature with samples of patients who received mesenchymal stem cells for the treatment and standard alloys without mesenchymal stem cells.
4. The literature contains complete details of the examination of the mesenchymal stem cells for the treatment of patients with COVID-19 severe/critical category.
The exclusion criteria used were:
1. Literature with samples of patients undergoing therapy without mesenchymal stem cells.
2. Literature with samples of patients with comorbid other infectious diseases.
3. Literature with a sample of patients whose primary diagnosis was not COVID-19.
4. Literature with samples of patients who did not receive health interventions.
2.1. Quality of Reporting
Generally, drug therapy using cells in several diseases has provided effective and real evidence [27]. Several cell-based treatments are currently being planned for many diseases other than COVID-19, namely the lungs [16, 26], cardiovascular [28], liver [28, 29], kidney [30, 31] and other diseases. However, some researches and clinical trials still need to be done further regarding its effectiveness and safety [32]. MSCs have a positive role in immunomodulatory effects through the secretion of various types of cytokines, such as paracrine secretion or making direct interactions with immune cells [33]. Due to its strong immunomodulatory abilities, treatment with MSCs is expected to have a better effect on preventing or attenuating cytokine storms [34].
The aim of this article was to review the scientific literature that has investigated the potential effects of using MSCs in the COVID-19 treatment for critically ill patients. Overall, MSCs treatment showed significant clinical effects in both patients with SARS-CoV-2 infection. The researcher obtained eight studies that meet the criteria. These studies came from the United States and China [35].
Research conducted by Sengupta et al. stated that patients who received therapy with MSCs had a greater survival rate of 80%, a cure rate of 71% (17 of 24), and a lower mortality rate of 16%. In addition, the clinical status of the patient and oxygenation level improved significantly, which was 192% (P < 0.001) [36]. In China, in a study conducted by Leng et al., the patients who had received MSC therapy within 2-4 days, all symptoms (high fever, weakness, shortness of breath) disappeared. There was an increase in oxygen saturation and faster time for RT-PCR conversion to negative, and there are no reports of side effects in patients who have received MSC therapy [37]. In addition, MSCs therapy showed good clinical outcomes, such as CD3+ T cells, CD4+ T cells, and CD8+ T cells were increased to normal levels. There is improvement in lymphopenia, reducing severity in critically ill patients and reducing mortality [33]. In concordance, other studies with COVID-19 patients in the critical category who received MSCs therapy also experienced clinical improvement and did not show allergic reactions after therapy [38, 39]. Another study conducted by Shu et al. stated that the cure rate of patients with MSCs was higher than the control group (p < 0.001); the mortality rate of patients with MSCs was also lower than the control group (p = 0.543), and the time to clinical improvement in the MSCs group was shorter than the control group (p=0.006) [40]. In a Study from China on critically ill COVID-19 patients, MSCs therapy significantly showed better clinical outcomes. MSCs therapy boosts immunity (CD4+ cells and lymphocytes), decreases inflammatory indicators (IL-6 and C reactive protein), and increases oxygen saturation (O2) and partial pressure (PO2) [41].
MSCs can help enhance or restrain the inflammatory process depending on their microenvironment. In conditions of an active immune system, MSC will release pro-inflammatory cytokines to help increase inflammation. However, if the response from the immune system is excessive, then the MSC will switch to secreting anti-inflammatory cytokines to suppress inflammation [41]. MSC can stimulate the process of immunomodulation through autocrine, paracrine, and endocrine pathways. MSC can regulate the occurrence of innate and adaptive immune responses, thereby reducing the production of proinflammatory cytokines. As explained by Xu et al (2022), MSC therapy can reduce local inflammation in the lungs and systemic inflammation, improve immune cell activation and reduce lung injury. MSC has also been shown to reduce the infiltration of macrophages, neutrophils, and dendritic cells in the lungs and reduce levels of MIP-2 TNF-α, IL-6, IL-1b, and IL-12p70 in bronchoalveolar lavage (BAL) fluid [42]
Giving stem cells can also improve lung function, improve symptoms, and suppress the inflammatory process that occurs. According to the research by Mahendiratta et al.,, MSC is able to reduce systemic inflammation and protect patients from COVID-19 infection. Intramuscular injection of mesenchyme-like cells taken from the placenta cures six critically ill COVID-19 patients in a trial conducted in Israel.
One of the complications of COVID-19 is excessive production of lung fluid and pulmonary edema, which impairs lung function. MSCs release keratinocyte growth factor (KGF), angiopoietin-1, and LXA4 in their exosomes. These factors activate the Na+- K+ pump, thereby reducing the permeability of the alveolar epithelium to proteins and fluids and inhibiting fluid accumulation in the lung tissue. Another complication of COVID-19 is lung fibrosis. These complications must be considered because they cannot be cured once they occur. MSCs prevent pulmonary fibrosis by two mechanisms: (a) differentiating into type II alveolar cells; (b) paracrine signals (such as KGF) that induce proliferation and inhibit apoptosis in type II alveolar cells, In addition to its effects on the immune system, MSC is able to promote capillary barrier re-formation, stop bacterial growth, and restore alveolar AT.
In COVID-19 patients, it was found that a set of growth factors and these cytokines are increased drastically, as in general pulmonary fibrosis. Administering stem cell therapy to COVID-19 patients will improve symptoms such as weakness, shortness of breath, and low oxygen saturation. On CT-scan examination, it was found that there was a faster recovery of lung inflammation in patients with stem cell therapy. According to the results of the previous meta-analysis, many critical-severe COVID-19 patients who received MSC therapy did not experience serious side effects. In addition, reductions in mortality and morbidity in COVID-19 patients were also found with this therapy.
In Indonesia, the application of MSCs therapy, which is conducted as an additional treatment for COVID-19 patients with critical categories, can increase the survival rate by 2.5 times higher than the control group (P = 0.047). In addition, patients receiving MSC therapy experienced a decrease in IL-6 significantly compared to the control group (p= 0.023).
MSCs secrete trophic and immunomodulatory factors. MSCs express their functions mainly through paracrine effects, i.e., secreting immunomodulatory cytokines, such as indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), IL-6 and IL-10 to balance pro and anti-inflammatory responses, as well as growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), insulin-like growth factor 1, and fibroblast growth factor 2 (FGF2) which promotes cell generation. On the other hand, MSCs can also be regulated by cell-to-cell contact, and they can secrete extracellular vesicles. These mechanisms support the notion that MSCs may reduce or even eliminate cytokine storm syndrome (CSS) in COVID-19 patients [40].
Additionally, MSCs release anti-inflammatory factors IL-10anf IL4 to repress the activation of lymphocytes and inflammatory cytokines such as IL-1-α-β, -6, -17, and TNF-α. On the other hand, MSCs prevent infection-induced damage to lung tissues by decreasing the excessive secretion of neutrophil extracellular traps at the infectious site. During bacterial infections, MSCs reduce inflammation and ameliorate tissue injury through at least the following mechanisms: (1) by diminishing the excessive production of neutrophils and enhancing neutrophil-mediated phagocytosis, (2) promoting macrophages to differentiate into M1, which induces phagocytosis and promotes bacterial clearance, and M2, which benefits tissue repair by attenuating inflammation at the injection site, and (3) promoting the proliferation of regulatory T cells and inhibiting at the infection site, and (4) promoting the proliferation of regulatory T cells and inhibiting the proliferation of effector T cells, thereby diminishing the immune response and ameliorating lung damage in ARDS [33, 34].
The average outcome of treatment studies with MSC revealed significant results. Like the results of the previous meta-analysis, many critically severe COVID-19 patients who received MSC therapy did not experience serious side effects. In addition, reductions in mortality and morbidity in COVID-19 patients were also found with this therapy [35-42]. Especially in clinical and symptomatic factors, such as CD3+ T cell, CD4+ T cell, and CD8+ T cell counts greatly increased to normal levels, indicating a reversal of lymphopenia, which is a common feature of COVID-19 patients and improvements related to disease severity and mortality [14]. Study characteristics of the treatment of mesenchymal stem cells in COVID-19 patients are presented in Table 1.
| S.No | Authors/Year | Country | Study Design | Types of Cell | Result Measurement | Conclusion |
|---|---|---|---|---|---|---|
| 1. | [36] | United States of America | Prospective cohort study | Exosomes derived from allogeneic BMMSCs | • Patients receiving treatment with mesenchymal stem cells had a higher survival rate of 80%, a cure rate of 71% (17 of 24), and a lower mortality rate of 16%. • The patient's clinical status and oxygenation improved significantly by 192% (P < 0.001). |
The therapeutic use of mesenchymal stem cells provides a clinical improvement in the treatment of COVID-19 |
| 2. | [37] | Chinese | Pilot Study |
ACE-Mesenchymal stem cell (MSC) |
• Within 2-4 days after receiving the intervention, all symptoms (high fever, weakness, shortness of breath) disappeared. • There are no reported side effects of mesenchymal stem cell therapy. • There is an increase in oxygen saturation. • And the time for RT-PCR conversion to negative is faster. |
There is a clinical improvement in the treatment of COVID-19 towards the use of mesenchymal stem cell therapy |
| 3. | [33] | Chinese | Case Report | hUCMSCs | • After the distribution of hUCMSCs, clinical outcomes such as CD3+ T cells, CD4+ T cells, and CD8+ T cells greatly increased to normal levels. • Shows improvement in lymphopenia. • And reduce severity and mortality. |
Mesenchymal stem cell therapy shows good clinical results in COVID-19 towards the use of mesenchymal stem cell therapy |
| 4. | [39] | Chinese | Case Report | UC-MSCs | • Lung function improved significantly • Absolute lymphocyte count, O2, and PaO2 increased. • Absolute neutrophil count and IL6 decreased. No allergic reaction was seen after the distribution of UC-MSC. |
Mesenchymal stem cell therapy shows good clinical results in COVID-19 patients with critical categories and severe lung inflammation |
| 5. | [38] | Chinese | Case Report | Umbilical cord Wharton’s jelly- derived MSCs (hWJCs) | • The patient's clinical symptoms are significantly reduced (out of breath, limp, increased lymphocytes) • Improved immune function (CD3+ T cells, CD4+ T cells, and CD8+ T cells) • Decreased inflammatory factors (IL-6 and TNF-α) |
There have been clinical improvements in the treatment of COVID-19 towards the use of mesenchymal stem cell therapy. |
| 6. | [40] | Chinese | Prospective cohort study | hUC-MSCs | • The cure rate of patients with hUC-MSCs was higher than the control group (p<0.001). • The mortality rate of patients with hUC-MSCs was lower than the control group (p=0.543). • Time to clinical improvement in the hUC-MSC group was shorter than in the control group (p=0.006). |
Mesenchymal stem cell therapy has good clinical effects and can reduce the death rate of COVID-19 patients. |
| 7. | [41] | Chinese | Case Report |
Mesenchymal stem cell (MSC) |
• Boost immunity (CD4+ cells and lymphocytes) • Lowers inflammatory indicators (IL6 and C reactive protein). • Increase saturation (O2) and partial pressure of oxygen (PO2) |
Mesenchymal stem cells therapy is able to increase the patient’s survival rate |
| 8. | [42] | Indonesia | Randomized Clinical Trial | Human Umbilical Cord Mesenchymal Stromal Cells (Huc-Mscs) | • Patient with MSC has a higher recovery level of 74% than control group who has 28,6% • Patient’s survival rate with MSC has 2,5 times higher than control group (P = 0.047) • Patient with MSC experiences IL6 drop significantly than control group (P= 0.023) |
Mesenchymal stem cells therapy is able to increase the patient’s survival rate |
*Huc-Msc (Human Umbilical Cord Mesenchymal Stromal Cells).
* hWJCs (Umbilical cord Wharton’s jelly- derived MSCs).
CONCLUSION
MSCs therapy has been found to be the most effective and promising treatment to cure COVID-19 in critically ill patients. It works in depressing the immune system overactivation, preventing lung fibrosis, and improving lung function. MSCs are also able to decrease cytokine levels without causing allergic reactions. Referring to all clinical effects caused, MSCs are expected to reduce the death rate of COVID-19 patients. However, it is too early to identify the potential therapeutic role of MSCs due to the fact that there are more mechanisms that need to go through a well-designed clinical trial, and more data are required to be collected for further analyses. Further research needs to be executed with MSC therapy tests on a large scale to validate its clinical improvements.
LIST OF ABBREVIATIONS
| MSCs | = Mesenchymal stem cells |
| WHO | = World Health Organization |
| COX-2 | = Cyclooxygenase–2 |
| NO | = Nitrous oxide |
| MODS | = Multiple Organ Dysfunction syndromes |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none


