All published articles of this journal are available on ScienceDirect.
Evaluating the Effect of Underlying Pulmonary Disease on the Clinical Outcome and survival among Patients with COVID-19: Using Propensity Score Matching
Abstract
Background
Coronavirus (COVID-19) is a life-threatening factor throughout the world. Having an underlying disease among the patients with this disease diminishes the clinical effectiveness and increases their mortality rate. Hence, the study was carried out to compare the clinical outcomes in patients with COVID-19 with and without pulmonary disease using propensity score matching.
Methods
This case-control study was conducted on 299 COVID-19 patients with pulmonary disease (case group) and 299 COVID-19 patients without pulmonary diseases (control group). Matching the patients in the case and control groups was done using propensity score matching. Logistic regression was used to assess the effect of factors on the patient's clinical outcome (recovery-death), and the Cox model was used to determine the factors affecting patient survival. Data were analyzed in R software.
Results
The mean (SD) of the patients' age in the case and control groups was 65.49 (15.55) and 65.67 (15.55), respectively. The results of the logistic regression model showed that age, pulmonary disease, nausea, and blood oxygen affect patient death. The results of the Cox proportional-hazards model indicated that the variables of age, blood oxygen, and pulmonary had a significant effect on patient survival.
Conclusion
Given the high mortality rate among patients with COVID-19 and chronic pulmonary disease, these patients are considered a high-risk group and need special care.
1. BACKGROUND/INTRODUCTION
COVID-19 is a highly contagious and fatal infection caused by severe coronavirus disease, the acute respiratory syndrome virus [1]. From October 31, 2020, the coronavirus spread rapidly throughout the world (in more than 236 countries) with the global mortality rate continuing to increase, with an unprecedented effect on global economic performance [2]. Older patients and patients with chronic underlying diseases who have a weaker immune system are especially at greater risk and are more vulnerable [3]. Some patients may develop pneumonia or end in multiple organ failure or even death, while most patients have mild symptoms, and the overall mortality rate of diagnostic cases has been 3.4% [4]. In patients with underlying disease and older ones, the mortality rate was about 20%-22.7% [5, 6]. Most of those dying from COVID-19 have a history of cancer, hypertension, diabetes, or other chronic illnesses [5]. Although the studies on the effects of underlying diseases on the risk of patients with COVID-19 are increasing, most studies have been reported unadjusted because of the small sample size [7-10]. Nonetheless, the studies published have shown a higher risk of mortality in those with chronic underlying diseases compared to the general population with COVID-19 [11-17].
A major limitation of retrospective cohort studies is that they are at high risk for many research biases. To remove the effects of confounders as much as possible and to check fewer variables in the multivariable models due to low sample size, propensity score matching for disease severity and other variables has been utilized in some observational studies [18-20].
Hence, the purpose of the study was to examine the effect of pulmonary disease on the outcome of patients' treatment and to evaluate the factors affecting the survival of patients with COVID-19 according to a case-control study in Hamadan.
2. METHODS
2.1. The Study Design and the Participants
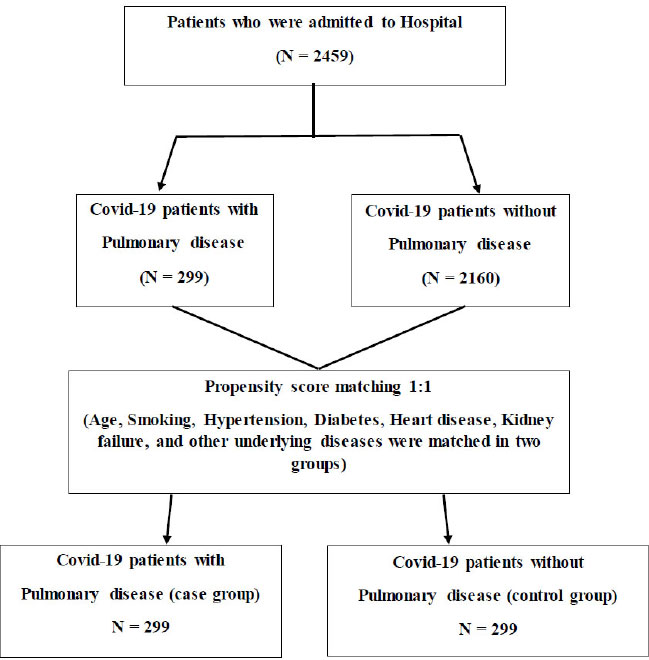
About 2459 patients who were admitted to Sina Hospital in Hamadan, Iran, from February 2020 to December 2020 due to COVID-19 disease were assessed in this study. Patients whose PCR test results were positive, had features compatible with COVID-19 at chest imaging (computed tomography, ultrasonography or radiography), or had severe clinical symptoms, such as shortness of breath or low blood oxygen levels, were included in our study. The underlying diseases of patients were extracted from medical records. In this case-control study, 299 pulmonary patients formed the case group, and 299 non-pulmonary patients were selected from 2160 non-pulmonary disease patients using the propensity score matching method based on age, smoking and other underlying diseases, including hypertension, diabetes, lung and heart disease as the control group. All patients participating in this study were not vaccinated. Fig. (1) shows how to select a control group. All demographic characteristics of the patient, clinical characteristics, underlying diseases, clinical signs upon diagnosis, measures taken, and vital signs were obtained from patients' medical records. Lab findings and computed tomography (CT) images were carried out during hospitalization. The study was confirmed by the Ethics Committee (IR.UMSHA.REC. 1399.1050).
In this study, we included hospitalized COVID-19 cases, both confirmed with PCR or serological tests and those exclusively diagnosed based on clinical criteria (i.e., symptoms, imaging, and laboratory results). Also, all patients who had incomplete information or were treated on an outpatient basis were excluded from the study.
2.2. Statistical Analysis
Quantitative variables were described by means (SD) and their mean was compared by t-test of two independent groups. Qualitative variables in both groups were described using frequency (%) and compared with the Chi-square test or Fisher's exact test. Matching was performed based on the propensity score by the nearest neighbor method with a ratio of (1: 1). Chi-square test and plotting were used for examining the variability of variables before and after matching. The aim was to achieve a balanced distribution of all the covariates in the propensity score-matched (PSM) cohort. Pulmonary diseases in this study included asthma 48%, COPD 41%, pneumonia 7%, and chronic bronchitis 3%. Fig. (2) shows the frequency of patients as well as the mortality rate of patients in different groups.
MatchIt, survival and survminer packages were used for matching and survival analyses. The logistic regression model was used to estimate the odds ratio of patients' death outcomes. Stepwise regression using the backward selection (Wald) method was also performed to obtain an optimal model and further validate the findings. The optimal model only included the variables that contributed significantly to the model. To compare the survival of patients for time from admission to death for patients who died (days), the Cox regression model was used. The findings were compared before and after matching. The analyses were carried out using R software version 3.6.2. The significance level of the tests was considered to be 5%.
3. RESULTS
Out of 2459 patients, 299 patients had pulmonary disease (case group), and 2160 did not. Table 1 shows the demographic and clinical characteristics of the patients. Before matching, Age, hypertension, and smoking in these subgroups were statistically significant (p <0.05). Matching was carried out based on age, smoking and all underlying diseases. After matching, no significant differences were observed between the demographic and clinical variables of patients.
The Cox proportional hazard model was turned to in the study to examine the effect of pulmonary disease on the survival of patients with COVID-19. The results of the univariable Cox regression model before and after matching for the pulmonary underlying disease are given in Table 2. Pulmonary disease before matching had no statistically significant effect on the survival of patients with COVID-19 (p = 0.18); however, after matching, the effect of this variable on survival was statistically significant (p = 0.01). Thus, one can state that the underlying pulmonary diseases affect the survival of patients with COVID-19. Table 3 presents the results of a simultaneous evaluation of these variables. The findings indicated that pulmonary disease, age and blood oxygen affected patient survival (p <0.05). The Hazard of death among the patients with the underlying pulmonary disease was 1.61 times higher than those without the disease. With a one-year increase in the patient's age, the hazard of death increased by 4%, and the hazard of death decreased by 5% with an increase of one unit in blood oxygen level. Furthermore, the survival function of the patients before (Fig. 3) and after matching (Fig. 4) in case and control groups using Kaplan-Meier plot was given for the time from admission to death (days). Fig. (4) shows that survival probability was statistically different in the two groups after matching, with the pulmonary disease patients having lower survival rates (P<0.05).

| Characteristics | Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|---|
|
Case Group (n=299) |
Group Control (n=2160) |
P-value |
Case Group (n=299) |
Group Control (n=299) |
P-value | ||
| ( SD ± mean ) Age | 65.49 ± 15.55 | 17.32 ± 59.66 | <0.01 | 15.55 ± 65.49 | 15.55 ± 65.68 | 0.85 | |
| Sex (male (%)) | 160 (53) | 1155 (53) | 1 | 160 (53) | 161 (54) | 1 | |
| Temperature (mean ± SD) | 37.32 ± 0.76 | 0.76 ± 37.33 | 0.84 | 37.32 ± 0.76 | 0.80 ± 37.38 | 0.38 | |
| Hypertension (yes (%)) | 122 (41) | 725 (34) | 0.02 | 122 (41) | 115 (38) | 0.62 | |
| Systolic BP (mean ± SD) | 19.84 ± 123.20 | 18.62 ± 121.10 | 0.08 | 19.84 ± 123.20 | 18.14 ± 124.70 | 0.33 | |
| Diastolic BP (mean ± SD) | 11.64 ± 76.07 | 11.73 ± 75.55 | 0.47 | 11.64 ± 76.07 | 11.67 ± 76.68 | 0.52 | |
| Smoking (yes (%)) | 61 (20) | 132 (6) | <0.01 | 61 (20) | 60 (20) | 1 | |
| Heart disease (yes (%)) | 53 (18) | 143 (6) | <0.01 | 53 (18) | 40 (13) | 0.18 | |
| Diabetes (yes (%)) | 51 (17) | 408 (19) | 0.49 | 51 (17) | 54 (18) | 0.83 | |
| Kidney failure (yes (%)) | 19 (6) | 85 (4) | 0.07 | 19 (6) | 9 (3) | 0.08 | |
| Other diseases (yes (%)) | 94 (31) | 751 (35) | 0.28 | 94 (31) | 105 (35) | 0.38 | |
| Death (%) | 72 (24) | 378 (17) | 0.01 | 72 (24) | 26 (9) | <0.01 | |
| Clinical Severity of Individual Patients | |||||||
| Blood oxygen (mean ± SD) | 11.98 ± 81.28 | 10.80 ± 84.35 | <0.01 | 11.98 ± 81.28 | 11.14 ± 84.58 | <0.01 | |
| Percentage of lung involvement (mean (SD)) | 72.36 ± 12.86 | 71.43 ± 15.07 | 0.24 | 72.36 ± 12.86 | 56.41 ± 16.65 | <0.01 | |
| Lymphocyte level (109/L) (mean (SD)) | 2.24 ± 1.01 | 2.34 ± 1.24 | 0.14 | 2.24 ± 1.01 | 3.78 ± 1.39 | <0.01 | |
| Measures taken in the Hospital | |||||||
| Length of hospitalization (mean ± SD) | 7.46 ± 6.10 | 7.34 ± 5.94 | 0.74 | 7.46 ± 6.10 | 6.25 ± 4.87 | <0.01 | |
| ICU admission (yes (%)) | 99 (33) | 609 (28) | 0.09 | 99 (33) | 55 (18) | <0.01 | |
| Oxygen therapy (%) | 286 (96) | 2050 (95) | 0.68 | 286 (96) | 280 (94) | 0.36 | |
| Mechanical ventilation (%) | 74 (25) | 369 (17) | <0.01 | 74 (25) | 26 (9) | <0.01 | |
| Length of ICU stay (days) (mean ± SD) | 2.48 ± 5.76 | 2.23 ± 5.48 | 0.47 | 2.48 ± 5.76 | 1.30 ± 4.29 | <0.01 | |
| Patient Clinical Signs | |||||||
| Asthma (yes) (%) | 225 (75) | 1233 (57) | <0.01 | 225 (75) | 184 (61) | <0.01 | |
| Diarrhea (yes) (%) | 28 (9) | 226 (10) | 0.63 | 28 (9) | 26 (9) | 0.89 | |
| Dry cough (yes) (%) | 101 (34) | 936 (43) | <0.01 | 101 (34) | 124 (41) | 0.06 | |
| Sputum cough (yes) (%) | 80 (27) | 372 (17) | <0.01 | 80 (27) | 59 (20) | 0.05 | |
| Muscle pain (yes) (%) | 117 (39) | 934 (43) | 0.20 | 117 (39) | 104 (35) | 0.31 | |
| Fever (yes) (%) | 166 (55) | 1159 (54) | 0.59 | (55)166 | 158 (53) | 0.57 | |
| Chills (yes) (%) | 164 (49) | 990 (64) | 0.36 | (49)164 | (46)126 | 0.12 | |
| Sore throat (yes) (%) | 15 (5) | 90 (4) | 0.60 | 15 (5) | 8 (3) | 0.20 | |
| Nausea (yes) (%) | 65 (22) | 489 (23) | 0.78 | 65 (22) | 56 (19) | 0.41 | |
| Headache (yes) (%) | 44 (15) | 374 (17) | 0.30 | 44 (15) | 47 (16) | 0.82 | |
| Fatigue (yes) (%) | 8 (3) | 61 (3) | 1 | 8 (3) | 12 (4) | 0.49 | |
| Vomit (yes) (%) | 58 (19) | 422 (19) | 1 | 58 (19) | 49 (16) | 0.39 | |
| Runny nose (yes) (%) | 1 (0) | 12 (0) | 1 | 1 (0) | 1 (0) | 1 | |

| Characteristics (Reference) | Before Matching by PSM | After Matching by PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Lower | Upper | Lower | Upper | |||||
| Sex | 1.21 | 1.00 | 1.47 | 0.05 | 0.70 | 0.61 | 1.39 | 0.92 |
| Age (male) | 1.03 | 1.03 | 1.04 | <0.01 | 1.04 | 1.02 | 1.06 | <0.01 |
| Hypertension (yes) | 1.22 | 1.01 | 1.48 | 0.04 | 0.99 | 0.66 | 1.50 | 0.97 |
| Smoking (yes) | 1.03 | 0.73 | 1.47 | 0.85 | 1.05 | 0.65 | 1.70 | 0.85 |
| pulmonary disease (yes) | 1.20 | 0.92 | 1.58 | 0.18 | 1.77 | 1.14 | 2.74 | 0.01 |
| Heart disease (yes) | 1.37 | 0.93 | 2.21 | <0.01 | 1.43 | 0.93 | 2.21 | 0.11 |
| Diabetes (yes) | 1.13 | 0.91 | 1.42 | 0.27 | 0.76 | 0.44 | 1.31 | 0.33 |
| Kidney failure (yes) | 1.24 | 0.80 | 1.92 | 0.34 | 1.19 | 0.48 | 2.93 | 0.71 |
| Cancer (yes) | 2.40 | 1.58 | 3.66 | <0.01 | 1.66 | 0.23 | 12 | 0.61 |
| Other diseases (yes) | 1.15 | 0.95 | 1.39 | 0.16 | 0.83 | 0.53 | 1.29 | 0.42 |
| Temperature | 0.93 | 0.83 | 1.1 | 0.30 | 0.99 | 0.77 | 1.29 | 0.97 |
| Blood oxygen | 0.97 | 0.96 | 0.97 | <0.01 | 0.95 | 0.94 | 0.96 | <0.01 |
| Asthmatic (yes) | 1.15 | 0.94 | 1.41 | 0.16 | 0.76 | 0.45 | 1.29 | 0.31 |
| Diarrhea (yes) | 0.96 | 0.68 | 1.34 | 0.81 | 0.98 | 0.45 | 2.12 | 0.96 |
| Dry cough (yes) | 0.77 | 0.63 | 0.94 | 0.01 | 0.93 | 0.61 | 1.42 | 0.74 |
| Muscles pain (yes) | 0.75 | 0.62 | 0.92 | <0.01 | 0.73 | 0.46 | 1.15 | 0.17 |
| Fever (yes) | 0.86 | 0.80 | 1.03 | 0.11 | 1.25 | 0.83 | 1.89 | 0.29 |
| Chills (yes) | 0.85 | 0.70 | 1.03 | 0.09 | 1.10 | 0.73 | 1.67 | 0.64 |
| Sore throat (yes) | 0.72 | 0.40 | 1.32 | 0.29 | 0.93 | 0.23 | 3.80 | 0.92 |
| Nausea (yes) | 0.96 | 0.77 | 1.20 | 0.75 | 1.56 | 1.00 | 2.45 | 0.05 |
| Vomit (yes) | 0.89 | 0.70 | 1.14 | 0.37 | 1.34 | 0.84 | 2.15 | 0.22 |
| Fatigue (yes) | 1.49 | 0.63 | 2.09 | 0.65 | 0.77 | 0.19 | 3.14 | 0.72 |
| Headache (yes) | 0.73 | 0.55 | 0.97 | 0.03 | 0.62 | 0.30 | 1.29 | 0.20 |
| Sputum cough (yes) | 0.88 | 0.68 | 1.13 | 0.31 | 0.76 | 0.45 | 1.29 | 0.31 |
| Characteristics (Reference) | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| *Age | 1.04 | 1.02 | 1.06 | <0.01 |
| pulmonary disease (yes)* | 1.61 | 1.02 | 2.52 | 0.04 |
| Blood oxygen level* | 0.95 | 0.94 | 0.97 | <0.01 |
| Nausea (yes) | 1.39 | 0.88 | 2.19 | 0.15 |

| Characteristics (Reference) | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| *Age | 1.07 | 1.05 | 1.10 | <0.01 |
| Sex | 1.05 | 0.68 | 1.67 | 0.81 |
| Smoking | 1.26 | 0.73 | 2.13 | 0.37 |
| High blood pressure (yes) | 1.18 | 0.75 | 1.85 | 0.47 |
| Temperature | 0.99 | 0.73 | 1.30 | 0.92 |
| Blood oxygen level* | 0.93 | 0.92 | 0.95 | <0.01 |
| pulmonary disease (yes)* | 2.27 | 1.43 | 3.70 | <0.01 |
| Kidney failure (yes) | 1.25 | 0.41 | 3.12 | 0.66 |
| Diabetes (yes) | 0.94 | 0.51 | 1.67 | 0.85 |
| Heart disease (yes)* | 2.27 | 1.39 | 3.70 | <0.01 |
| Cancer (yes) | 0.77 | 0.04 | 4.35 | 0.81 |
| Other diseases (yes) | 0.94 | 0.58 | 1.51 | 0.82 |
| Asthmatic (yes)* | 1.96 | 1.18 | 3.45 | 0.01 |
| Diarrhea (yes) | 0.79 | 0.32 | 1.69 | 0.58 |
| Dry cough (yes) | 1.01 | 0.63 | 1.56 | 0.98 |
| Muscles pain (yes) | 0.66 | 0.40 | 1.05 | 0.09 |
| Fever (yes) | 1.03 | 0.66 | 1.61 | 0.89 |
| Chills (yes) | 0.99 | 0.63 | 1.54 | 0.95 |
| Sore throat (yes) | 0.51 | 0.08 | 1.75 | 0.36 |
| Nausea (yes)* | 2.04 | 1.23 | 3.33 | <0.01 |
| Vomit (yes)* | 1.89 | 1.12 | 3.12 | 0.01 |
| Fatigue (yes) | 0.59 | 0.09 | 2.13 | 0.49 |
| Headache (yes) | 0.48 | 0.21 | 0.97 | 0.06 |
| Sputum cough (yes) | 0.70 | 0.39 | 1.20 | 0.22 |
| Characteristics (Reference) | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age* | 1.07 | 1.05 | 1.09 | <0.01 |
| pulmonary disease (yes)* | 2.27 | 1.35 | 3.90 | <0.01 |
| Nausea (yes)* | 2.15 | 1.20 | 3.81 | <0.01 |
| Blood oxygen* | 0.94 | 0.93 | 0.96 | <0.01 |
Additionally, unadjusted logistic regression was used after matching to assess the covariates of patients with COVID-19 on clinical outcomes (to identify significant variables that affect patient recovery) (Table 4). The effect of other diseases and laboratory and clinical symptoms were adjusted with multivariate logistic regression, whose results are given in Table 5. The results showed that having chronic pulmonary disease is an influential factor in the outcome of clinical of patients, so the odds of death in patients with the pulmonary disease is 2.27 times higher than in patients without it. Furthermore, based on the adjusted regression outcomes, besides pulmonary disease, age, nausea and blood oxygen level significantly affect patient mortality rate (p <0.05).
4. DISCUSSION
In this case-control study, demographic, clinical and laboratory characteristics of 299 patients with chronic pulmonary disease and 299 without COVID-19 patients in Hamadan were examined. Then, the effects of the factors that affect the odds of death among them were determined using a logistic regression model and the factors affecting on survival of patients were evaluated using the Cox proportional hazard model.
Although age had been matched between case and control groups in the study, it was associated with patient survival; that is, the hazard of death in patients with COVID-19 increased with an increase in age. As the increase in age decreases the immune function of the body widely [15], older people and those with underlying diseases are affected by more severe infections of the COVID-19 virus and are more likely to experience more adverse consequences [21, 22]. The cause of higher mortality rate in pulmonary patients in other studies was reported to be their advanced age [23, 24]. Additionally, the study results indicated that gender has no relationship with mortality that is in line with the results of other studies [16-20, 25-33].
Some other studies have approved the relationship between chronic diseases like diabetes and hypertension and mortality rate among COVID-19 patients [7-11, 18]. As the effect of other diseases had been modified in the study, the relationship between chronic pulmonary disease and clinical outcome with survival rate of patients with COVID-19 was significant and was in line with some studies too [34-36]. The mortality rate of the patients with pulmonary diseases was twice as high as other patients in the present study. Some studies revealed that viral infections could be related to exacerbation of pulmonary disease [14]. Furthermore, using immuno- suppression to treat pulmonary patients might increase COVID-19 risk [37].
Nonetheless, an unadjusted meta-analysis evaluated the patients with underlying diseases of COVID-19, arguing that many prevalent underlying diseases might be risk factors for exacerbating COVID-19 [8]. While reporting the results of a modified analysis, Guan et al. claimed that COVID-19 patients with any underlying disease had a lower prognosis compared to other patients [9]. In a study of 17 million patients in the UK, Drack et al. revealed that patients with chronic pulmonary disease were at higher risk than other COVID-19 patients (Relative Risk 1.95 (95% CI: 1.86-2.04)) and even the risk of death in them is higher than patients with cancer or heart disease [38]. In this study, the hazard of death among the pulmonary patients was 1.61 (confidence interval: 0.62-1.02) equal to other patients. Esposito et al. in America showed the mortality rate among patients with COVID-19 with chronic pulmonary disease was 3.2 times higher than in non-pulmonary patients [34]. The odds of mortality in pulmonary patients were 2.27 times higher than other patients in our study.
In the present study, the patients' main symptoms were 68.4% dyspnea, 54.2% fever, 45.5% chills, 37.6% dry cough, and 37% muscle pain in all patients. In Yang et al., the symptoms were 78% fever, 74% dry cough, 37% pain, 37% sputum cough, and 35% dyspnea [8]. In Tian et al., the critical symptoms were fever, dry cough, muscle pain, and sputum cough [39]. In Yang et al. and Tian et al., fever and dry cough were the most important clinical symptoms among the patients [8, 39]. In other studies, similar to our study, dyspnea, cough and fever have been the key symptoms of the patients [7]. These symptoms have been considered as the factors affecting patient survival in some studies [40]. In the present study, only nausea was significant in the logistic regression model and was effective in the odds of patients' mortality. Logistic and Cox regression models revealed that blood oxygen level affects the survival of patients, and other symptoms do not significantly affect patient survival. Likewise, Park et al. argued that symptoms like fever and dry cough can be considered as the primary causes of COVID-19 disease; however, when the symptoms worsen and become more severe, respiratory symptoms like dyspnea and lack of saturated oxygen can lead to death [41].
At the beginning of the coronavirus pandemic, most of the patients assessed in this study underwent drug therapy such as Azithromycin, Ceftriaxone, Kaletra, Lopinavir/ritonavir, Hydroxychloroquine, Ribavirin, Ceftriaxone and Vancomycin. However, patients today have more treatment options in the battle against coronavirus disease [42-47].
CONCLUSION
This study examined the effect of pulmonary disease on COVID-19 patients’ survival and clinical outcomes by adjusting the effect of various factors. The findings revealed that patients with pulmonary disease will be at a higher mortality risk compared to other patients with COVID-19. Hence, they are naturally a vulnerable population with a higher mortality rate compared to the general population and thus require intensive care. Therefore, screening these patients and their identification and vaccination could diminish the mortality rate among these patients.
LIMITATIONS OF THE STUDY
This study had some limitations. First, the lack of detail in some clinical information of the patients made it impossible to evaluate the effect of this information on the investigated outcomes. Second, this was a retrospective study with underlying disease (such as underlying pulmonary disease) extracted from medical records, and we could not analyze the effects of the severity of COPD and other types of pulmonary disease on COVID-19. Third, this study was a single-center study and focused on one province of Iran (Hamadan) instead of all over the country. Therefore, certain aspects cannot be generalized. Finally, the diagnosis of types of pulmonary disease could have been underestimated, given that spirometry was not performed during the pandemic period, and the disease might have been even more prevalent.
LIST OF ABBREVIATIONS
| HR | = Hazard Ratio |
| OR | = Odds Ratio |
| COVID | = Coronavirus Disease |
| CT | = Computed Tomography |
| PSM | = Propensity Score Matched |
| PCR | = Polymerase Chain Reaction |
| SD | = Standard Deviation |
| COPD | = Chronic Obstructive Pulmonary Disease |
| CI | = Confidence Interval |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The proposal of this study was approved by the Institutional Review Board (IRB) of Hamadan University of Medical Sciences (ethical code: IR.UMSHA.REC.1400.004, project no: 14000207892).
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
All individual participants included in the study gave their informed consent for inclusion in the study and for publication of the manuscript.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and materials that support the findings of this study are available from the corresponding author [G.R] upon request.
FUNDING
Not applicable.
CONFILCT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the Vice-Chancellor of Research and Technology, Hamadan University of Medical Sciences, for the approval and support of the study. This article is part of project number 9912199270 with the specific Ethics ID code IR.UMSHA.REC.1399.1050.


