All published articles of this journal are available on ScienceDirect.
Phenylketonuria Incidence based on the Results of the Neonatal Screening Program and Evaluation of this Program-based Surveillance Data System in Sirjan City from 2012 to 2019
Abstract
Background
PKU is the most common hereditary metabolic disease. Considering the severe complications caused by the lack of timely diagnosis of this disease, it is important to carry out the newborn screening process properly.
Aim
This study aims to determine the incidence of phenylketonuria (PKU) based on the results of the neonatal screening program and evaluate the indicators of this program in Sirjan City from 2012 to 2019.
Methods
The present study was across-section descriptive, and all screening babies were examined from 2012 to 2019. Screening information was obtained from data recorded in the neonatal screening program and patient information from the national form of epidemiological investigation of genetic diseases in the Kerman Health Department. Excel software was used to draw charts. Descriptive statistics were used to describe the variables. Information was provided as a frequency table and chart.
Results
The overall screening coverage was 95.3%. Among this, 93.2% of infants were screened during the 3 to 5 days of life. The incidence of PKU was found to be 1.33 in 10,000 live births. Around 0.44% of the samples were inappropriate, and the percentage of inappropriate samples was from the beginning of the program and monitored across each year. On the first visit to the PKU treatment center, 50% of infants were below 2 weeks. About 100% of patients identified in screening were the first child in the family, and around 83.3% of parents were consanguineous.
Conclusion
The incidence of PKU in Sirjan is in line with the national average. Implementation of the screening program showed a significant impact on the timely diagnosis and the onset of patients. It is necessary to increase household awareness about the consequences of consanguineous marriages, especially in areas with a high prevalence of PKU.
1. INTRODUCTION
PKU, a metabolic disorder, is inherited in an autosomal recessive manner [1]. PKU is a disorder where the body lacks the enzyme phenylalanine hydroxylase. This enzyme is needed to convert the amino acid phenylalanine into tyrosine. Without this enzyme, the level of phenylalanine in the body rises, leading to issues in normal metabolism and potential brain damage [2]. The child who is affected starts off being submissive from birth. If the child is not identified and treated, it leads to the development of neuronal issues and will gradually worsen over time [3, 4]. Today, within the initial days after a baby is born, individuals with PKU are diagnosed through a screening test. This test involves taking a few drops of blood from the baby's heel and measuring the level of phenylalanine present [5, 6].
The primary treatment for this disease involves following a suitable diet with restricted phenylalanine intake under the guidance of a nutritionist. This dietary adjustment, implemented during the first two weeks of life, helps to mitigate the effects of the disease and promotes normal growth and development [7, 8].
According to the conducted studies, the occurrence of this disease varies among countries. In Thailand, the lowest recorded incidence of this disease is 1 in every 327,740 live births [9], and the highest incidence is 1 in 2600 live births in Turkey [10]. PKU is also recognized as the most frequently inherited metabolic disease in Iran [11, 12]. The occurrence of this disease is estimated to be 1 in every 7000 live births across the entire country [12]. Due to the high occurrence of marriages with in the family, this field has one of the highest recorded statistics [13]. According to a study conducted in Iran, it was found that 71% of the families with PKU patients mainly due to consanguineous marriage [14]. Studies conducted in Iran have reported different incidences of this disease [15-17]. The prevention and control program for PKU was introduced in Iran in 2007 with the aim of reducing the impact of this disease [18, 19].
There are strategies to tackle PKU, such as offering complete clinical services at PKU treatment centers, providing genetic counseling, and supporting the parents and relatives of patients who are at risk to decrease the number of people affected by PKU. Due to limited laboratory resources in the entire country, newborn screening for early detection of the disease was initially available only at six universities. However, starting from 2012, it was gradually expanded to the entire country, including the province of Kerman. This expansion was necessary to ensure standardized clinical services were available [20]. In order to prevent serious complications caused by delayed diagnosis of a disease, it is crucial to ensure that the newborn screening process is carried out with high quality. To achieve this, it is necessary to review the performance indicators of the screening program and identify any potential reasons for not achieving the desired goals. By understanding the current situation, appropriate interventions can be implemented promptly. This study was conducted to determine the occurrence of PKU and assess the indicators of newborn screening programs in the population served by the Kerman University of Medical Sciences.
2. METHODS AND MATERIALS
This cross-sectional retrospective study was conducted to examine the health system and newborn screening from 2012 to 2019. The researchers obtained permission from the ethics committee of the Kerman University of Medical Sciences Research Deputy. They collected information is mainly based on the data obtained during newborn screening. The patients' details were extracted from the national form of the epidemiological investigation of genetic diseases. It contains information on the patients' gender, date of birth, and the condition of their physical siblings. The form was completed by an expert from the genetics program at the Sirjan Health Center and approved by the genetics program expert from the Kerman Health Department. The information was gathered from the patients' family, genetic files, household health files, and patient treatment files at the PKU treatment center.
This study focused on Iranian patients who were treated at the PKU treatment center, and the disease was confirmed by a pediatrician based on the HPLC analysis. To ensure privacy, only the first author of the article had access to the epidemiological survey forms. The incidence rate was calculated using the number of live births obtained from the Civil Registry website.
The following formula was used to calculate the incidence rate:
The number of positive hospital cases identified from newborn screening/living birth in the same year *10,000.
Excel software was used to draw graphs. Descriptive statistics were used to describe the variables. The information was presented in the form of frequency tables and graphs.
2.1. Newborn Screening Process
The screening test for newborns is conducted between the third day (72 hours after breastfeeding) to the fifth day after birth, provided that the baby has been adequately fed. To perform the test, 5 drops of blood were taken from the baby's heel on 903 Whatman filter paper, as instructed by the guidelines followed in the country. The amount of phenylalanine in the blood was then determined using the calorimetric method. If the level of phenylalanine was high (more than 4 mg/dL), it was considered a positive screening result. To further confirm, the test was conducted using the high-performance liquid chromatography (HPLC) method after taking another blood sample on filter paper. This sample was then sent to an approved laboratory. If the phenylalanine level, as determined by the HPLC method, was equal to or greater than 3.4 mg/dL, it was considered a positive confirmation result. These individuals were then referred to a specialized PKU hospital in the center of the province. Only after a specialist doctor confirmed the diagnosis through further tests (like what), the individuals were officially recognized as confirmed patients.
3. RESULTS
A total of 44,791 newborns were included in this study, of which 42,686 (95.3%) underwent screening during 2012 – 2019. Out of the screened infants, 39,802 (93.2%) participated in the screening program within 3 to 5 days of birth. The phenylalanine level of 42,651 (99.9%) newborns in the screening test was found to be less than 4 mg/dL. However, 35 babies had a positive screening result and were re-tested. After measuring the phenylalanine level using the HPLC method, 7 babies were confirmed to have PKU and were referred to a treatment center.
Additionally, 6 babies were diagnosed with PKU based on the opinion of a pediatrician. The incidence rate of PKU was found to be 1.33 per 10,000 live births. The results of the newborn screening and the incidence rate can be seen in Table 1.
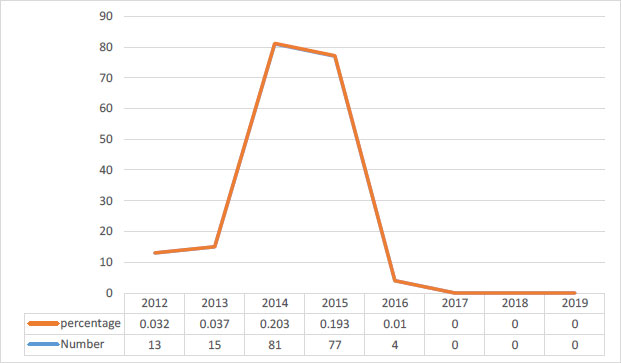
A total of 1753 babies, which accounts for 4.10% of the total, required resampling. Out of these, 1563 babies (89.1%) were re-sampled due to special conditions, such as dialysis, while 190 babies (10.8%) were rejected because the initial samples were inappropriate. The reasons for the inappropriate samples included transfusion, exchange, lack of feeding with milk or feeding with substances, low protein, and other medical reasons. In total, 0.44% of the samples were deemed inappropriate. Fig. (1) illustrates the decreasing trend of the percentage of inappropriate samples over time, starting from the integration of the program until the time of the study. For cases where the HPLC test showed levels higher than 3.4 mg/dL in 3 infants (50%), the first visit to the selected hospital occurred within the first 2 weeks of their lives (Fig. 2). All parents of these infants who showed elevated levels of phenylalanine were referred to the genetic counseling center. Additionally, 100% of the patients and their parents were referred to health service centers and received ongoing care. It is worth noting that all of the patients identified in the screening were the first children in their respective families, and 83.3% of the patients' parents were relatives.
| Date | Newborns | Number of Newborns Screened (Percentage) |
Number of Newborns Screened during first 3 to 5 Days (Percentage) |
Phenylalanine Levels (At Screening) |
Phenylalanine Levels (At Diagnosis Confirmation) |
Number of Patients | Incidence in 10000 live Births | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <4 mg/dl |
4-19.9 mg/dl | ≥ 20 mg/dl | <3.4 mg/dl |
3.4-19.9 mg/dl | ≥ 20 mg/dl | ||||||
| 2012 | 5533 | 3935(71.1) | 3811(96.8) | 3928 | 7 | 0 | 7 | 0 | 0 | 0 | 0 |
| 2013 | 5852 | 5621(96) | 5517(98.1) | 5613 | 7 | 1 | 5 | 0 | 3 | 3 | 5.12 |
| 2014 | 5971 | 5758(96.4) | 5566(96.6) | 5756 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| 2015 | 6147 | 6065(98.6) | 5612(92.5) | 6062 | 2 | 1 | 2 | 0 | 1 | 1 | 1.62 |
| 2016 | 5936 | 5850(98.5) | 5633(96.3) | 5846 | 4 | 0 | 3 | 1 | 0 | 0 | 0 |
| 2017 | 5316 | 5334(100.3) | 5181(97.1) | 5332 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| 2018 | 5220 | 5228(100.1) | 4090(78.2) | 5224 | 4 | 0 | 3 | 0 | 1 | 1 | 1.91 |
| 2019 | 4816 | 4895(101.6) | 4392(89.7) | 4890 | 5 | 0 | 4 | 0 | 1 | 1 | 2.07 |
| Total | 44791 | 42686(95.3) | 39802(93.2) | 42651(99.9) | 33 | 2 | 28 | 1 | 6 | 6 | 1.33 |

4. DISCUSSION
The purpose of this study was to investigate how common PKU is and assess the effectiveness of the newborn screening program in Sirjan City. The study analyzed data from the start of the screening program until 2019.
According to the results of this study, 95.3% of infants participated in the screening program. Similar studies, including Ganji et al. in Chaharmahal Bakhtiari (100%) [21], Saadat Nasab et al. in South Khorasan (100%) [22], Rezabigi et al in Kerman (92%) [23], and Mahmoudi et al in Tehran (91.4%) [24], have reported that infants participated in newborn screening programs with different percentages. Also, Brazil, China, and Iraq have reported screening rates of 94.5%, 93.5%, and 71.7% [25, 26]. Interpreting these different findings in screening coverage in different studies can point to differences in parental literacy and awareness levels, ongoing parental education by the health system about the importance of infant screening, and different years of conducting these screenings.
In the current study, the screening rate was 71.1% in the program integration year and 101.6% at the time of the study. One of the reasons for the low screening coverage in the first year of the implementation of this program is that this screening program started in the fourth month of the year. Therefore, infants who were born before that date were not included in screening, and the high rate of screening in subsequent years indicates the active participation of parents in newborn screening programs. The proximity of several villages in other cities to Sirjan and household preferences for choosing the closest city for neonatal sampling may have influenced the high proportion of newborns tested in the final year of the study, which is consistent with the results of Carvalho et al. In Brazil, the testing rate increased from 54% in 2001 to 80.2% in 2005 [27]. A study conducted in Iraq found that coverage for newborn screening increased from 61% in the year the program started to 73.3% at the time of the study [28]. In this study, there was an increase in the number of people screened in the subsequent years compared to the start of the program, but this increase in the number of people screened was less than the number of people screened in our study. In the interpretation of this finding of the current research, it can be pointed out that in Iran, awareness sessions are held for all pregnant women in the last months of pregnancy, focusing on newborn screening after the birth of the baby. This matter probably had an effect on increasing the number of screenings.
In this study, 93.2% of infants aged 3 to 5 years were tested. Timely screening decreased from program initiation to the time of study. Therefore, it has dropped from 96.8% at the time of program integration to 89.7% in 2019. In a study by Rezabigi et al., 80% of infants were tested with Kerman at the time. Although the percentage of timely screening was lower compared to the present study, contrary to this study, screening on the 3rd to 5th day of birth had an increasing trend [23]. According to the findings of the current research, it can be concluded that it is necessary to give parents the necessary training regarding the importance of timely screening. In this study, all the positive screening cases were tested for confirmation of the diagnosis, and there was no case of parental non-cooperation or failure to respond to an urgent call from the health center. In a study conducted in Iraq, 681 infants in the screening program for PKU, hypothyroidism, and galactosemia were reported to be positive, and 25 families were not informed to perform the confirmation test due to the lack of response to the phone call or the wrong phone number, and 238 families within the presence of information, they did not participate in the confirmation test [28] and the severe complications of these diseases. In addition to receiving several safe contact numbers, it is necessary to obtain the address of the parents so that in case of non-response, they can be actively followed up and the opportunity for timely diagnosis and treatment is not lost for any baby. Also, in order to increase the willingness of all families in the test to confirm the diagnosis, the necessary training should be provided to the families regarding the importance of diagnosing these diseases. Therefore, it is necessary to raise public awareness about the importance of screening at the community level.
In this study, 0.44% of the samples were not accepted, and all infants whose samples could not be tested were asked to be resampled. Because of the agency's sensitivity at the start of the screening program, the proportion of ineligible samples was small in the first few years, but most of the ineligible samples were associated with the middle years of program implementation, and thereafter percentage decreased to zero in 2017. It is possible that the number of inappropriate samples increased during his second and third years of the program because the sampler who received the necessary training early in the program transferred to other departments. Given the confidential nature of this program, the use of experienced samplers and ongoing training ensured no inappropriate samples were taken. This research finding shows that the method of sampling and the use of experienced samplers are among the important things in the PKU screening program. Therefore, the use of experienced and trained samplers can increase the accuracy of screening. In a study conducted in Kerman, he reported an unfit sample rate of 0.34%. According to national guidelines, the average improper sampling across Iran is 1.31%, and the expected volume of improper sampling is estimated to be less than 3% [29]. This indicates that this metric is desirable in this and the above studies.
In this study, 83% of patients were identified and referred to a PKU treatment center before 3 weeks of age. In a study by Rezabeighi et al., 61% of affected infants were identified before 3 weeks of age and referred to a PKU treatment center [23]. Implementation of screening programs in Iran has impacted the early detection and treatment of patients with PKU. Prior to the introduction of screening programs, patients were older, under-diagnosed, and most had severe complications. For example, in a study in Mazandaran, the median age of diagnosis for patients before the integration of the newborn screening program was 20 months [30], whereas in Mexico, the age of diagnosis for patients without a newborn screening program was 20 years. Met. 2 years and 8 months [31].
In this investigation, the frequency of PKU was determined to be 1.33 per ten thousand live births. Rezabeighi et al. conducted a study in Kerman City, where the incidence rate was found to be 1.35 in ten thousand live births [23]. Similarly, Taj-Aldeen et al. conducted a study in Iraq, where the incidence rate was 1.3 in 10,000 live births [28]. These findings are in agreement with the results of the present study. According to recent reports from medical sciences universities throughout the country, the national average incidence of PKU is 1.23 per 10,000 births [32]. However, this figure has been reported differently in various studies. For instance, Motamedi et al. reported an incidence rate of 1.91 per 10,000 live births in Lorestan [33], while Abbas Khanian reported a rate of 0.66 per 10,000 live births in Mazandaran [34]. This difference in the findings can be related to the different time and geographic locations in sampling, as well as cultural issues and the level of parents' awareness of the importance of screening and participation in the screening program.
In the current investigation, it was observed that in certain years under study, the incidence of PKU was reported to be zero. This issue can be attributed to the implementation of the prevention program in Iran in 2007. This prevention program included the identification of carrier couples, prenatal diagnosis tests, and abortion treatment, which ultimately led to accurate screening for PKU and a reduction in the rate of PKU. Daneshi et al. demonstrated in their research that the average expected incidence rate in Kerman was 1.36 patients per ten thousand births without the implementation of the prevention program, which was reduced to 1.13 per ten thousand births with the program [23]. All the patients identified in the screening were the first child of the patient in the family. In a way, the prenatal screening program for PKU has been able to prevent the birth of another sick child in families with a patient with PKU by identifying carrier couples. Daneshi et al.'s study revealed that 97% of the patients born in Kerman after the integration of the prevention and control program in the health system were the first child of the patient in the family, and their parents were unaware of their condition until then [23]. Therefore, considering the challenges related to the care of these patients, it can be concluded that the health service providers have explained the importance of the screening program to the parents. The result of this awareness has been more cooperation among parents in identifying patients in the newborn screening program and using screening services during pregnancy. The present study found that 83.3% of the patients' parents were consanguineous, which is consistent with other studies conducted in Iran [13, 19, 35, 36]. A systematic review conducted by El-Metwally revealed that most of the parents of PKU patients in Arab countries were consanguineous [37]. In the Vela-Amieva study in Mexico, only 2 out of the 50 families of PKU patients were consanguineous [31].
CONCLUSION
The coverage of screening within the population under coverage was found to be favorable. However, there was a decreasing trend in performing the screening on time, which necessitates special attention and interventions to increase this index. The implementation of the newborn screening program within the healthcare system has had a significant impact on the timely diagnosis and treatment of patients. The percentage of inappropriate samples was lower than the national average. Within this study, it was observed that a majority of the PKU incidence in Sirjan city is mainly due to consanguinity. It is important to note that carrier couples are unaware of their status until the birth of their first child with the disease. Therefore, increasing awareness among families regarding consanguineous marriages, particularly in areas with a high prevalence of PKU, can significantly help in controlling PKU.
LIMITATIONS OF THE STUDY
Considering that secondary data are used in this study, the data are based on current reports and contents recorded in the system, and the researcher does not have much power to control the quality of the information in the collection process, so it is possible that the information collected especially in the screening part is not accurate. Also, it was not possible to describe the screening data based on gender, place of residence, and the reason for re-screening based on the specific conditions of the baby.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article reports the results of a research project approved by Kerman University of Medical Sciences with the code of ethics (IR.KMU.REC.1398.553).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All human procedures followed were per the guidelines of the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
In order to comply with ethical considerations in this research, the information of the participants was kept confidential, and other people were not able to access this information. The names and surnames of the participants were not used for data collection, and data collection was done after obtaining the code of ethics from the Kerman University of Medical Sciences.
STANDARDS OF REPORTING
STROBE guideline has been followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [S.D] upon reasonable request.
FUNDING
This research was done with the financial support of Kerman University of Medical Sciences, Funder ID. 1398.553, Awards/Grant number. 1398.553.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This article is the result of the research project approved by the Vice-Chancellor of Research and Technology of Kerman University of Medical Sciences, for which we are deeply grateful. We thank and appreciate the cooperation of the health department of Kerman University of Medical Sciences and the staff of the genetic counseling center.


