All published articles of this journal are available on ScienceDirect.
The Prevalence of HIV Load Suppression and Related Factors Among Patients on ART at Phedisong 4 Clinic, Pretoria, South Africa
Abstract
Background:
Globally, the benefits of viral load suppression in improving the lives of people living with HIV/AIDS have been established. In 2010, the South African Government decentralised ART to the primary care level. This study intended to determine the effect of this decentralisation in achieving viral load suppression among patients.
Objective:
To determine the prevalence of HIV viral load suppression and factors related to the suppression among patients initiated on ART at Pedisong 4 clinic, Tshwane District in Pretoria.
Methods:
A prospective cohort study was conducted on 98 patients initiated on ART between 01 November 2012 and 30 April 2013. Based on the viral load results, they were divided into those who achieved Viral Load Suppression (VLS), and those who did not (NVLS). Analyses were done using SAS® (version 9.2) for Microsoft software. A p < 0.05 was considered significant.
Results:
Ninety patients (91.8%; 95%CI, 84.7% – 95.8%) achieved viral load suppression while eight (8.2%; 95%CI, 4.2% – 15.3%), did not. Of the 98 patients, 63 (64%) were female. In the NVLS group, the female to male ratio was 7:1 (p = 0.038). There was no relationship between viral load suppression and patients’ baseline characteristics, behavioural characteristics and clinical characteristics (p > 0.05). ART adherence reported in both patient groups was ≥ 87.0%.
Conclusion:
There was good viral load suppression in patients initiated on ART at Pedisong 4 clinic. Patients’ baseline, behavioural and clinical characteristics were not related to viral load suppression, necessitating further large sample size studies in various health facilities.
1. BACKGROUND
Globally, the benefits of Human Immunodeficiency Viral (HIV) load suppression in prolonging and improving the lives of People Living With HIV/AIDS (PLWHA) have been demonstrated [1, 2]. The link between inadequate adherence to treatment regimens and lack of viral load suppression has been shown in literature whereby inadequate adherence negatively influenced viral load suppression, resulting in poor patient clinical outcomes [3, 4].From various studies, there are other factors that impact on viral load suppression, namely patients’ socio-demographic, behavioural, psychosocial and clinical characteristics [5-8]. At the study site, Phedisong 4 clinic it was observed that some patients did not achieve adequate viral load suppression following review at six months post-ART initiation.
Baseline socio-demographic characteristics have been shown to influence viral load suppression in patients [5, 9, 10]. In terms of age, a multi-centre observational study conducted in 13 American clinical sites reported that age was independently linked with viral load suppression, where the median age of people who did not attain viral load suppression was 41.1 years versus 47.1 years for those who did [5]. A survey conducted in Kenya, Malawi, Zimbabwe and South Africa investigated the association between age and viral load suppression in patients initiated on antiretroviral treatment and reported that viral load suppression improved with increasing age [9].
A Brazilian study showed that married patients or those in committed relationships exhibited higher rates of HIV viral load suppression as compared to those who were in unstable relationships, single or widowed [10]. Regarding the level of education and employment, a study conducted in eight United Kingdom clinics between 2011 and 2012, found that education below university level, unemployment and having an increased financial hardship were associated with virological non-suppression in patients on ART [11]. However, contradictory results were observed in the study by Wiewel et al., where poverty, unemployment and education level were not associated with viral load suppression [12].
A number of behavioural factors associated with viral load suppression among People Living With HIV/AIDS (PLWHA) have been identified [6, 13]. In a South African study, fifty percent of patients who engaged in risky sexual behaviour (unprotected vaginal or anal sex during which a condom was not used or used inconsistently) did not achieve viral load suppression [6]. The ASTRA study from the United Kingdom confirmed that viral load suppression was better amongst patients who did not engage in risky sexual behaviour [13]. Since patients with HIV/AIDS are still stigmatized in many societies, the viral infection poses a major barrier to disclosure [14]. Disclosure has been found to be the first step in gaining support from families and significant others [15]. However, limited data exist about the association of disclosure with viral load suppression, but lack of family support has been reported to have a negative association with viral load suppression [16].
Jordan et al. studied the clinical correlates of viral load suppression among 100 HIV positive patients who received ART and used substances like cigarettes. Although cigarette smoking was the most prevalent (84%) substance used in their cohort, the study showed no correlation between cigarette smoking and viral load suppression [7]. In another study, patients on ART who admitted to daily alcohol use had an almost four-fold rise in the odds of detectable HIV viral load as compared to patients on ART who did not use alcohol (OR=3.81, p=0.01, 95%CI = [1.42-11.48]) [8] Depression in patients on ART was associated with decreased amounts of natural killer cells, resulting in increase in the activated CD8 T lymphocytes and viral loads [17].
From the aforementioned, a number of factors associated with viral load suppression among PLWHA have been identified at various settings. The studies did not produce the same findings. This study was aimed at determining the extent to which these factors were related to viral load suppression among patients initiated on ART at our setting. It is hoped that the findings of this study will guide health care professionals and policy makers to identify and closely monitor patients on ART who are at risk of poor or suboptimal viral load suppression, in the interest of optimum treatment outcome.
2. METHODS
2.1. Study Area and Period
The study was conducted at Phedisong 4 clinic, which offers various primary health care services to patients, including ART. It is located in the Ga-Rankuwa Township of the Gauteng Province, about 37 km north of Pretoria, the capital city of South Africa. At the time of the study, it served an estimated population of 2 921 488 [18]. Odi District Hospital (23 km away) and a tertiary hospital, Dr George Mukhari Academic Hospital (5km away) served as the referral centres for this clinic.
2.2. Study Design
We conducted a prospective cohort study among patients down referred primarily from the aforementioned referral centres for ART initiation.
2.3. Study Population
At the time of the study, the clinic managed an average of seven patients per week who were referred from other health care centres for ART initiation. That amounted to 28 patients per month. To obtain a sample size with 95% confidence level and 10% error margin, the Epi Info (version 7.0) software was used yielding a sample size of 93 patients. To account for possible drop-outs over-sampling was done over a period of six months (1st November 2012 up to and including 31st March 2013) which yielded 155.
2.4. Sampling Procedure
The inclusion criteria were the age ≥ 18 years, patients initiating ART, patients who were ART naïve and patients who had undergone the mandatory three adherence counselling classes recommended by the South African Department of Health [19].
Of the 155 eligible patients for ART, 20 did not meet the inclusion criteria and 13 declined participation. We, therefore, enrolled 122 patients for the study (79% response rate of eligible patients). In addition, another 24 patients did not attend the follow up viral load measurements at six months after ART initiation as follows: Defaulters (9), deceased (3), transferred to another health facility (3) and discontinued participation (9). Fig. (1) VLS was defined as a viral load of < 40 copies/millilitre and NVLS was a viral load >1000 copies/millilitre, based on. viral load measurements by the South African National Health Laboratory Services (NHLS) using the m2000 Real Time HIV-1 Viral Load System® supplied by Abbott [20].

2.5. Data Collection
A research assistant assisted the patients to complete a standardised, structured questionnaire provided in either English or Setswana, the predominant local language. The principal researcher trained the research assistant in recruitment and data collection procedures for this study. The baseline characteristics questionnaire contained questions on age, gender, marital status, highest educational level attained and employment status. At the inception of the study, we collected these variables from all 122 patients who initiated ART at the clinic.
At six months’ evaluation, data on behavioural and clinical characteristics were available from 98 patients who remained in the study. Behavioural characteristics entailed reported use of condoms, presence or absence of life-time sexual partners, cigarette smoking, use of alcohol, HIV status disclosure, an expression of the level of satisfaction about family support, as well as admission to feeling sad in the previous two weeks. Clinical characteristics were based on patient’s clinical condition according to the World Health Organization (WHO) HIV staging, the Antiretroviral Treatment (ART) combination the patient had been receiving since treatment initiation and the presence of any co-morbid illnesses.
Patients who admitted to alcohol use completed the CAGE test questionnaire to establish harmful alcohol use. CAGE stands for “need to Cut down on alcohol”, Annoyed by being criticised for taking alcohol”, feeling Guilty about one’s drinking” and “needing alcohol first thing in the morning to steady one’s nerves (Eye opener)”. Each variable when present is allocated “1” point. A score of 0-2 indicates non-harmful alcohol use; 2-3 indicates harmful alcohol use and 4 - alcohol dependence [21]. Therefore, a total score ≥ 2 was considered clinically significant as it indicated harmful alcohol use, with a sensitivity of 93% and a specificity of 76% 21].
Patients who answered “Yes” to the question “Have you been feeling sad in the past week” were further requested to complete an internationally validated tool, namely the Centre for Epidemiological Studies Depression Scale (CES-DS) to screen them for clinical depression. The CES-DS questionnaire consists of 20 questions with a total possible score of 0 to 60. A total score ≥ 16 is considered as pointing towards depression [22].
We allocated a special code to each patient for identification at six months review when s/he came for routine measurement of HIV viral load as per the South African National Department of Health guideline protocol [19]. The adherence of each patient was assessed by means of the Morisky Medication Adherence Scale (MMAS-4) [23]. This scale is a generic self -reporting measurement of patient behaviour in taking medication. It consists of four questions with a scoring of 0 for “Yes” and 1 for “No”, with a total range of 0-4 points. A score of 0 indicates high adherence, 1-2 medium adherence and 3-4, low adherence [23].
2.6. Data Analysis
We compared percentage outcomes in the two groups (VLS versus NVLS). All the categorical variables mentioned under “data collection” were compared by the use of Fisher’s exact test which was appropriate for the sample size below 100 respondents [24]. Statistical tests were two-sided and a p-value < 0.05 was considered statistically significant. The SAS® (version 9.2) for Microsoft statistical software was used for all analyses.
2.7. Ethical Considerations
The Medunsa Research and Ethics Committee (MREC) approved the study with the clearance certificate number MREC/M/265/2012: PG. The Facility Manager at Phedisong 4 clinic gave permission to conduct the study at the clinic. All respondents gave consent for the study. Participation was voluntary, patients were informed that they were free to discontinue from the study after enrolment and their discontinuation would not compromise their clinical care at the health facility. The researchers assured them of anonymity and confidentiality of collected data.
3. RESULTS
The profiles of the two groups of patients (90 versus 8) are reflected in the following tables and graphs, with significance tested by the Fisher Exact test appropriate for small samples. Because of the imbalance in the size of the two groups (90 versus 8), the power of the Fisher Exact test to verify the difference between the two groups was low in relation to the different variables. However, these results should be regarded as descriptive and not as conclusive.
A total of 90 out of 98 patients (91.8%; 95% CI: 84.7% – 95.8%) of the patients who formed the sample size achieved viral load suppression which reflected the prevalence of HIV load suppression in this setting. This proportion was significantly different to those who did not (p < 0.001). Table 1 outlines the distribution of the baseline characteristics between the group that achieved Viral Load Suppression (VLS) and the group that did Not achieve Viral Load Suppression (NVLS). When considered according to age-groups, there was no significant difference between the two groups (p = 0.483). The mean age was also comparable between the two groups. There were more females than males in both groups albeit with no statistical difference between them (p = 0.253). In both the VLS and NVLS groups the female to male ratio was statistically significant [(56; 62%), p = 0.003] versus [(7; 88%), p = 0.001], respectively. The most frequent level of education among the patients was high school in both the VLS and NVLS groups: 66 (73.4) and 5 (62.5), respectively. There were no significant differences in the remaining baseline characteristics: marital status, level of education and employment status (p > 0.05).
| Baseline Characteristics |
Achieved Viral Load Suppression (VLS) n (%) |
Did Not Achieve Viral Load Suppression (NVLS) n (%) |
p-Values* |
|---|---|---|---|
| Viral load suppression | 90 (91.8) | 8 (8.2)) | < 0.001 |
|
Age (Years) ≤ 20 21 – 30 31 – 40 41 – 50 51 – 60 ≥ 61 Mean Standard Deviation |
0 (0) 23 (26) 38 (39) 20 (22) 8 (8) 1 (1) 37 9.33 |
0 (0) 2 (25) 4 (50) 2 (25) 0 (0) 0 (0) 35 8.07 |
0.483 |
|
Gender (%) Female Male |
56 (62) 34 (38) |
7 (88) 1(12) |
0.253 |
|
Marital status (%) Single Divorced Married Separated Widowed Cohabiting Question not answered |
65 (72.2) 2 (2.2) 15 (16.7) 3 (3.3) 0 2 (2.2) 3 (3.3) |
5 (62.5) 0 1 (12.5) 0 0 1 (12.5) 1 (12.5) |
0.536 |
|
Highest Level of Education (%) None Primary Secondary High school Post matric Question not answered |
2 (2.2) 6 (6.7) 6 (6.7) 66 (73.4) 8 (8.8) 2 (2.2) |
0 0 2 (25.0) 5 (62.5) 0 1 (12.5) |
0.372 |
|
Employment (%) No Yes |
50 (55.6) 40 (44.4) |
5 (62.5) 3 (37.5) |
1.000 |
Table 2 shows that the reported consistent use of condoms was similar in the VLS and NVLS groups [45(50%) versus 4(50%)], respectively. In both the VLS and NVLS groups, the highest proportion of sexual partners ranged from 2-4 partners (50; 55.5%) versus (5; 62.5%), respectively, with no significant difference between them (p = 0.76). All the patients in the NVLS group admitted to cigarette smoking 8 (100%) versus 78 (86.7%) in the VLS group. The use of alcohol in the VLS group was 15 (16.7%) and none in the NVLS group.
| Behavioural Characteristics |
Achieved Viral Load Suppression (VLS) (n=90) |
Did Not Achieve Viral Load Suppression (NVLS) (n=8) | p-Value* |
|---|---|---|---|
| Use of condoms n (%) Always Never Occasionally No response |
45 (50.0) 25 (27.8) 6 (6.7) 14 (15.5) |
4(50.0) 3(37.5) 0(0) 1(12.5) |
0.83 |
| Life-time number of sexual partners n (%) 1 2-4 >5 No response |
4(4.4) 50(55.5) 21(23.3) 15(16.8) |
0(0) 5(62.5) 1(12.5) 2(25.0) |
0.76 |
| Cigarette smoking n (%) Yes No No response |
11 (12.2) 78 (86.7) 1 (1.1) |
0(0) 8(100.0) 0(0) |
0.59 |
| Use of alcohol n (%) Yes No CAGE score (14/15) ≥ 2 |
15(16.7) 75(83.3) 14 (94.3) |
0(0) 8(100.0) 0(0) |
0.35 |
Table 3 demonstrates that among the 15 patients who used alcohol in the VLS group, 14 had a CAGE score ≥ 2 (implying harmful alcohol use).
| Alcohol Use | CAGE Score | Number of Respondents (CAGE < 2) |
Number of Respondents (CAGE ≥ 2) |
Total Number of Respondents |
|---|---|---|---|---|
| Non-harmful alcohol use (< 2) |
0 | 1 | ||
| 1 | 0 | |||
| Harmful alcohol use (≥ 2) |
2 | 8 | ||
| 3 | 6 | |||
| 4 | 0 | |||
| Total | 1 | 14 | 15 | |
Table 4 shows the level of satisfaction with family support as reported by 83 (92.2%) in the VLS group and 8 (100.0%) in the NVLS group. The level of disclosure of the HIV status to family members was equally high in both groups, 81 (90.0%) in the VLS group and 8 (100.0%) in the NVLS group. Patients who admitted feeling sad in the previous week in the NVLS group were 2 (25.0%) versus 7 (7.8%) in the VLS group. However, the difference between these two groups was not statistically significant (p = 0.41). Using the Centre for Epidemiologic Studies Depression Scale (CES-DS), only three of the nine patients scored ≥ 16 at baseline as depicted in Table 4. The depression rate in this cohort was 3/98 (3.1%). One of these three patients did not achieve viral load suppression and answered “No” to the same question at six months review.
| Family Support and Disclosure | Achieved Viral Load Suppression (VLS) (n=90) |
Did Not Achieve Viral Load Suppression (NVLS) (n=8) |
p-Value* |
|---|---|---|---|
| Satisfied about family support n (%) Yes No No response |
83(92.2) 3(3.3) 4(4.4) |
8(100) 0(0) 0(0) |
1.00 |
| Disclosure of HIV status n (%) Yes No |
81(90) 9(10) |
8(100) 0(0) |
1.00 |
| Depression | |||
| ‘YES’ to feeling sad in the past week Yes No |
7(7.8) 83(92.2) |
2(25) 6(75) |
0.41 |
| Screened for depression CES-DS score > 16 CES-DS score < 16 |
n=7 2 (28.6) 5 (71.4) |
n=2 1 (50) 1 (50) |
0.57 |
Table 5 shows that the majority of the patients were within World Health Organization (WHO) stages II and III, and the percentages were comparable between the two groups 40 (44.4%) versus 4 (50%) and 42 (42.2%) versus 4 (50%) respectively (p > 0.05).
| WHO Clinical Staging | Achieved Viral Load Suppression (VLS) (n=90) |
Did Not Achieve Viral Load Suppression (NVLS) (n=8) | P-Value* |
|---|---|---|---|
| Stage I | 3(3.3) | 0(0) | 0.60 |
| II | 40(44.4) | 4(50) | 0.76 |
| III | 42(46.7) | 4(50) | 0.85 |
| IV | 5(5.6) | 0(0) | 0.50 |
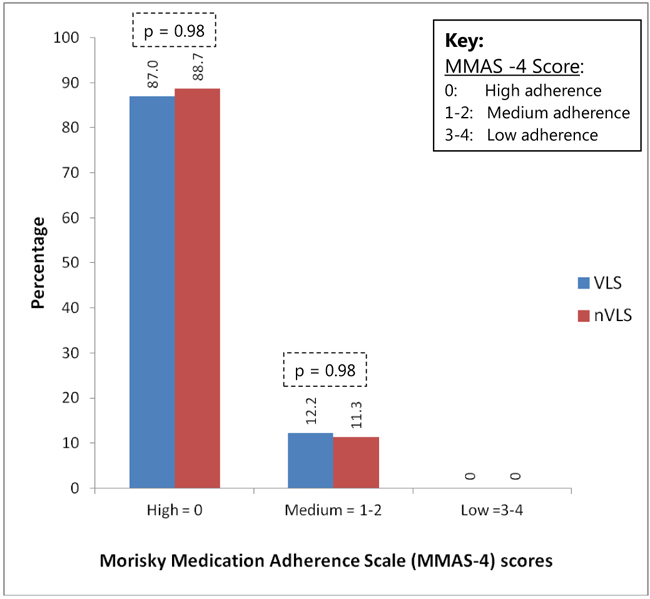
The Fig. (2) below shows the self-reported levels of adherence to ART by the patients. There was no significant difference (p = 0.98) between the VLS and NVLS patients. The majority of patients in the VLS and NVLS group had a high MMAS-4 score of zero in 87.0% and 88.7%, followed by a low medium MMAS-4 score of 1-2 in 12.2% and 11.3%, respectively. None of the patients fell in the low MMAS-4 score of 3-4 in both groups.
4. DISCUSSION
This preliminary study has shown a viral load suppression success rate of 92% six months after ART initiation. This was possibly linked to the generally high self-reported level of adherence to medication (≥ 87%). There was no relationship between viral load suppression and patients’ baseline characteristics, behavioural characteristics, reported family support, disclosure of HIV status to family, a positive screening for depression and WHO clinical staging. Ninety-eight patients were retained on ART at six months evaluation. A study with a comparable sample size of 71 patients reported virological failure to be associated with depression, young age and low adherence to HIV therapy in contrast to our study findings where this association was not found. The researchers attribute this difference to the 29 months follow up period in their study versus six months in the current study [25].
In the current study, the majority of patients were in their late 30’s with the mean ages of 37 and 35 years in the group that achieved viral load suppression and the group that did not, respectively. Studies have shown that the older age group (> 40 years) is associated with better viral load suppression among patients on ART [5, 9, 26] which, as already mentioned above, was not the case in this study. A high proportion of patients accessing ART were female (64%) which was comparable to the systematic review study by Muula et al. [27]. In their study, the reason advanced for the sex difference was that women displayed a high health seeking behaviour than males, which may also be the same explanation in our study.
Seven of the eight patients who did not achieve viral load suppression were females. This was consistent with another study on the interplay of baseline characteristics factors on virological suppression among out-patients who attended an HIV clinic in the USA, where failure of viral load suppression was observed mostly among women [28]. The higher viral load suppression among men was also observed in a multi-center cohort study in Canada that found the male sex as one of the predictors of the likelihood of viral load suppression [29]. The failure of viral load suppression in 8/98 (8%) was comparable to the cross-sectional study from Papua New Guinea by Gare et al. where evidence of virological failure was shown in 12/95 (12%) of their patients [30].
In our study, patient behaviour entailed reported use of condoms, life-time number of sexual partners, cigarette smoking and use of alcohol. The effect of proper and consistent condom usage in reducing HIV transmission has been demonstrated in many studies [31, 32]. In this study, an equal percentage (50%) of patients in the VLS and NVLS groups respectively reported that they used condoms consistently, while almost a third in each category reported that they never used condoms. However, the two categories did not differ significantly with respect to condom use. The most frequent number of life-time sexual partners indicated by both patient groups was two to four. The study did not inquire about condom usage in this regard. The study conducted in North Carolina (USA) by Peters et al. [33], although focussing on men who have sex with men (MSM), demonstrated the need for tracing and treatment of infected patient partners to break the cycle of infection with the virus.
A study has demonstrated that cigarette smoking in patients with HIV is associated with the development of opportunistic infections (oral candidiasis and community acquired pneumonia), lung cancer and obstructive lung disorders [34]. In the current study, all patients in the NVLS group indicated that they did not smoke cigarettes, but one in ten in the VLS group admitted to cigarette smoking. There was therefore no association between cigarette smoking and viral load suppression, in keeping with Kabali et al., who investigated cigarette smoking and HIV disease progression and found no association between the two [35].
Harmful alcohol use among PLWHA has been associated with unprotected sexual behaviour [36], and poor ART adherence [37], leading to failure of viral load suppression [38]. In our study, over 90% of patients who admitted to use of alcohol reported harmful alcohol use (CAGE score ≥ 2). However, since they were in the group of patients who achieved viral load suppression, alcohol use could not be directly linked to the failure of viral load suppression.
Syed et al. reported disease non-disclosure to be associated with fear of stigma and family emotions [39]. HIV/AIDS stigma, in particular, poses a major barrier to disclosure [40]. However, the disclosure is identified as the first step in gaining support from family and significant others [41]. Family support and care are vital in ensuring patient ART adherence, as reported by Afolabi et al. in Osogbo, Nigeria [42]. The South African HIV Clinicians Society encourages disclosure as a vital component of HIV/AIDS management as it facilitates patient directed support [43]. In our study, we found that over 90% of the patients who achieved viral load suppression, and 100% the patients who had not achieved viral load suppression indicated that they had disclosed their status to their families. These patients also indicated that they were satisfied with the family support they were receiving. Consequently, in this study, the failure of viral load suppression among the 8% cannot be explained by lack of HIV status disclosure or lack of family support.
The effect of depression on HIV viral load has been demonstrated in a few studies linking symptoms of depression with detectable viral loads and virological non-suppression [44, 45]. In the current study, the majority (≥ 75%) of the patients in both groups indicated that they did not feel sad in the previous week. Among the seven in the VLS group, only two had a CES-DS score above 16, while among the two in the NVLS group, only one had a CES-DS score above 16. The results in the current study did not link depression with viral load suppression.
There was no relationship between the WHO HIV clinical staging of patients and their viral load suppression in our study. We recorded five (5%) patients who had HIV WHO clinical stage IV among the patients who had achieve viral load suppression, and none among the patients who had not achieved viral load suppression. The fact that the five patients who had HIV WHO clinical stage IV achieved viral load suppression tallied with the findings of Cescon et al., where the WHO clinical stage IV at baseline was found to be a predictor of the likelihood of viral load suppression [29].
Regarding adherence, both the patients in the VLS and NVLS groups reported high adherence rates, with no difference between the two groups at six months evaluation. However, other studies have demonstrated a direct correlation between adherence and viral load suppression [46, 47]. Nilsson et al. had a relatively larger sample size than our study (144 patients) [39], while Li et al. followed their patients beyond 18 months [40]. We think our different study findings can be ascribed to the methodological differences in the length of patient follow-up between the cited studies and the current study.
5. STRENGTHS AND LIMITATIONS
As far as the authors are aware, this study on viral load suppression is the first to be conducted in the Tshwane health district of Pretoria, South Africa. It can be used as a reference for studies investigating another aspect of the same phenomenon. However, since the sample size was small, the study findings could not be generalised to other districts in South Africa. A large variety of possible factors related to viral load suppression from the literature were taken into consideration. The MMAS-4 score used to assess adherence to treatment is a self-reported screening tool, therefore inaccurate reporting by patients in both groups cannot be excluded. The patient review time of six months was relatively shorter compared to other studies, where the cohorts were followed for a minimum of a year [48, 49]. Furthermore, an expression of satisfaction about family support could have been measured more objectively rather than being quantified it as either “Yes” or “No”.
6. RECOMMENDATIONS
This study demonstrated that there was a significantly higher proportion of female patients who did not achieve viral load suppression among the patients in the NVLS group. Further enquiry is needed to establish possible explanations for this. None of the patient characteristics identified through literature search were related to viral load suppression in the current study. We recommend that qualitative studies be conducted in our setting so as to explore the phenomenon with the hope of identify any unidentified local peculiarities.
CONCLUSION
The high prevalence of HIV load suppression six months after ART initiation demonstrated in this study was possibly linked to the generally high self-reported level of adherence to medication. There was no relationship between viral load suppression and patients’ baseline characteristics, behavioural characteristics, reported family support, disclosure of HIV status, depression, ART regimen and WHO clinical staging. However, among the patients who did not achieve viral load suppression, there was a significantly higher percentage of female compared to male patients, and a significantly higher percentage of single compared to other marital status categories.
AUTHORS’ CONTRIBUTION
M.N.J. conceptualised the research idea, L.H.M. supervised the Master of Medicine (Family Medicine) registrar and drafted the manuscript. M.N.J. and G.A.O. reviewed the draft manuscript and G.A.O. edited the final draft. All authors approved the final manuscript for publication in TOPHJ.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Medunsa Research and Ethics Committee (MREC) approved the study with the clearance certificate number MREC/M/265/2012: PG. The Facility Manager at Phedisong 4 clinic gave permission to conduct the study at the clinic.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
All respondents gave consent for the study. Participation was voluntary, patients were informed that they were free to discontinue from the study after enrolment and their discontinuation would not compromise their clinical care at the health facility. The researchers assured them of anonymity and confidentiality of collected data.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was conducted in partial fulfilment of the requirements for the award of the Master of Medicine (Family Medicine) degree for the first author at the University of Limpopo, Medunsa Campus, Pretoria, South Africa [now known as Sefako Makgatho Health Sciences University (SMU)]. The researchers acknowledge the immense contributions of Professor HS Schoeman for the data analyses.


