All published articles of this journal are available on ScienceDirect.
Oxytetracycline-Protein Complex: The Dark Side of Pet Food
Abstract
Background:
Worldwide antibiotic abuse represents a huge burden, which can have a deep impact on pet and human health through nutrition and medicalization representing another way of antibiotic resistance transmission.
Objective:
We aimed our research to determine a possible complex formation between biological bone substrates, such as proteins, and Oxytetracycline (OTC), an approved antibiotic for use in zootechny, which might determine a toxic effect on K562 cells.
Method:
Cell viability and HPLC-ESI/QqToF assays were used to assess potential toxicity of bone extract derived from OTC-treated chickens according to standard withdrawal times and from untreated chickens at 24, 48 and 72h of incubation.
Results:
Cell culture medium with ground bone from chickens reared in the presence of OTC (OTC-CCM) resulted significantly cytotoxic at every incubation time regardless of the bone concentration while cell culture medium with ground bone from chickens reared without OTC (BIO-CCM) resulted significantly cytotoxic only after 72h of incubation. HPLC-ESI/QqToF assay ruled out the possible presence of OTC main derivatives possibly released by bone within culture medium until 1 μg/mL.
Conclusion:
The presence of a protein complex with OTC is able to exert a cytotoxic effect once released in the medium after 24-48h of incubation.
1. INTRODUCTION
Some studies reveal that the widespread antibiotic use in agriculture and aquaculture might contribute to the development of resistance to antibiotics commonly used in human medicine [1]. This issue becomes important particularly in zootechny due to its accumulation in animal feed and food with potential chronic consequences deriving from its ingestion to the species fed with these foods [2].
Nowadays, antimicrobials use represents a serious concern particularly in two correlated fields i.e. medical and agricultural [2-5]. In poultry, for instance, antibiotics are used to promote growth and to treat, control, and prevent overcrowding diseases [2, 6, 7]. A routinely exposure to antibiotics induce a selection for resistant bacteria that can persist on meat and in animal waste with a vertical transmission through maternal generations of breeding stocks [7]. Such bacteria can get in contact with humans in food-animal production facilities, in meat processing plants but also consuming contaminated meat [6, 8-10]. Despite there is not a common consensus on the use potential antibiotic resistance elicited by antibiotic used in animal food many countries have made substantial efforts to reduce the overall use of antibiotics in food-producing animals, in the attempt to decrease the antibiotic resistance in animals, the environment, and in human beings.
Moreover, Mueller et al. hypothesized that food allergens e.g. beef, fish and chicken could drag antibiotics and hormones thus representing the cause for the onset of dermatological symptoms in cats [11]. Among pharmacologically active substances, tetracyclines (in particular oxytetracycline, OTC) and their metabolites present in meats and meat-based foods for humans and pets were considered and studied [9, 10, 12].
We firstly hypothesized and observed the role of OTC as an underlying cause of some chronic inflammatory pathologies in vivo and in vitro as described by Di Cerbo et al. [8, 9, 13-17]. Due to its low cost and high efficacy [18], OTC is widely employed in the intensive farming of poultry [2], livestock [19] and aquaculture [20]. However, OTC has a high affinity for calcium, mainly present within bones, and a very low and long clearance in treated animals [21]. Further, pet food production, which mainly relies on poultry by-products [22], also avails itself of an important percentage of bone meal (20-30%) [8] with a consequent dragging of OTC residues that are frequently found within commercially available diets [10].
Despite the setting of maximum residue limits in foods by Food and Drug Administration [23] and World Health Organization [24] OTC residues may still persist since bone is not considered as an edible tissue, thus making commercial pet food potentially harmful [25].
In this paper, based on our previous results [6, 21], we aimed our research to determine possible interactions between biological substrates, such as proteins, and OTC, which might lead to an increased or decreased toxic effect on K562 cells mediated by the antibiotic. For this purpose we evaluated the potential toxicity of bone extract derived from OTC-treated chickens according to standard withdrawal times and from untreated chickens.
2. MATERIALS AND METHODS
2.1. Cell Culture
K562 myelogenous leukemia cell line, were purchased from American Type Culture Collection (ATCC) (LGC Standards srl, Milan, Italy). K562 cells were grown in RPMI supplemented with 10% Fetal Bovine Serum (FBS) 100 g/mL streptomycin, 100 U/mL penicillin, 2 mM glutamine (Euroclone Spa, Milan, Italy). The cells were cultured in a humidified incubator at 37 °C with 95% and 5% CO2.
2.2. Conditioned Culture Medium Preparation
Conditioned Culture Medium (CCM) was obtained by incubating 10 mL of a RPMI 1640 cell culture medium with 124, 90, 6, 2.48 mg and 124 μg of sterilized ground bone from chickens reared in the presence (OTC-CCM) or absence (BIO-CCM) of treatments with OTC and constantly shaken for 24-48-72 h at 37° C. After incubation, both CCMs were filtered through 0.45 and 0.20 μm syringe filters (Sartorius Stedim Biotech, Goettingen, Germany) to remove any residual ground bone particles and microbial [21].
2.3. Determination of Cell Viability
Cell viability was assessed after 24-48-72 h of continuous exposure with the aforementioned concentrations. A Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) was used to measure the cytotoxicity on K562 cells. Briefly, the K562 cells were plated on 96-well plates (Euroclone, Milan, Italy) at concentration of 5000 or 10000 cells/cm2. After exposure to desired concentrations of the different compounds, 10µl of CCK-solution was added to each well and incubated for a period of 2 h at 37°C. Finally, absorption was measured at 450 nm using a multiplate reader Multiscan FC (Thermo Scientific, USA). Dimethyl Sulfoxide (DMSO) 3% was used as toxic reference drug. Cell viability was expressed as a percentage of that of the untreated cells (Control). For each concentration of tested compounds, mean values of the mean absorbance rates from four wells were calculated.
2.4. HPLC-ESI/MS Analysis
CCM samples were analyzed using a HPLC-ESI/MS, analyses were performed on a 1200 Series HPLC coupled to either a 6520A Quadrupole/Time-of-Flight high mass spectrometer (QqToF) or a 6410B Triple Quadrupole mass spectrometer (QqQ), (both from Agilent Technologies, California, USA) via an electrospray ion source (ESI). On the HPLC-ESI/QqToF system, the chromatographic separation was carried out with 4 mL injection volume on an Agilent Zorbax SB-C18 30 x 2.1 mm ID 3.5 mm ps column. Elution was performed at T=25°C, with a flow rate of 0.3 mL/min. were used as a mobile phase. A linear gradient elution starting at 0.5 min going from 2% (B) up to 40% (B) in 11 minutes then up to 95% (B) in 6 minutes was performed using water with 0.5% formic acid (A) and Acetonitrile (B) as mobile phase components. The mass spectrometer was operated in positive-ion mode (ESI+) using “Auto MS/MS” acquisition with MS and MS/MS scan ranges being 50<m/z<1000 Th and selection of the top 3 most abundant mono-charged ions from each MS scan for their subsequent MS/MS spectra acquisition (active exclusion was enabled after 1 spectrum and released after 0.15 min). Targeted MS/MS analyses on the HPLC-ESI/QqQ included chromatographic separation on an Agilent Zorbax SB-C18 30 x 2.1 mm ID 3.5 mm ps column at 0.3 ml/min with the following gradient (mobile phases: A: water + 0.1 FA%, B: acetonitrile + 0.1 FA%): 0’ 98:2 A:B, kept for 0.5’ then B% was raised to 40% in 11’, then B% was raised to 95% in 3’, hold for 6’ then columns was reconditioned at the starting conditions, for a total runtime of 32 minutes.
ESI source was operated in positive mode at 3.5 KV, the Gas Heater was set to 300°C, the gas flow was 8 l/min and the nebulizer pressure was set to 25 psi.
Three transitions for OTC were chosen for Selected Reaction Monitoring, as shown in the Table (1).
| Precursor (m/z) | Product (m/z) | Fragmentor (V) | CE (V) | Dwell Time (ms) |
|---|---|---|---|---|
| 461.5 | 426.4 | 106 | 17 | 400 |
| 461.5 | 286.1 | 106 | 25 | 100 |
| 461.5 | 201.2 | 106 | 29 | 100 |
A matrix-matched calibration curve was prepared, ranging from 1 ppb to 1 ppm OTC concentration on a diluted and filtered CCM; samples were filtered, diluted at the same dilution factor of the calibration curve and analyzed. For standard-addition method, the sample was spiked with OTC ranging from 1 ppb to 1 ppm.
2.5. Statistical Analysis
All data are presented as the mean ± SD of at least three different experiments done in quadruplicate. One-way ANOVA analysis of variance with Dunnett’s post-test, were performed to compare differences between the groups, as indicated in the figures (Graph-Pad 6 Software Inc., San Diego, CA, USA). P values < 0.05 were considered significant.
3. RESULT
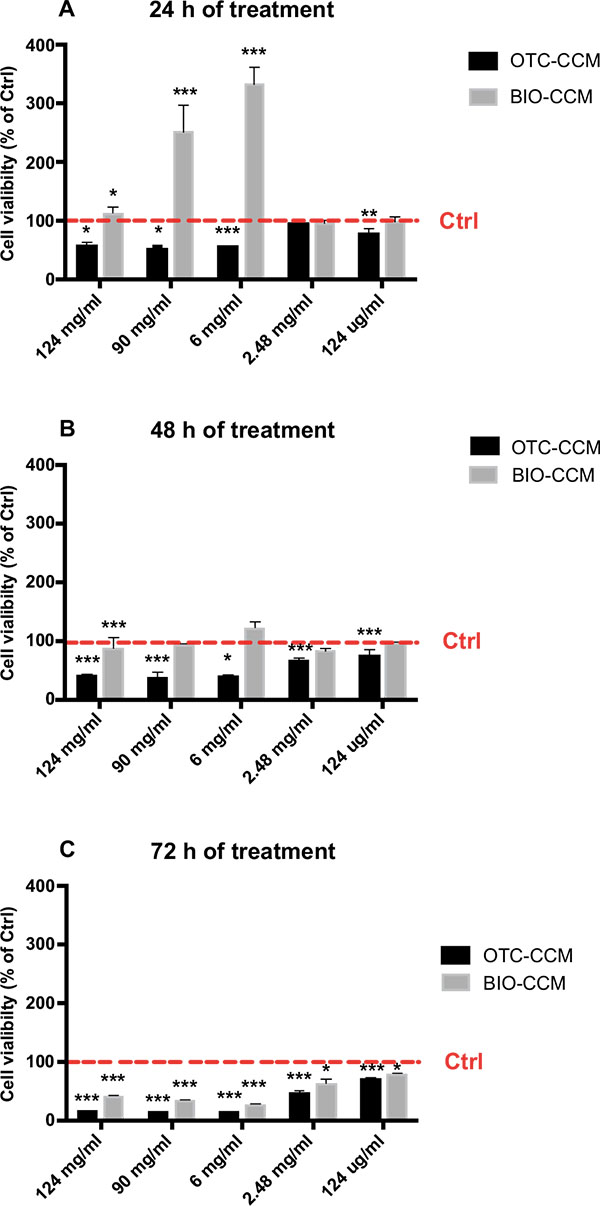
Here we found that OTC-CCM resulted significantly cytotoxic at every incubation time regardless of the bone concentration while BIO-CCM resulted significantly cytotoxic only after 72h of incubation Fig. (1).
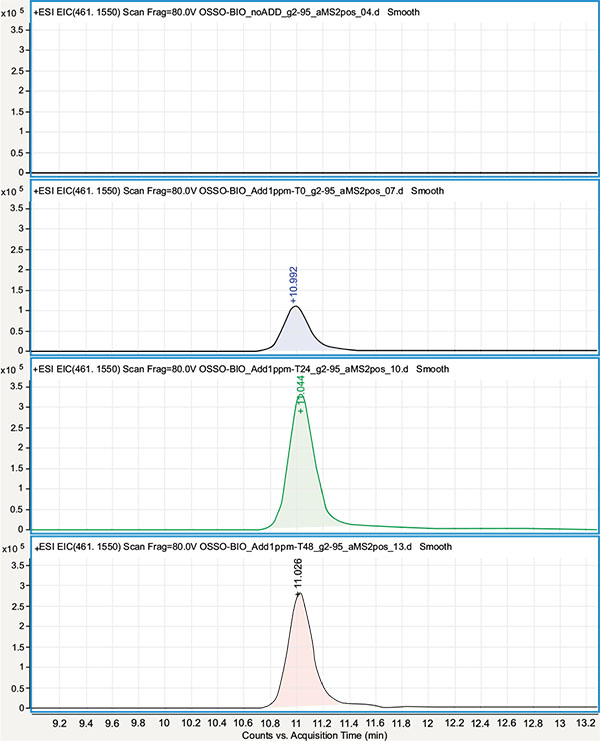
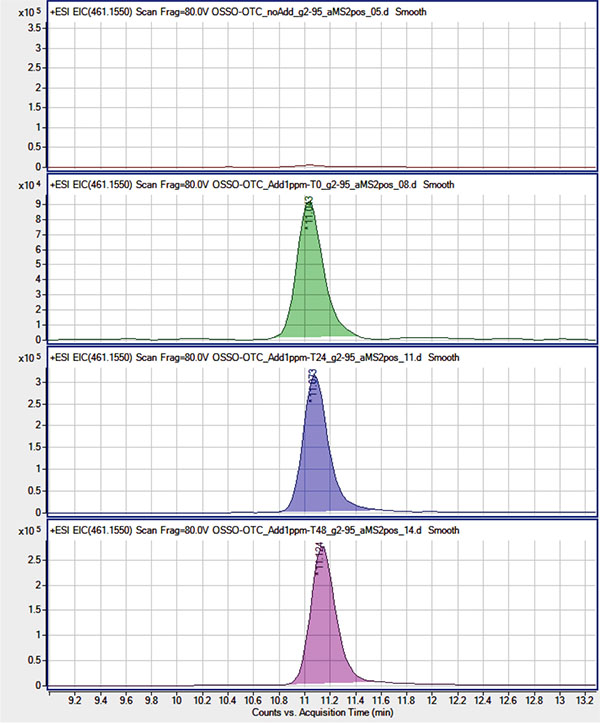
Qualitative analysis was performed using the HPLC-ESI/QqToF assay in order to test for the presence of OTC (at a concentration ≥ 1mg/mL) and its main derivatives N-Demethyloxytetracycline, N-Didemethyloxytetracycline, and Apooxytetracycline. To this purpose, their presence was tested using their molecular ion signals eventually revealed on their respective Extracted Ion Chromatogram (EIC) in the ESI(+) MS experiments. Due to the QqToF high resolution and mass accuracy (< 5 ppm) each EIC was obtained using a narrow extraction window (10 ppm) centered on the molecular ion theorical m/z value. In order to account for the eventual suppression of OTC signals at the 1 mg/mL concentration level, due to matrix effect in extracted samples or for its degradation/combination in the extracted sample environment, extracted samples were as well spiked with OTC at 1 μg/mL and analyzed right away (t0) and after 24 and 48 hours (t 24, t 48). Neither OTC nor its derivatives were revealed in non-spiked OTC-CCM and BIO-CCM extracted samples Figs. (2, 3) while OTC molecular ion ([M+H]+ at m/z= 461.155) was clearly revealed in t 0, t 24 and t 48 spiked samples with roughly the same signal intensity (within the instrument variability). This suggested the OTC concentration being substantially below the tested 1 μg/mL level, while reasonably excluding strong degradation/combination reactions of OTC in the extracted matrix environment at least within the tested 48 hours period.
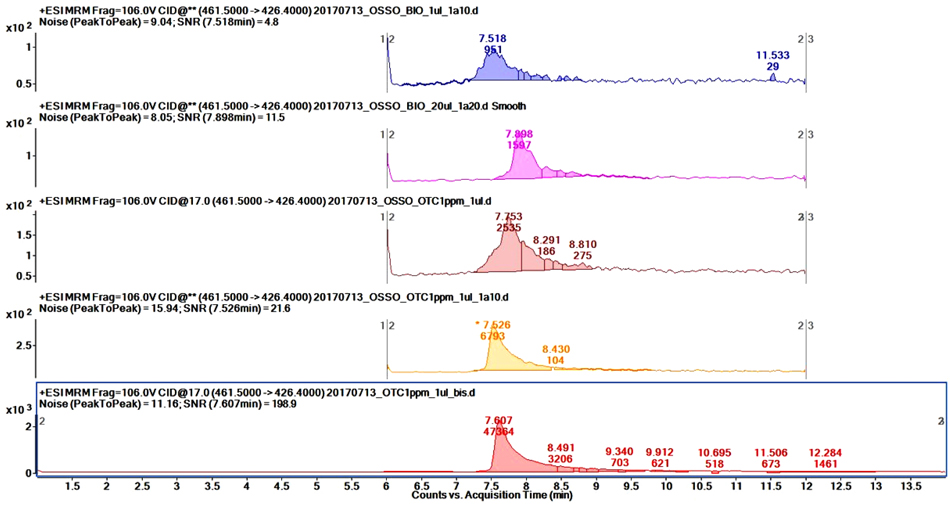
Targeted MS/MS analyses were performed on the HPLC-ESI/QqQ to quantitate OTC in bone samples. Preliminary tests showed a heavy matrix effect, thus we optimized the dilution factor and injection volumes for best results, ending up with a 20-fold dilution and 20 μl of diluted sample injected.
Matrix-matched calibration curve showed a good fit (r2 ≥ 0.999); unfortunately, the sample signal was below the quantification limit. In order to increase the sensitivity because of the low concentration of OTC in analyzed samples, standard-addition method was applied. In this case, by extrapolating the intercept of the curve on the y-axis we were able to determine OTC concentration. By correcting the value for the dilution factor, the final determined concentration was 200 ng/ml. As a proof of concept, we ran the same standard addition analysis on the organic bone extract; the curve in this case showed an intercept with the y-axis significantly closer to the origin, indicating that the concentration of OTC (if any) was significantly lower in this extract compared to the bone extract Fig. (4).
4. DISCUSSION
Previously published data showed a significant cytotoxic effect of OTC powder at 24, 48 and 72h at a concentration of 150 μg/mL (according to the amount of OTC expected to be provided to chicken during a standard treatment and disclosed on the antibiotic package) and at 48 and 72h at 75 and 35 μg/mL [8]. Moreover, the cytotoxic effect was clearly visible at OTC concentrations far below those previously investigated [10].
We recently claimed the unavoidable occurrence of oxytetracycline as a contaminant of meat, bone meal and poultry by-products, which are widely present within commercially available pet diets and human foods (mainly würstels and sausages) [8, 10]. In addition, we reported a discrepancy among OTC kibble concentration, OTC serum concentration of dogs with dermatitis and otitis, diarrhea and general anxiety and in vitro OTC, 20% liquid or powder, last cytotoxic concentration [10]. In fact, OTC kibble concentration was 19 μg/mL, serum oxytetracycline concentration was 0.22 μg/mL while in vitro last cytotoxic concentration was 35 μg/mL for the OTC powder and 87.5 μg/mL for the 20% liquid OTC.
Despite our in vitro data are in agreement with those previously published [6, 10], it is worth noting that HPLC-ESI/QqToF clearly evidenced the lack of OTC and its main derivatives within conditioned medium until 1 μg/mL. However the results obtained with Triple Quadrupole mass spectrometer (QqQ) showed the presence of an OTC-protein complex
This latter result helped us to rule out the toxic role of OTC itself and allowed us to speculate the presence of a protein complex inside bone with OTC able to exert a cytotoxic and pro-inflammatory effect once released in the medium after 24-48h of incubation. Moreover, it is reasonable to hypothesize that most of symptoms observed in the dogs of our previous paper might be due to an overall inflammatory condition elicited by the daily intake of kibbles made of chicken-derived bone with OTC. Although more researches are needed in order to figure out the chemical composition of the complex and the molecular mechanism involved in the cell toxicity, our results revealed a new possible explanation linked to the side effects of chicken-derived bone with OTC.
CONCLUSION
Our research represents a further insight into the overall landscape of antimicrobials abuse pointing out the possible consequences on pets and humans well being with particular regard to the actual antibiotic resistance spread. Unfortunately, WHO and FDA have set high OTC minimal residual limits within organs of farmed animals and do not consider bone as a possible veihicle of contamination since considered as not edible [8, 26]. In this view our findings rise new questions concerning the interactions between antibiotics, i.e. OTC, and organic substrates. Indeed our results pointed out the formation of a complex which induce cell toxicity even more then antibiotic alone. The further question to be answerd is wether this complex is able to induce antibiotic resitence too.
Since WHO ruled out an agenda on the study and the prevention of antimcorobial resistence (AMR) in the different fields where the antibiotic were used, further studies with more detailed evaluations are needed to support our preliminary observations, and to confirm a possible new scenario in the AMR.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Decleared none.


