All published articles of this journal are available on ScienceDirect.
Incidence Of Central Line-associated Bloodstream Infections In Intensive Care Units In A Private Hospital (Cairo, Egypt)
Abstract
Objective:
The study aimed to measure the incidence, risk factors and most frequent causative organisms of central line-associated bloodstream infections (CLA-BSI) in the Medical/Coronary and Surgical Intensive Care Units (ICUs) at a private hospital.
Methods:
This prospective study included 499 patients and was conducted between April 2014 and September 2014 in the Medical/Coronary ICU and Surgical ICU of a private hospital in Cairo, Egypt.
Results:
Approximately 44% of all the patients admitted to the ICUs underwent Central Venous Catheter (CVC) insertion. The overall incidence density rate of CLA-BSI was 6 cases per 1000 central line-days. The central line utilization rate was 0.94 per 1000 patient-days. The mortality rate among cases with CLA-BSI was 16.8% (95% CI: 13.6% – 20.4%) during the study period. Risk factors for CLA-BSI were detected by univariate analysis and included associated co-morbidities such as heart failure, APACHE II scores of >15, an ICU stay of 5 days or more, duration of CVC placement, subclavian insertion of CVCs, and mechanical ventilation. Additionally, logistic regression analysis identified a long ICU stay of 5 days or more, mechanical ventilation and the presence of heart failure as the only significant predictors. Gram-negative bacteria, especially Enterobacter (36.8%: 95% CI: 16.3%– 61.6%), Pseudomonas aeruginosa (21.1%: 95% CI: 16.0% - 45.5%) were the predominant organisms detected in CLA-BSI cases.
Conclusion:
CLA-BSI is an important cause of mortality in ICU patients. The infection rate is considerably higher than that in recent studies from developed counties, but it is still lower than the rates reported in comparable published studies in Egypt. Strict adherence to the standard infection prevention practices for critically ill patients is highly recommended.
1. INTRODUCTION
The use of vascular catheters is common in both inpatient and outpatient care. In the United States, it is estimated that almost 300 million catheters are used each year, nearly 3 million of which are Central Venous Catheters (CVCs), also known as central lines [1]. However, their use is associated with a risk of bloodstream infection caused by microorganisms that colonize the external surface of the device or the fluid pathway when the device is inserted, as well as an infection that occurs over the course of use [2]. CVCs are the most frequent cause of Healthcare-Associated bloodstream Infections (HAIs) [3].
The Central Line-Associated Bloodstream Infection (CLA-BSI) is a primary bloodstream infection (i.e., there is no apparent infection at another site) that develops in a patient with a central line in place within the 48-hour period before the onset of the bloodstream infection that is not related to infection at another site [4].
Healthcare-Associated Infections (HAIs) occur throughout the world and affect hundreds of millions of patients each year [5]. These infections are not only costly to individuals and health care systems but can significantly increase morbidity and mortality in developed and developing countries [6]. Seriously ill patients are particularly vulnerable to serious complications due to HAIs, likely due to factors such as progressively more invasive medical technology and complex medical procedures, increasing immunocompromised status and old age, and the rising incidence of antimicrobial resistance [7].
In the United States, the CDC estimates that 5% to 10% of hospitalized patients develop HAI [8]. CLA-BSI is the most common cause of HAI in the bloodstream, according to the United States Centers for Disease Control (CDC). Approximately 80,000 CLA-BSI cases are observed in ICUs in the United States each year. CLA-BSIs are serious but can often be prevented when evidence-based guidelines are followed for the insertion and maintenance of central lines. Following such guidelines can considerably reduce the risk of infection and mortality in patients [9]. CLA-BSIs also increase the cost of health care and prolong hospital lengths of stay by up to three weeks [10]. A single incident of CLA-BSI can cost as much as US $56,000 to treat according to some studies once the cost of pharmacy charges, catheter changes, lab tests and an additional day in the ICU are summed [11]. Nearly one-third of these HAIs were due to CLA-BSIs, with an associated case fatality rate of 12.3% [12]. In developing countries, mortality rates may be as high as 50% [7].
In Egypt, a study was conducted in the ICUs of 3 hospitals at Cairo University, and CLA-BSI rates varied widely, from 2.9 to 14.3 per 1,000 central line-days, with an overall rate of 9.1/1,000 central line-days [13].
Available data on the global impact of HAIs have been more limited, particularly in many resource-constrained areas. Low- and middle-income countries generally do not have adequate resources to conduct HAI surveillance. Researchers who have attempted to quantify HAI rates in developing countries have found these rates to be much higher than those in developed countries, and their impact on patients and health care delivery systems is both severe and underestimated [14].
The current study was performed to determine the incidence, risk factors and most frequent causative organisms of CLA-BSIs in the Medical/Coronary and Surgical ICUs of a private hospital in Cairo, Egypt.
2. SUBJECTS AND METHODS
2.1. Study Design
A prospective cohort study was conducted in which all patients admitted for more than 48 hours between April 2014 and September 2014 to the Medical/Coronary ICU and Surgical ICU of a private hospital were monitored for the occurrence of CLA-BSI.
2.2. Study Location
The study was conducted in the ICUs of a private hospital accredited by the Joint Commission International. The hospital extensively collaborates with the Cleveland Clinic Foundation, the top cardiac hospital in the U.S. The hospital has two ICUs, one medical ICU for medical and coronary cases and a surgical ICU. In each ICU, there are 16 beds. Twelve of these beds are isolation beds arranged in separate cubicles, two of which are for airborne isolation with negative pressure and ante-rooms. Four beds in each ICU are in the bay area besides each other with a separation distance of 3.5 meters and a water-proof curtain.
In the hospital, the Nosocomial Infection Committee implemented a registration system for infection control surveillance of HAIs in 2009. The registration is based on nosocomial infections suspected or diagnosed by a system of physicians and liaison nurses. The surveillance of blood culture results is carried out monthly to identify causative agents and antibiotic sensitivity patterns of isolated organisms. Monthly denominator sheets are completed by infection control doctors for the calculation of the incidence rate in patient-days and the device-associated infection rate.
2.3. Study Population
The infection surveillance cohort consisted of all patients admitted to the Medical/Coronary and Surgical ICUs who had central venous lines for at least 48 hours. The infection surveillance was carried out over a period of 6 months, from April 2014 to September 2014.
2.4. Inclusion Criteria
ICU patients with a central venous catheter in the hospital with no infection at the time of admission to the ICU and no remote site of infection who were admitted to the ICU for at least 48 hours.
2.5. Exclusion Criteria
Patients who died or were discharged within 48 hours of admission and patients without a central venous line were excluded.
2.6. Sample Size
The sample size was calculated to obtain 2000 device-days to yield at least 14 cases of infection with a rate of 7/1000 device-days and a 95% CI of 4-11 infected cases per 1000 device-days (according to the calculated rate of the last year). The average number of device-days was 5 days/case in the last year; therefore, approximately 400 cases were needed [15]. These patients were monitored for six months from the start of the study in April 2014 to September 2014 until one of the following events occurred: episodes of CLA-BSI, patient discharge or transfer to another ward or hospital, removal of the catheter or death in the hospital.
2.7. Methods
2.7.1. Study Tools
2.7.1.1. Worksheets
I- Worksheet 1: Used to record the following data on each ICU patient with a central line: Administrative data: Patient name, medical record number, hospital admission date, ICU admission date and discharge date; Demographic data: Gender, age, medical history and co-morbidities; and Admission diagnosis: Chronic Obstructive Pulmonary Disease (COPD), liver cell failure, heart failure, immuno-compromised state, end-stage renal disease, Diabetes Mellitus (DM), hypertension, smoking, and chronic neurodevelopmental problems.
- Interventions performed on the patient in the unit: Insertion of invasive devices, e.g., a central venous catheter, mechanical ventilation or urinary catheter.
- Exposure to risk factors for infection: Type of catheter, insertion site, APACHE II score on the 1st day of ICU admission, duration of catheter placement, hospitalization days before ICU admission, length of stay, previous hospitalization, inserted medical devices at admission, repeated central line insertion during the same ICU admission.
- Central Line-Associated Bloodstream Infection (CLA-BSI) criteria: Date of suspected CLA-BSI, symptoms (e.g., fever, chills and hypotension) and laboratory criteria. Only cases with positive blood cultures were included (laboratory-confirmed cases), e.g., a known blood pathogen was isolated from one or more blood cultures and the isolated pathogen was not related to an infection at another site or a common skin contaminant (e.g., diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci, or micrococci) and was cultured from two or more blood cultures drawn on separate occasions 1 day apart.
- Outcome of stay in the ICU: e.g., discharge, death, transfer to another ward or transfer to another hospital.
II- Monthly denominator sheet: Completed to calculate suitable rates; denominator data (i.e., patient days, central line days, urinary catheter days and mechanical ventilation days) were recorded daily by hospital staff on a denominator reporting form, and some data were selected from the form for analysis.
2.8. Blood Tests
Blood cultures and sensitivity for ICU patients with a central line were performed if the patient was admitted from another hospital, was found to have any signs of central line infection, had manifestations of sepsis or septic shock or received follow-up treatment for positive blood cultures.
2.9. Data Management
The collected data were reviewed for accuracy and completeness. Missing data were completed by revisiting the unit and were obtained from the hospital records. Data were then coded, entered and analyzed with SPSS (Statistical Package for Social Science) version 23.
Quantitative variables were recoded to qualitative variables (e.g., APACHE II score was converted into two categories (high and low-risk groups) based on a cut-off value of 15.
APACHE II (Acute Physiology and Chronic Health Evaluation II) is a severity-of-disease classification system, one of the several ICU scoring systems. It is applied within 24 hours of the admission of a patient to an ICU, and an integer score from 0 to 71 is computed based on several measurements; higher scores correspond to more severe disease and a higher risk of death.
2.10. Calculation of Rates
Steps for calculating the central line-associated bloodstream infection/central venous catheter-related BSI rate (CVCR BSI rate):
All patients admitted to different adult ICUs were monitored daily by attending physicians for the subsequent development of nosocomial BSI, which was required to meet at least one of the following criteria: Criterion 1: Patient has a recognized pathogen cultured from one or more blood cultures and the organism cultured from blood is not related to an infection at another site. Criterion 2: Patient has at least one of the following signs or symptoms: fever (> 38°C), chills, or hypotension.
A common skin contaminant (e.g., diphtheroids, Bacillus spp., coagulase-negative staphylococci, or micrococci) is cultured from two or more blood cultures drawn on separate occasions. The signs and symptoms of infection appear 48 hours after admission, and there are no signs or symptoms of infection at the time of admission, demonstrated by the patient’s history and clinical examination [16].
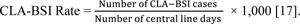 |
Quantitative data are presented as the range, mean, median, and Standard Deviation (SD), and qualitative data are presented in simple frequency tables. A chi-square test was used to compare qualitative data between groups, and a t-test was used to compare quantitative data.
The adjusted risk factors for CLA-BSI were obtained using the logistic regression analysis. The dependent variable was the presence and absence of CLA-BSI in all the patients. All variables described previously were considered as possible candidates for the final model. Wald Backward Method was used to build the final model. The calibration of the final model was assessed using the Hosmer and Lemeshow goodness-of-fit test. The test assesses whether or not the observed event rates match expected event rates in subgroups of the model population. Hosmer-Lemeshow statistic is compared to a chi-squared distribution; models for which expected and observed event rates in subgroups are similar (not significant) are called well calibrated [15]. A p-value < 0.05 was chosen as the level of significance
3. RESULTS
Overall, 1140 patients were admitted to the ICU in the follow-up period; specifically, 43.7% of all the patients admitted had CVC insertion (499 patients), 300 were admitted to the SICU and 199 were admitted to the MICU/CCU. Males constituted 72.3% of the studied subjects. The age of the studied patients ranged from 20-98 years (mean ± SD was 58.2 ± 14.6 years), the median hospital stay before ICU admission was 2 days, and the median ICU stay was 5 days. The highest incidence of CLA-BSI in the SICU was in the months of August, July and May (12.9, 7.9, and 7.8/1000 CL-days), and the lowest incidence was in April and September (4.2, 3.9/1000 CL-days). The total CLA-BSI rate was 7.9/1000 CL-days. The highest incidence of CLA-BSI in the MICU/CCU was in the months of May and June (6.4, 5.5/1000 CL-days), and the lowest incidence was in August and September (2.7, 0/1000 CL-days). The total CLA-BSI rate was 3.8/1000 CL-days. The overall CLA-BSI rate for both ICUs was 6/1000 CL-days (Table 1).
| ICU Type | Surgical ICU (n=300) | Medical/Coronary ICU (n=199) | Both ICUs (n=499) |
|||
|---|---|---|---|---|---|---|
| Months | CVCR BSI* rate | 95% CI | CVCR BSI rate | 95% CI | CVCR BSI rate | 95% CI |
| April n=52 | 4.2 | 0.1-23.3 | 3.5 | 0.09-19.7 | 4.4 | 0.5-15.7 |
| May n=50 | 7.8 | 0.9-28.0 | 6.4 | 0.7-22.9 | 7.3 | 1.9-18.6 |
| June n=45 | 6.3 | 0.7-22.7 | 5.5 | 0.6-19.6 | 6.2 | 1.6-15.7 |
| July n=49 | 7.9 | 0.9-28.2 | 4.8 | 1.2-21.6 | 6.8 | 1.4-19.7 |
| August n=53 | 12.2 | 2.5-35.2 | 2.7 | 0.06-14.8 | 7.1 | 1.9-18.0 |
| September n=51 | 3.9 | 1.1-32.3 | 0 | 0 | 3.7 | 0.4-13.4 |
| Total n=300 | 7.9 | 4.0-13.6 | 3.8 | 1.5-7.7 | 6 | 3.5-9.2 |
A higher rate of CLA-BSI infections was observed among patients with an ICU stay ≥ 5 days, APACHE II scores of >15, subclavian insertion of a CVC compared with other sites of CVC insertion, and mechanical ventilation; the presence of medical devices, such as a urinary catheter or a central line, on admission and place of referral were not related to CLA-BSI occurrence (Tables 2-4).
| Variable | Infection No. % |
No infection No. % |
Chi-square | P value |
|---|---|---|---|---|
| Gender Males (n=361) Females (n=138) |
13 (3.6) 6 (4.3) |
348 (96.4) 132 (95.7) |
0.1 | 0.6 |
| Type of ICU: Surgical (n=300) Medical/Coronary (n=199) |
12 (4.0) 7 (3.5) |
288 (96.0) 192 (96.5) |
0.07 | 0.7 |
| Previous hospitalization (n=95) | 4 4.2 | 91 95.8 | 0.05 | 0.8 |
| COPD (n=42) | 2 4.8 | 40 95.2 | 0.1 | 0.7 |
| Liver failure (n=84) | 3 3.6 | 81 96.4 | 0.01 | 0.9 |
| Heart failure (n=48) | 5 10.4 | 43 89.6 | 6.3 | 0.01* |
| Immunosuppression (n=18) | 1 5.6 | 17 94.4 | 0.1 | 0.6 |
| Renal failure (n=90) | 4 4.4 | 86 95.6 | 0.1 | 0.7 |
| DM** (n=221) | 10 4.5 | 211 95.5 | 0.5 | 0.4 |
| Hypertension (n=274) | 10 3.6 | 264 96.4 | 0.04 | 0.8 |
| Smoking (n=255) | 6 3.9 | 149 96.1 | 0.002 | 0.9 |
| Neurological disease (n=54) | 2 3.7 | 52 96.3 | 0.002 | 0.9 |
| Variable | Infection Mean ± SD |
No Infection Mean ± SD |
t test | P value |
|---|---|---|---|---|
| Age (years) | 58.4 ± 17.6 | 58.1 ± 14.4 | 0.06 | 0.9 |
| Hospital stay (days) before ICU admission | 2.8 ± 5.0 | 4.2 ± 7.5 | 0.7 | 0.4 |
| ICU stay days | 15.7 ± 9.6 | 6.4 ± 5.4 | 6.9 | 0.000 |
| APACHE II score* | 13.8 ± 6.8 | 9.8 ± 6.7 | 2.4 | 0.02 |
| CVC days | 15.7 ± 9.6 | 6.0 ± 5.2 | 7.5 | 0.000 |
| Variable | Infection No. % |
No infection No. % |
Chi-Square | P value | ||
|---|---|---|---|---|---|---|
| ICU length of stay <5 days (n=299) ≥5 days (n=200) |
4 15 |
1.3 7.5 |
295 185 |
98.7 92.5 |
12.4 | 0.000* |
| APACHE II score Low risk <15 (n=363) High risk ≥15 (n=114) |
10 9 |
2.8 7.9 |
353 105 |
97.2 92.1 |
5.9 | 0.01* |
| Inserted Medical devices Medical devices (n=340) Urinary catheter (n=287) Central venous line (n=310) Mechanical ventilation (n=294) |
15 13 13 16 |
4.4 4.5 4.2 5.4 |
325 274 297 278 |
95.6 95.5 95.8 94.6 |
1.0 0.9 0.3 5.2 |
0.3 0.3 0.5 0.02* |
| Site of CVC insertion Jugular (n=430) Subclavian (n=53) Femoral (n=2) Mahurkar (n=14) |
12 6 0 1 |
2.8 11.3 0 7.1 |
418 47 2 13 |
97.2 88.7 100 92.9 |
9.8 | 0.02* |
| Place of admission Unknown (n=6) Other hospital (n=33) Same hospital (n=364) Home (n=96) |
0 2 15 2 |
0 6.1 4.1 2.1 |
6 31 349 94 |
100 93.9 95.9 97.9 |
1.5 | 0.6 |
In the multivariate logistic regression analysis, an ICU stay of 5 days or more, heart failure and mechanical ventilation were the only independent risk factors for the occurrence of CLA-BSI in ICU patients (Table 5).
| Variable | No of cases with risk factor/ all participants | No of cases with risk factor/ all cases with CLA-BSI | P value * | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|
| ICU stay of at least 5 days | 15/19 | 200/499 | 0.002 | 6.3 | 2.0-19.4 |
| Heart failure | 5/19 | 90/499 | 0.03 | 3.4 | 1.1-10.6 |
| Mechanical ventilation | 16/19 | 294/499 | 0.02 | 4.3 | 1.2-15.4 |
Regarding the fate of admitted patients, 72.7% were discharged to the same hospital, 1.6% were discharged to another hospital, 8.9% were discharged home, and 16.8%; (95% CI: 13.6% – 20.4%) of the patients died.
The highest reported causative organisms of CLA-BSI were the following: Enterobacter (36.8; 95% CI: 16.3%– 61.6%), Pseudomonas (21.1; 95% CI: 16.0% - 45.5%), Enterococcus fecalis (15.8), Acinetobacter (10.5) Klebsiella pneumoniae (10.5) and Staph aureus (5.3).
4. DISCUSSION
The study findings agreed with those of recent studies conducted in Latin America; Asia; Africa, including Egypt; Europe; Lebanon; Kuwait; and the Philippines [18-21].
In contrast to the current study findings, lower CLA-BSI rates were reported in the Netherlands and USA [22-24].
The highest CLA-BSI rate (4.3/1000 CL-days (95% CI 3.7–5.0) was found in the medical ICUs, and the rate in the surgical ICUs was 3.5/1000 CL-days (95% CI 3.2-3.7).
The higher rate of CLA-BSI in the current study than in developed countries could be explained by the fact that ICU patients in developing countries are at an increased risk for infection. Several studies have identified some of the main factors associated with high CLA-BSI rates in ICUs in limited-resource countries. In the few instances in which infection control programs are regulated, compliance with the rules is poor. In addition, infection control surveillance and hospital accreditation are not mandatory at the national level. Compliance with hand hygiene measures is highly variable in most hospitals [25].
The nurse-to-patient ratio is likely a major factor contributing to higher ICU CLA-BSI rates considering the higher ratio between nursing staff and patients in limited-resource countries than in health care facilities in developed countries [26]. Finally, our CLA-BSI rates may be higher than those in developed countries because the ICU in our center admits many patients who are terminally ill with advanced chronic illnesses, as represented by high APACHE II scores; who are referred from other hospitals; receive multiple courses of antibiotics; and are colonized and/or infected with multi-drug-resistant pathogens. In addition, clinical practice guidelines for CVC insertion were not fully implemented in our hospital during the study period, as evidenced by the high proportion of insertions in the internal jugular instead of the subclavian vein and the high central line utilization rate.
Higher CLA-BSI rates were reported in a prospective cohort device-associated HAI surveillance study carried out in Egypt [27] and in other countries [13, 28-30].
Several factors probably contributed to the observed lower CLA-BSI rates in this study than in other published studies in Egypt, many of which are specific to the country and the hospital setup itself. In Egypt, guidelines for specific infection control practices are in place, but national infection control surveillance is not conducted. Our hospital was granted accreditation by the Joint Commission International, which adheres to the rigorous infection control program currently in place. Practice guidelines for the prevention of CLA-BSI and other device-associated HAIs have now become central to the care of patients in ICUs. Other hospitals in the country are also working toward accreditation.
It was difficult to draw conclusions regarding the overuse of CVCs because there was no accurate risk adjustment for illness severity for the total population. However, the high central line utilization ratio in the current study indicates a potential target for future efforts directed at CLA-BSI reduction, a reduction in their use or a limitation in the duration of their use.
4.1. Risk factors for CLA-BSI in ICU patients
The current study revealed that the ICU length of stay was longer in patients with CLA-BSI than in non-infected patients (RR=5.9; 95% CI=1.9-18.2), which was in agreement with the findings of other studies [31, 19]; a long length of stay increases the risk of exposure and the number of CVC days, which have been recognized as risk factors for CLA-BSI.
The current study showed a statistically significant association between the occurrence of CLA-BSI in ICU patients with higher mean APACHE II scores. The current findings agree with those of Fuzheng et al. [32]. Therefore, the prevention of CLA-BSI should be a priority in critically ill patients with complicated diseases.
The severity of illness could explain the higher risk among CLA-BSI patients, as sicker patients tend to stay longer in the ICU. However, some studies have suggested that the assessment of severity at admission may be inadequate because severity could change prior to infection [33]. As a daily severity score was not recorded during the study period, we were unable to compare and control for severity at infection onset.
Moreover, there was a statistically significant association between the occurrence of CLA-BSI and heart failure as a chronic underlying condition, coinciding with the results of a case-control study that found that medical cardiovascular disease was a newly identified independent risk factor in patients with CLA-BSI. However, there was no relationship between diabetes or solid-organ transplant and the development of CLA-BSI [31].
Medical cardiovascular disease was a novel risk factor that was independently associated with CLA-BSI. Antibiotic use in this population may be lower if hemodynamic abnormalities were attributed to cardiac causes rather than to infection. Additionally, chronic low cardiac output may cause tissues to be more susceptible to the proliferation of pathogenic bacteria.
The current study revealed that mechanical ventilation on admission to the ICU was an independent risk factor associated with the occurrence of CLA-BSI based on the multivariate analysis, in agreement with studies performed in Egypt, South India and Mexico [31, 34, 35]. Mechanical ventilation suppresses the normal defense mechanism of patients and is associated with a poor general health status. No simple mechanism can explain this association except the severity of illness of patients requiring prolonged mechanical ventilation [36].
The current study findings revealed that a prolonged catheter dwell time was associated with an increased risk for CLA-BSI. This finding was in agreement with a previous study [31].
Further studies are needed to explore CVC dwell time and the risk for CLA-BSI. This association may be influenced by many other variables, including the patient population, catheter type, frequency of catheter access, catheter maintenance practices, and indications for catheter insertion.
4.2. Bacteriological Profile of CLA-BSI Causative Agents in ICU Patients
The current study revealed that Gram-negative organisms are the most common organisms associated with the incidence of CLA-BSI in ICU patients. The current study findings also reveal that Enterobacter spp. and Klebsiella pneumoniae were the most common causative agents for CLA-BSI episodes in the SICU and MICU/CCU patients, respectively.
It may be prudent to turn our attention to infections caused by these other pathogen groups that may require different approaches to prevention, e.g., optimizing central line maintenance practices [37].
The current study findings are similar to the findings of other studies [13, 19, 38, 39].
In contrast, Gram-positive skin organisms are often the most commonly reported causative microorganisms of bloodstream infections [34, 40, 41]. The infection rate is considerably higher than that in recent studies from developed counties but is the same as that in developing countries. Nevertheless, it was still lower than the rates reported in comparable studies in Egypt.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Approval from the Ain Shams University Ethical Review committee and from the medical council in the hospital was obtained to conduct the research.
HUMAN AND ANIMAL RIGHTS
No animals/ humans were used for the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the participants prior to publication.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


