All published articles of this journal are available on ScienceDirect.
Indicators of Adrenal Insufficiency in TB-suspect Patients Presenting with Signs and Symptoms of Adrenal Insufficiency at Three South African Hospitals in Pretoria
Abstract
Background:
Primary adrenal insufficiency occurs when the function of the adrenal cortex to produce cortisol is impaired. Infections, such as disseminated Tuberculosis (TB) and malignancies, are the major causes of Adrenal Insufficiency (AI) in developing countries. AI is characterized by specific symptoms, signs, and laboratory findings.
Objective:
To determine indicators of AI in TB-suspect patients presenting with signs and symptoms of AI.
Methods:
A cross-sectional study was conducted at the primary health care ward of Dr. George Mukhari Academic Hospital, Jubilee District Hospital, and Odi District Hospital. The population comprised all TB-suspects, from whom a sample of 75respondents was obtained. A researcher administered questionnaire was used to collect data related to their signs, symptoms, and laboratory findings.
Results:
Of the 75 respondents, 47 (62.37) and 28 (37.3%) were classified as Adrenal Sufficiency (AS) and AI, respectively. The most occurring symptoms were craving for salt, dry, itchy skin, and vomiting (prevalence: 79.7%, 68.1%, and 69.0%, respectively). Signs or symptoms by themselves did not discriminate persons with a high likelihood of AI. However, a fasting serum glucose (≤ 5.25 mmol/L), a positive GeneXpect, a low CD4 count (≤ 274.5 cells/ml), with a combination of signs and symptoms (9.5) constituted a discriminator for AI in TB-suspect patients (87.5% likelihood).
Conclusion:
A low fasting serum glucose, a positive GeneXpect, a low CD4 count with a minimum of ten signs and symptoms constitute a discriminator for AI in TB-suspect patients, necessitating treatment initiation to save patient lives in laboratory resource-limited settings.
1. INTRODUCTION
Primary adrenal insufficiency (Addison’s disease) named after Thomas Addison was first described in 1855 [1]. It is a
rare disease with an estimated incidence of 2/10 000 in the general population [2, 3]. In Western countries, it has been estimated at 39 to 144 per million people [4, 5].There is a paucity of literature on Addison’s disease in Africa, with a study conducted in a major South African teaching hospital recording 50 cases over a period of 17 years (1980-1997) [6]. Addison’s disease is caused by many factors, e.g. auto-immune adrenalitis, Tuberculosis (TB), systemic fungal infections, AIDS opportunistic infections (cytomegalovirus, bacteria and protozoa), Kaposi’s sarcoma, metastatic carcinoma (lung, breast, and kidney) and lymphomas [1]. All these causes involve the adrenal cortex as a whole, resulting in the deficiency of cortisol and aldosterone production, with varying severity [7].
There is a high prevalence of granulomatous infectious adrenal disease (tuberculosis, fungal infections, cryptococcus, histoplasmosis) in resource-limited countries [8-10], including South Africa.TB, through dissemination, can destroy the adrenal glands in an infected individual [11]. Although the incidence of Adrenal Insufficiency (AI) due to TB of the adrenal glands has been shown to decrease with the improved treatment for TB, it is still the predominant cause of Addison's disease in poorly resourced countries [12]. Furthermore, it has been advised that AI can be considered in all TB patients who have persistent symptoms like nausea, vomiting, hypotension, and hyponatremia resistant to saline infusions [13].
Since Addison’s disease, like syphilis, tends to mimic other conditions, it has been found that at the time of its diagnosis, about 20% of the subjects had the disease for longer than five years as a result of physicians’ failure to recognise it or false diagnosis as psychiatric and/or gastrointestinal disorders [14, 15].This represents a missed opportunity since it has been demonstrated that the clinical conditions of patients with signs and symptoms of AI have been improved markedly when started on glucocorticoid and mineralocorticoid replacement therapy [16].Therefore, a clinician should develop a high index of suspicion for AI in patients presenting with signs and symptoms suggestive of AI, with a history of chronic infections (tuberculosis, fungal infections, cryptococcus, histoplasmosis), systemic diseases (sarcoidosis, systemic lupus erythematosus), and malignancy (breast cancer, lung adenocarcinoma) [17].
A TB-suspect has been defined as “Any person who presents with symptoms or signs suggestive of TB. The most common symptom of pulmonary TB is a productive cough for more than two weeks, which may be accompanied by other respiratory symptoms (shortness of breath, chest pains, hemoptysis) and/or constitutional symptoms (loss of appetite, weight loss, fever, night sweats, and fatigue)” [18]. The researchers observed that a number of patients admitted in the primary health care settings presented with signs and symptoms suggestive of this condition. Furthermore, the researchers also observed that a patient presenting as a TB-suspect also showed signs and symptoms of AI.
This study was conducted to determine indicators ofAI (signs and symptoms and certain laboratory findings) among TB-suspect patients admitted to the settings mentioned below.It was hoped that this determination would enable early detection and treatment of patients with AI as it has been shown that delaying treatment with corticosteroids can lead to the deterioration of their clinical condition culminating in shock and death [19].
2. MATERIALS AND METHODS
2.1. Study Design and Setting
A cross-sectional study was conducted at the primary health care (also called the level one) ward number 35 of Dr. George Mukhari Academic Hospital (DGMAH), which is a tertiary hospital located in Pretoria, as well as two referring district hospitals: Odi District Hospital (ODH) and Jubilee District Hospital (JDH), which are located 60km and 15km away, respectively, from the tertiary hospital. All three settings are located on the outskirts of the city of Pretoria in South Africa. At the time of data collection for this research study(2014/15), there were 40 available beds in ward 35) [20], 216 at ODH [21] and 446 at JDH [22].
2.2. Study Population and Sampling Strategy
The study population consisted of all TB-suspect patients admitted to the three hospitals over a six months’ period (1st September 2014 - 28th February 2015), which worked out to the following numbers: DGMAH (ward 35), 31 patients, ODH, 23 and JDH 38, giving a total of 92 patients. Each respondent was allocated one data collection sheet. Seventeen data collection sheets had areas of missing data and were excluded for data analysis, resulting in 75 datasheets remaining, which were analysed.
2.3. Data Collection and Measurement
Three medical officers (one for each setting) were hired to collect data. They used the researcher-administered data collection sheets. Data comprised baseline characteristics (age, sex, marital status, and educational level); symptoms of AI (dry itchy skin, muscle and joint pains, tiredness, craving for salt, loss of libido in males, amenorrhoea in females, dizziness, loss of weight and nausea and vomiting) and signs of AI (systolic hypotension, low pulse volume, tachycardia, hypothermia, mucosal and skin hyperpigmentation and general body wasting) derived from a literature review [23-25].We conducted serum cortisol, sodium, potassium and fasting serum glucose to establish patients’ electrolyte status vis-à-vis cortisol levels. Furthermore, we conducted GeneXpert, CD4 cell count, lipoarabinomannan (LAM), and TB blood culture for possible confirmation of TB infection, given that the targeted patients were TB-suspects.
We used the low-dose (1µg/ml intravenously) short corticotropin (Synacthen®) stimulation test [26]. This was motivated by the reality that the low-dose alternative was affordable in our setting [27]. We administered the test drug to patients between 07h00 and 09h00 when the serum cortisol has been shown to be at its highest in a given patient (101 - 536 nmol/L) [28].The low-dose synacthen solution was constituted as follows: one ampoule of 250µg of synacthen was diluted into 249ml of sterile 0.9% saline solution to obtain a concentration of 1µg/ml. This procedure was carried out by the three data collectors in the wards under sterile conditions, each using a graduated measuring jar to prepare the solution. The remaining solution was stored in 50ml syringes kept in the medicines refrigerator,as it has been shown that the solution can be kept for up to 60 days at a temperature of 4 degrees [27].Two blood samples from each patient were taken to measure the pre-corticotropin and post-corticotropinserum cortisol levels. The time interval between the first and second specimens was 30 - 60 minutes [1]. It has been shown that among individuals with normal adrenal gland function, the corticotropin stimulation test elicits a rise in blood cortisol level, while those with a primary or secondary form of AI respond poorly or not at all [29].
All the assays were performed in one reference laboratory, the National Health Laboratory Service (NHLS), at Dr George Mukhari Academic Hospital. The blood specimens were processed together with the routine specimens sent to the laboratory, with no specific identity. In that way, the laboratory technicians were blinded to the study samples. The paired pre- and post-injection samples were run in the same batch. The Coefficient of Variation (CV) for cortisol on the Abbot Architect instrument was as follows: Level 1 = 105 nmol/L (3.95%), Level 2 = 558 nmol/L (2.12%) and Level 3 = 703.7 nmol/L (2.01%).
2.4. Statistical Analysis Plan
Descriptive statistics was performed to describe the characteristics of the study sample. Measures of central tendency and dispersion were obtained for continuous variable and frequency distributions for categorical variables. Chi-square tests were performed to compare the prevalence of the signs and symptoms experienced by the respondents between those who tested positive or negative for AI. Multiple logistic regression analyses were performed to determine if signs and symptoms were significantly correlated to AI, which was defined by the morning serum cortisol of<500 mmol/L, as recommended by the Endocrine Society [27]. The SPSS version 25 computer software was used for data analysis. The level of statistical significance was set at p-value < 0.05.
Additional variables were derived using the signs and symptoms as clinical factors (data not shown). Signs Burden Index (SiBI) was computed by summing all seven signs, coded 1 if a sign was present and 0 if absent. Signs burden was then dichotomized into“low” and “high”. Signsburden score above the median (5 signs) was classified as “high” and “low” otherwise. Symptoms Burden Index (SyBI) was computed by summing all 12 symptoms, coded 1 if a symptom was present and 0 if absent. The symptoms burden was then dichotomized into“low” and “high”. A symptomburden score above the median (5 symptom) was classified as “high” and “low” otherwise. A Sign and Symptom Burden Index (SSBI) was computed by summing all 18 signs and symptoms. The signs and symptoms burden was also dichotomized into “low and “high”.
Since the thresholds and scaling of the potential indicators of AI were arbitrary, given no standard cut-off values in the literature. The alternative analytic approach to analysing the data was to use the Classification Regression Tree (CART) [30], which determines cut-off values for the indicator variables. CART is a predictive algorithm, which explains how an outcome (target) variable's values can be predicted based on other values (independent factors). It is a ‘decision tree’ where each fork is a split in a predictor variable, and each node at the end has a prediction for the outcome variable. At each node of the decision tree in this study, the following information is provided:
- Attribute of the outcome variable expressed as class= 0 or 1, where 1 denoted attribute of interest. So in this case, class = 0 represents a group of participants with AS and class = 1 represents a group of participants with AI.
- The indicator variable that results in the branching of the tree (the threshold that results in the split is provided).
- Class membership, number of cases (number of participants with the outcome of interest) - in this case AI, and the percent of cases.
- The statistic “W” which is the weight that equates to the sample size at each split. The starting value of W is equal to N.
- The total sample size, N, of the cases in both groups.
3. RESULTS
Of the 75 study respondents 47 (62.7%) and 32 (37.3%) were classified as having AS and AI, respectively (AI definition = serum cortisol level < 500nmol/L) [27]. Respondents with AS were, on average, seven years older than those with AI. The mean age of the study sample was 40.3 years, with a standard deviation of 15.7 years. The gender distribution of patients with AS and AI was similar (AS: females=41.3% and AI: female=46.2). The distribution of marital status was also similar between those with AS and AI (Table 1).
Between the two groups (AS versus AI), there was no obvious difference in their levels of sodium, potassium and serum glucose. The proportion of respondents who tested LAM positive was insignificant (< 1.0%). The proportion of respondents who tested positive for TB blood culture was higher among the AI group compared to their counterparts (56.2% versus 43.8%). Respondents with AI had lower values of baseline and post-injection serum cortisol and higher CD4 counts (Table 2).
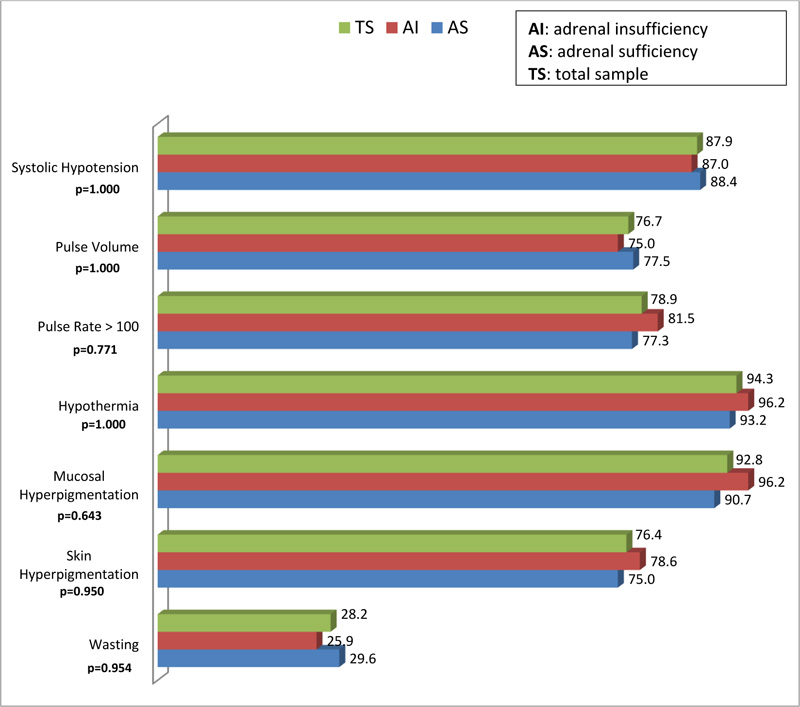
Fig. (1) provides a graphical depiction and comparison of the physical signs of respondents with AS and AI. There was no statistical difference in the physical signs exhibited by the respondents. Of the seven physical signs assessed, wasting was the least frequent sign among both groups of respondents. The following signs occurred slightly more frequently in the AI group compared to the AS group: systolic hypotension, hypothermia, mucosal hyperpigmentation, skin hyperpigmentation, pulse rate > 100, and wasting. None of these results demonstrated significant statistical significance (p > 0.05).
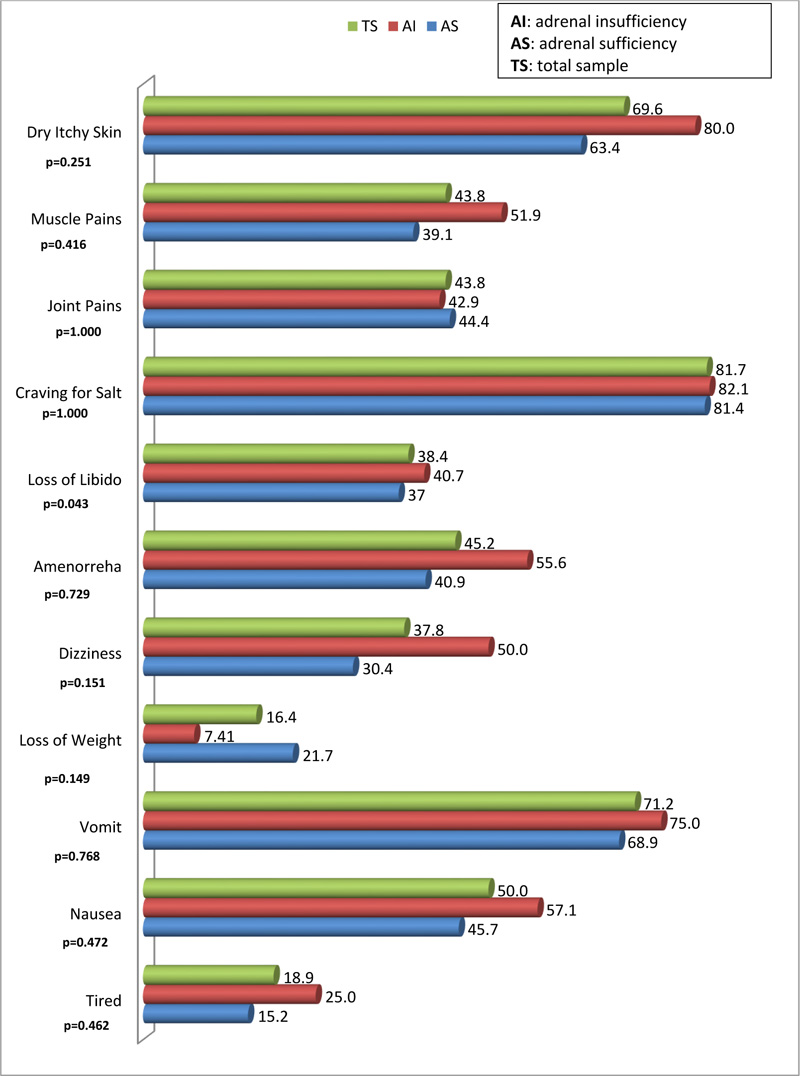
Of the 11 symptoms, only the occurrence of loss of libido was statistically different between respondents with AI compared to those with AS (40.7% versus 38.4%; p=0.043). The least occurring symptoms were loss of weight and tiredness, with a prevalence of 16.9% and 19.4%, respectively. The most occurring symptoms were craving for salt, dry, itchy skin and vomiting with a prevalence of 79.7%, 68.1%, and 69.0%, respectively (Fig. 2).
Based on the dichotomized values of the following indicators: Symptoms Burden, Signs Burden, Signs & Symptoms Burden, Sodium (mmol/L), fasting serum glucose (mmol/L), CD4 count by means of the median values; the presence or absence of the following signs: loss of libido and weight loss; and potassium and change in serum cortisol (post-pre) in 1 standard deviation units we found no significant correlated to AI status. The data for the non-significant crude and age-sex-adjusted associations are not shown.
| Socio-demographic Factors |
Total Sample (n=75) 100% |
Adrenal Sufficiency (n=47) 62.7% |
Adrenal Insufficiency (n=28) 37.3% |
P-value |
|---|---|---|---|---|
| Statistic | Statistic | Statistic | ||
| Age (in years), M± SD | 39.7±15.0 | 40.7±14.2 | 37.9±16.4 | 0.4387‡ |
| Gender, n | 72 | 46 | 26 | 0.8797 |
| Male | 56.9 | 58.7 | 53.9 | - |
| Female | 43.1 | 41.3 | 46.2 | - |
| Marital Status, % | 73 | 45 | 28 | 0.8486⌂ |
| Single | 67.1 | 66.7 | 67.9 | - |
| Married | 27.4 | 26.7 | 28.6 | - |
| Divorced/Separated | 5.5 | 6.7 | 3.6 | - |
| Educational Attainment, % | 69 | 46 | 23 | 0.1344⌂ |
| No Formal Education | 6.9 | 6.5 | 8.7 | - |
| Primary School | 23.3 | 30.4 | 8.7 | - |
| Secondary School | 57.5 | 47.8 | 73.9 | - |
| Tertiary Education | 12.3 | 15.2 | 22.2 | - |
| Clinical/Laboratory Data | Overall Total | Adrenal Sufficiency | Adrenal Insufficiency | P-value |
|---|---|---|---|---|
| Baseline Serum Cortisol Levels (mmol/L), MD (IQR) | 398.0 (255.0, 509.0) |
473.0 (355.0, 573.) |
255.0 (168.0, 343.0) |
<0.0001 |
| Post-Injection Serum Cortisol Levels (mmol/L), MD (IQR) | 537.0 (453.0, 632.0) |
606.0 (543.0, 674.0) |
425.5 (392.5, 464.0) |
<0.0001 |
| Δ Serum Cortisol Levels (mmol/L) M±SD | 147.3 ± 122.7 | 150.2 ± 117.0 | 142.1 ± 142.1 | 0.7980 |
| Sodium (mmol/L), MD (IQR) | 135.0 (131.0, 139.0) |
135.0 (130.0, 139.0) |
137.0 (133.0, 139.0) |
0.2488 |
| Potassium (mmol/L), M± SD | 3.99 ± 0.77 | 6.0 ± 2.9 | 5.5 ± 1.8 | 0.3486 |
| Fasting serum Glucose (mmol/L), MD (IQR) | 5.1 (4.6, 6.1) | 5.5 (4.7, 6.4) | 4.9 (4.4, 5.5) | 0.1705 |
| CD4 count MD (IQR) | 187.0 (42.0, 565.0) |
125.5 (29.0, 456.0) |
197.0 (44.0, 713.0) |
0.4522 |
| Lipoarabinomannan (LAM), MD (IQR) | 1.0 (1.0, 1.0) |
1.0 (1.0, 1.0) |
1.0 (1.0, 1.0) |
0.8647 |
| GeneXpert- Positive Results, n (%) | 13 (16.7) | 7 (14.9) | 6 (21.1) | 0.4672 |
| Burden of Symptoms, M± SD | 4.8 ± 2.4 | 4.6 ± 2.4 | 5.3 ± 2.4 | 0.2091 |
| Burden of Signs, M± SD | 4.9 ± 1.4 | 5.0 ± 1.5 | 4.9 ± 1.2 | 0.7204 |
| Burden of Signs & Symptoms, M± SD | 9.6 ± 3.2 | 9.5 ± 3.1 | 10.1 ± 3.1 | 0.4235 |
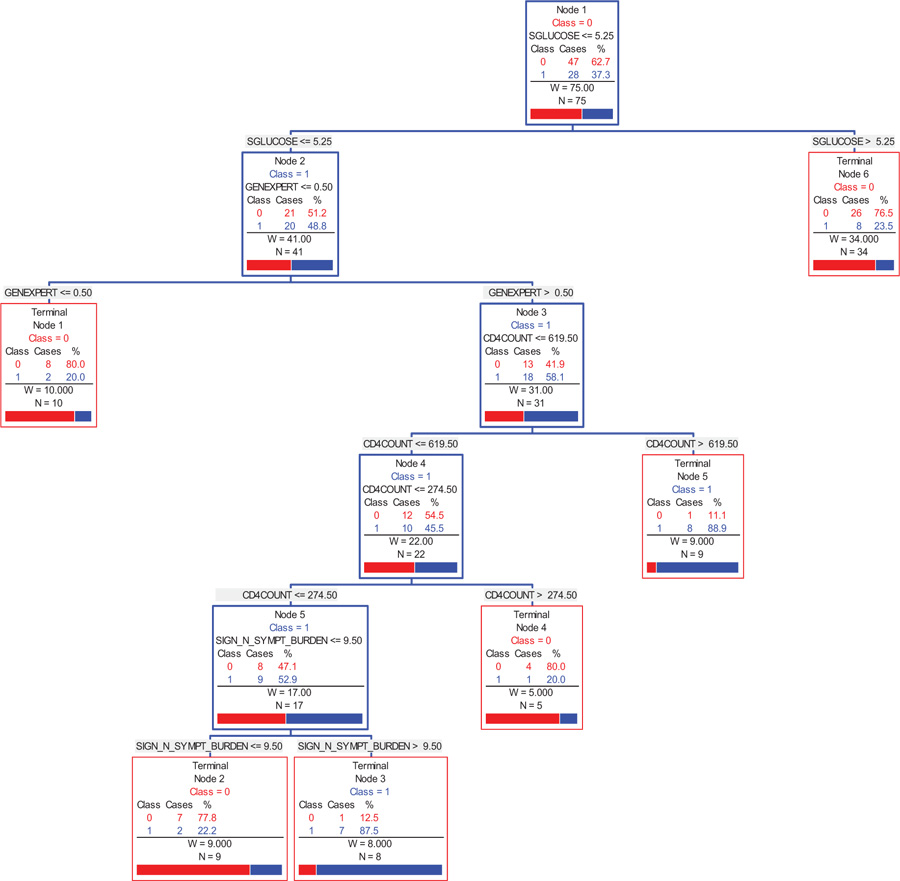
Fig. (3) provides a graphical summary of the selected indicators, in the order of importance, as to the correlates that discriminate between a person with AI and those with AS. The CART methodology [30] indicates that efficiency (low cost) of the significant indicators is obtained by a six-node decision tree, which generates the combinations and values of the indicators that provide an 87.5% likelihood of a participant having AI. The CART analysis suggests that the indicators (independent variables) and their corresponding cut-off values used for splitting the participants into groups are as follows: fasting serum glucose (5.25 mmol/L), GeneXpert (0.5), CD4 count (619.5 cells/ml) then CD4 count (274.5 cells/ml), and sign and symptoms burden (9.5). Regarding the CD4 count, the program used two discriminating values, firstly the CD4 ≤ 619.5 cells/ml, and further refined it to the CD4 ≤ 274.5 cells/ml. In summary, one can infer the following classes of participants with the corresponding likelihood of having AI (Fig. 3A):
- A person with a fasting serum glucose ≤5.25 mmol/L, GeneXpert > 0.5, CD4 count ≤ 619.5 cells/ml, then CD4 count ≤ 274.5 cells/ml, and sign and symptoms burden > 9.5, is 87.5% likely to have AI.
- A person with a fasting serum glucose ≤5.25 mmol/L, GeneXpert > 0.5, CD4 count ≤ 619.5 cells/ml, and then CD4 count ≤ 274.5 cells/ml, is 52.9% likely to have AI.
- A person with a fasting serum glucose ≤5.25 mmol/L, GeneXpert > 0.5, and CD4 count ≤ 619.5 cells/ml, is 45.5% likely to have AI.
- A person with a fasting serum glucose ≤5.25 mmol/L, and GeneXpert > 0.5, is 58.1% likely to have AI.
- The principal indicator, fasting serum glucose places a person with value ≤5.25 mmol/L, at a 48.8% likely of having AI.
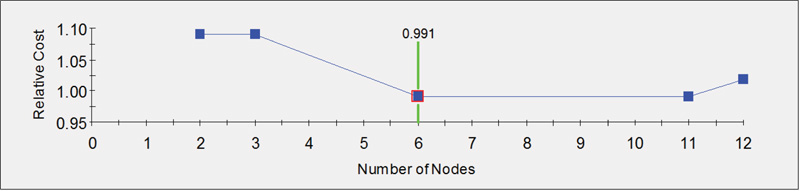
Fig. (3B) provides a graphical display of the measure of efficiency in group classification based on the number of nodes of the decision tree. The relative cost is inversely related to efficiency. Hence, in this case, the most efficient classification (minimum relative cost) is achieved with six nodes.
4. DISCUSSION
The discussion below focuses on the respondents’ baseline characteristics, laboratory investigations for AI and TB, signs and symptoms of AI as well as regression analyses models, and the CART analyses, which derived correlates of AI from exposure factors.
Regarding baseline characteristics, the study has shown that the proportion of males with AS was higher than their female counterparts, although not statistically significant, and the opposite maintained for those with AI. According to literature, there is no sex difference in Addison’s disease as a result of disseminated infections or metastatic conditions [31]. However, it needs to be born in mind that 70% of patients with autoimmune AI in poly-glandular autoimmune syndromes are predominantly female, while those with isolated autoimmune AI are predominantly male (71%) in the first two decades of their lives [32, 33]. The explanation for these sex differences is unknown [34].
Respondents with AI had lower values of baseline and post-injection serum cortisol and higher CD4 cell counts. The negative linear correlation between CD4 cell count and serum cortisol as it was found in the current study, has also been reported in literature [35]. The tests to confirm TB infection among the TB-suspect patients had a poor yield in the current study. There were no significant differences between the AS and AI groups regarding sodium, potassium, and serum glucose levels. This is contrary to a study that found the opposite: hyponatremia, hyperkalemia and hypoglycemia accompanying low cortisol levels in patients with AI [25]. The findings by Li, et al. [25] confirm the normal physiological response to deficient cortisol and aldosterone levels caused by adrenal gland infiltration. This finding could mean that in the setting of the current study, these laboratory findings should not be prioritized for consideration of AI among TB-suspect patients.
There was no significant difference between the AS and AI groups regarding the physical signs assessed. The researchers are of the view that the smaller sample size of 75 respondents may account for the non-significant differences findings, compared to studies with larger sample sizes [26, 36]. However, there were comparable findings between the current study and the study conducted in South Africa in 2013, regarding hyperpigmentation (93.3% versus 76.0%) and weight loss (40.6% versus 25.0%) among AI and AS patients, respectively [24]. Furthermore, in the current study, systolic hypotension, hypothermia and tachycardia occurred slightly more frequently in the AI group. Of the seven physical signs assessed, wasting was the least frequent sign among both groups of respondents, which went contrary to the researchers’ expectations, as TB is frequently associated with weight loss [23].On the whole, even though the difference of the signs of AI between the AS and insufficiency groups was not statistically significant, the overall picture is that the latter group exhibited more signs of AI compared to the former Fig. (1).
The symptoms which occurred more frequently were dry, itchy skin, craving for salt, nausea and vomiting, while the least occurring were perceived loss of weight and tiredness, with a higher proportion among the AI group. Physiologically, the dry itchy skin is caused by dehydration and the bile salts and acids [37].The accumulation of bile salts and acids in patients with AIis caused by cholecystitis [25].Craving for salt, a symptom of mineralocorticoid deficiency occurred in more than 80% in the AI group. It has been suggested that clinicians should instruct patients presenting with AI “to take salt and sodium-rich foods ad libitum” [38].These patients (adults) should be started on fludrocortisone 50-200 µg [38].Nausea and vomiting occurred in about 57% and 75%, respectively, in this study. It occurs as a result of electrolyte imbalance [39]. These two symptoms are part of the gastrointestinal clinical features of AI and have been found to be accompanied by abdominal pains and diarrhea, alternating with constipation in 56%-92% of patients [40]. An incidence of gastrointestinal symptoms of about 20% has been reported in other literature [41], while a study conducted in South Africa, reported an incidence of nausea and vomiting of more than 40% of the respondents [24]. These prevalence figures are lower than those obtained in the current study. Therefore, the current study provides another upper limit of the range of occurrence of nausea and vomiting in patients with AI in South Africa. In the current study, the only symptom that occurred more significantly among the AI group was the loss of libido. The association of Addison’s disease and neuropathy has already been demonstrated elsewhere [42].
Data analysis towards the derivation of predictor signs and symptoms for AI (especially in resource-limited settings in laboratory investigations) did not yield the desired outcome. Consequently, we decided to pool the signs and symptoms to develop the Signs and Symptoms Burden Index, as described in the literature [43]. Using this approach, the high burden of signs only, symptoms only and signs and symptoms were not significantly associated with AI (data not shown). However, the Classification and Tree Regression (CART) analyses [30] suggested that the following four indicators: serum glucose, GeneXpect, CD4 count (repeated at different thresholds) and signs and symptoms burden are significant correlates, which in combination discriminate between a person with a higher likelihood of having AI. This heightens the clinician’s index of suspicion in a TB-suspect patient presenting with signs and symptoms of AI. Indeed, evidence indicates that treatment should be initiated without delay when AI is suspected, so as to save the affected patient’s life [44].
Sub-Saharan Africa is a resource-limited region, including the availability of laboratory facilities [45]. The poor supply manifests itself more in remote and underserved areas [46]. Therefore, a TB-suspect patient presenting with signs and symptoms of AI, a decreased serum glucose (< 5.25), positive GeneXpert and CD4 count ≤ 274.5 cells/ml should raise a high index of suspicion for AI to the attending clinician who should commence treatment, without first conducting the ACTH stimulation test to confirm AI [16].
5. STRENGTHS AND LIMITATIONS
This was a preliminary, small sample size study conducted in only three primary health care settings in the Pretoria area of South Africa. To our knowledge, this is the first study in a resource-limited setting to demonstrate the usefulness of combining signs and symptoms and basic laboratory findings into indices in determining laboratory-confirmable AI among TB-suspect patients. There may have been inaccuracies in the method used to prepare the low dose(1µg/ml) synacthen solution by the three data collectors, as one study has demonstrated [47].
CONCLUSION
The study has demonstrated that using signs or symptoms by themselves does not discriminate persons with a high likelihood of AI. However, low fasting serum glucose, a positive GeneXpect, a low CD4 count with a combination of a minimum of ten signs and symptoms does constitute a discriminator for AI in TB-suspect patients. Therefore, a combination of these indices could be useful to raise the clinician’s index of suspicion for AIin resource-limited settings and contribute to patient care.
AUTHORS’ CONTRIBUTIONS
Conceptualization and investigation were performed by Langalibalele H Mabuza. Langalibalele H Mabuza wrote the initial draft. Data analysis and interpretation were done by Daniel F Sarpong.
Langalibalele H Mabuza, Daniel F Sarpong are responsible for writing, reviewing and editing of this manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Medunsa Research and Ethics Committee (MREC), South Africa, which endorses the latest declaration of Helsinki, approved the study with the clearance certificate number MREC/M/219/2010:IR.
HUMAN AND ANIMAL RIGHTS
Not applicable
CONSENT FOR PUBLICATION
Participation was voluntary, and respondents signed the informed written consent to participate. The researchers assured each respondent of anonymity and confidentiality.
AVAILABILITY OF DATA & MATERIALS
Not applicable.
FUNDING
This study was funded through the VLIR (Belgium) Grant Number: ZA2020IUC021A102.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The research team would like to thank Drs T Bongongo, C Barua, DK Nzaumvila, and J Reynecke for their involvement in data collection. We would also like to thank the staff at the National Health Laboratory Service (NHLS) for their advice and assistance with sample analysis: Mr. Mzimasi Gcukumana (Senior Manager: Communication, Marketing & Public Relations, NHLS), Mrs. Caren Smit (Communication Administrator, NHLS), Dr. Siphokazi Gwiliza (Senior Pathologist, NHLS) and Professor Donald Tanyanyiwa (Head of Department: Chemical Pathology, Sefako Makgatho Health Sciences University& NHLS).


