All published articles of this journal are available on ScienceDirect.
Impact of Sleep Disturbances on the Quality of Life Among Schizophrenic Out-patients of Jimma University Medical Center, Southwest Ethiopia: Hospital Based Cross-sectional Study
Abstract
Aim:
Current study was aimed to assess the impacts of sleep disturbances on patient’s quality of life.
Background:
Schizophrenia is a syndrome, which affects sleep. Up to 80% of schizophrenic patients complain of sleep disturbances which affect the quality of life
Objectives:
To assess the association of sleep disturbances and quality of life and other contributing factors among schizophrenic patients on follow-up treatment at Jimma University Southwest Ethiopia.
Methods:
A cross-sectional study with a consecutive sampling of 411 out-patients at Jimma University medical center was employed from April 21-June 20, 2019. Sleep disturbances and the quality of life were assessed by Pittsburgh sleep quality index and WHOQOL-BREF, respectively. Epi data version 3.1 and SPSS version 23.0 software was used. Chi-square and independent samples t-test were used for association and P-value < 0.05 was considered for statistical significance.
Results:
Most participants had sleep disturbances and the mean score of positive scale on PANSS was higher for patients with sleep disturbances. About one-fourth of the patients had very good subjective sleep quality and > 85% of sleep efficiency was reported by 139 participants. More than half (51.1%) of the subjects had used sleep medication and the majority (64.7%) of them were reported daytime dysfunctions in the past month. The social domain (M±SD=3.92±2.51, t=8.46, p= <0.001, eta2=0.15) and overall WHOQOL (M±SD=57.60±16.87, t=9.24, p= < 0.001, eta2= 0.17) score had a large difference of means and about 15% and 17% of the variance in sleep disturbance have been explained.
Conclusion:
Generally, the finding of the current study was in agreement with most of the previous studies and sleep disturbances respectively moderate to significant effects on the patient’s quality of life.
1. INTRODUCTION
1.1. Sleep Disturbances in Patients with Schizophrenia
Schizophrenia is a syndrome and serious psychotic illness which comprises a number of problems. It interferes with the patient’s life and affects a wide range of domains like emotion, behavior, perceptions, thoughts, and sleep. Sleep is a physiological process in which awareness of external stimuli is decreased and is crucial to the optimal functioning of the body and mind [1, 2].
Sleep disturbances are distressing symptoms that are common among various ranges of psychiatric disorders and affect up to 80% of schizophrenic patients [3]. It gives ways to the maintenance of psychotic symptoms and functional disabilities [4-6]. Difficulty in initiating sleep, early awakening and inability to fall asleep back, and reduced slow wave-sleep were repeatedly documented by polysomnography at any phase of the illness although its relation to the Quality Of Life (QOL) is not well known in the current setting [7]. Data were limited in Ethiopia concerning the sleep disturbances in patients with schizophrenia and previously, it was reported that the use of antipsychotics was significantly related to disturbed sleep like sedation [8] and the presence of these side effects along with other factors had related to poor medication adherence [9].
1.2. Sleep Pattern and Quality of Life Among Schizophrenic Patients
QOL is a multidimensional complex issue, a holistic approach and broader concept which contains social, functional, physical, economical, and emotional well-being of a person. It comprises different medical and psychosocial aspects such as daily living activities, awareness of own health status, psychological well-being and satisfaction with life and difficult to explain. Unlike previous decades, it is getting attention currently because of growing concerns about the disappointing life of patients with chronic mental illnesses like schizophrenia [10-12].
Health Related-Quality of Life (HRQOL) is a subjective awareness of the patient’s own health and illness and its management. This can be widely affected by sleep disturbance, although their relation is poorly studied in developing countries [13, 14].
Sleeping under the recommended time (five hours or less) is linked with different negative consequences of one’s health like poor mental health, increased severity of symptoms, causing chronic medical illness, low quality of life, and elevated suicide rate [15, 16]. As a report indicated, patients with schizophrenia with all types of sleep disturbances more likely had poor quality of life and they had a suicidal attempt and committed suicide about 13 times more than those without sleep disturbances [17].
Decreased Non-rapid Eye Movement (NREM) and Rapid Eye Movement (REM), fragmented sleep, altered sleep timing; reduced sleep continuity and sleep disturbance are highly prevalent in schizophrenia and negatively affect the quality of life [18]. Sleep disturbances affect large number of schizophrenic patients and cause psychological distress, social impairment, cognitive functioning problems, and disturbing health-related quality of life [19].
Disturbed sleep and sleep disorders linked to dangerous accidents, decreased productivity at work, and disturbed psychological functioning. Literature showed that the work-related accident is doubled in people with sleeping difficulty and prolonged sleep disturbance has negatively affected individual well-being and quality of life. It is also reported that about 40% of people with sleep disturbances, especially insomnia were diagnosed with at least one psychiatric illness [20]. It is also reported that sleep disturbance worsens all domains of quality of life independent of comorbid illness and good sleep quality instantly helps in the sustainment of physical functioning and mental wellbeing [21].
Despite the association of sleep disturbances with wide ranges of symptoms like excessive tension, drowsiness, bad mood and self-harm, little is known regarding the concerning patterns of sleep disturbances in a variety of psychiatric illnesses in the sub-Saharan countries [22].
Although it is often dominated by other clinical concerns like positive symptoms of psychosis, sleep problems are common among schizophrenic patients and linked to weight gain risk, impaired cognition, and lower quality of life and affect more than 90% of psychiatry hospitalized patients [23]. A study that was conducted on 20 individuals with schizophrenia reported as patients with schizophrenia had longer sleep period, sleep latency and time spent in bed and fragmented sleep. This can cause chronically disturbed sleep, which highly affects their social interactions, productivity, and quality of life [24].
Another study among 617 schizophrenic patients showed that 78% of sleep disturbances and revealed as insomnia and delayed sleep onset were frequent, and they had severe symptoms and poorer functioning than patients without sleep disturbances [4]. One study of sleep diary and wrist-actigraphy recordings also showed that patients with schizophrenia had poor sleep efficiency, irregular sleep-wake cycle, delayed sleep onset and high score on PSQI than healthy subjects and healthy subjects had a higher score on all domains of QOL compared to schizophrenic patients [25].
Another study reported elongated sleep latency, decreased sleep efficiency and increased nighttime awakening [26] and another study showed as almost half the patients had poor sleep quality and 24.7% of them were reported to have excessive daytime dysfunction, which has an impact on their daily living functioning and disturbed HRQOL [27]. One systematic review also reported that sleep disturbances are high (75-80%) among schizophrenic patients with severe psychotic experiences and 50% of them had insomnia and 48% of them had reported nightmares [5].
It is also reported that patients with schizophrenia had increased sleep latency, repeated sleep interruption and about 50-70% of them had sleep disturbances [28], and they had problems with sleeping, and had irregular sleep patterns, which cause them daytime dysfunctions and emotional instability [29]. As the literature showed, schizophrenic patients who had low educational status and lower economic status and those living in a large family had been associated with more sleep disturbances [30] and other results revealed that older age was linked to low risk of sleep disturbances and women sex associated with longer sleep time, shorter sleep latency and higher sleep efficiency [31, 32]. It is also reported that sleep disturbances were associated with older age (especially insomnia), unemployment and marital status, urban residence and female gender [33, 34].
Patients with sleep disturbances and poor sleep quality had a lower score on all domains of QOL and daytime dysfunction accounted for 12.6% of QOL index variance [35] and the score of QOL was reduced for patients with sleep disturbances [7]. Schizophrenic patients with daytime dysfunction and insomnia had a lower score of physical and social domains of QOL and the score was also lower on most domains of QOL for patients with poor sleep quality [14]. It was also reported that at least 29% of schizophrenic patients had no sleep disturbances and for those with sleep disturbances, the score of HRQOL was decreased significantly on all domains [21], and patients with any sleep disturbance had decreased quality of life when compared with those without sleep disturbances [36].
It was reported as patients with prominent positive symptoms had higher daytime dysfunctions [37], and patients with more severe positive symptoms had worsened sleep disturbances than those with prominent negative symptoms [38]. However, another study reported as no significant difference was found between patients with prominent positive and negative symptoms concerning sleep quality and sleep disturbances except that patients with prominent negative symptoms had reported poor social functioning [39].
Concerning antipsychotic medications, olanzapine post-treatment patients had shown increased total sleep hours, increased sleep efficiency and decreased sleep latency [40] and literature also reported that typical antipsychotics had the effects of increasing sleep efficiency, stage 4 sleeps and decreased REM latency in a short-term administration and atypical antipsychotics showed the effects of increasing total sleep time and sleep efficiency except for risperidone which has no such effects [41]. It is also reported as atypical antipsychotics like olanzapine and risperidone have the ability to improve sleep quality in schizophrenic patients than typical antipsychotics [42]. Although knowing the magnitude of sleep problems and their association with quality of life is vital to manage, the study was lacking in the current setting to our best knowledge. So, the current study is aimed to assess sleep disturbance and its association with quality of life among schizophrenic patients and try to fill gaps in the area.
2. MATERIALS AND METHODS
2.1. Study Design and Period
The current study is part of a previous study that was under process for publication elsewhere. It was a cross-sectional study conducted at Jimma University specialized teaching hospital, psychiatric clinic among 411 out-patient schizophrenia aged 18 years and above from April 21-June 20, 2019. The minimum number of required sample size for this study was determined by using the formula to estimate a single population proportion using the following assumptions.
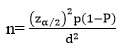 |
Where; n= minimum sample required
ϖ Z α/2 = standard score value for 95% confidence level=1.96
ϖ P= Estimated prevalence of sleep disturbances in schizophrenic patients (taken as 50% since no published material was found in our country and comparable countries among the same subjects as far as searched).
ϖ d= is margin of error (5%)
Using the above assumptions; n = [(1.96)2 x 0.5 (1-0.5)] / (0.05)2 n= 384
By adding 10% (384 x 0.10 = 38) of non-respondent, the final sample size was (384+38) = 422 although the response rate was 97.4% and accordingly 411 patients were finally included for analysis. Participants were included by using consecutive sampling technique and data were collected by face to face interview using structured and pre-tested questionnaires and card review for diagnosis. Patients who were acutely disturbed and those with other psychiatric comorbidity were excluded.
2.2. Data Collection Instruments
Questionnaires have contained socio-demographic characteristics of the participants, tools to assess sleep pattern, quality of life and severity of psychotic symptoms.
Sleep disturbance was assessed by Pittsburgh Sleep Quality Index (PSQI), which is a highly used tool to measure seven domains: sleep duration, sleep disturbances and sleep latency, use of sleep medication, daytime dysfunction, habitual sleep efficiency, and subjective sleep quality over the last month. Sleep disturbance is assessed by using nine different questions, which have 0-3 score each. Participants who had scored at least one positive response were identified as had some sort of sleep disturbance and the severity of the disturbance is increased with the score [43].
Quality of life was measured by the world health organization's quality of life scale-brief version (WHOQOL-Brief), which has 26 self-administered generic items. It is a valid tool cross-culturally and suitable to assess QOL in schizophrenic patients. The tool assesses four domains: physical health, psychological health, social relationships and environmental domain. Its score is a Likert form from 1 (very dissatisfied) to 5 (very satisfied) [44, 45].
The severity of psychotic symptoms (positive, negative and general psychopathology) of schizophrenia was assessed by the Positive and negative syndrome scale (PANSS), which is a 7-point rating instrument and contains 30-items grouped into P1-P7, N1-N7 and G1-G16 [46].
2.3. Data Quality Control
Forth and back translation of English versions questionnaires to local language (Amharic) was conducted. The pretest was done to clarify the difficulty of data collection instruments and collected data were checked for clearness and consistency on daily bases.
2.4. Data Processing and Analysis
Data were entered into Epi data version 3.1 and analyzed by SPSS version 23.0. Chi-square test was used for the association of sleep disturbance and categorical variables. Cramer’s V test was considered for effect size. For continuous variables, independent samples t-test was employed to calculate the mean difference and eta squared was considered to evaluate effect size by considering the quality of life as an outcome variable. We considered these methods rather than multivariate regression as we included view variables to consider the specific relationship between sleep disturbances and quality of life. Overall, 95% confidence interval and p-value < 0.05 was considered for statistical significance.
All assumptions were checked accordingly and Hosmer and Lameshow were calculated to check model fitness.
3. RESULTS
Finally, 411(97.4%) participants were included in the analysis and the majority (70.6%) of them were male. Almost half (49.5%) of the study subjects were currently living in urban residence. From the total participants, about one-third of them were followed only by primary school and 25.5% of study subjects were never married (Table 1).
In general, most (71.3%) of the patients had reported sleep disturbances and more than one-third of them slept for more than 7 hours/day during the past month. Slightly about one-fourth of the patients had reported very good subjective sleep quality and > 85% sleep efficiency was reported by 139 (33.8%) generally. More than half (51.1%) of participants had used any sleep medication in the past month and majority (64.7%) of study subjects were reported daytime dysfunction. (Table 2)
| Variables | Category | Frequency (%) |
|---|---|---|
| Ages (Years) | 18-44a 45-64 |
228 (55.5) 183 (45.5) |
| Sex | Male Female |
290 (70.6) 121 (29.4) |
| Educational status | No formal education Primary (1-8) Secondary (9-12) Above secondary |
94 (22.9) 137 (33.3) 110 (26.8) 70 (17) |
| Occupation | Government employee Self employed Jobless |
121 (29.4) 192 (46.7) 98 (23.8) |
| Place of residence | Urban Rural |
203 (49.4) 208 (50.6) |
| Marital status | Never married Married Divorced Widowed |
105 (25.5) 170 (41.4) 89 (21.7) 47 (11.4) |
| Family size | 1-5 ≥6 |
229 (55.7) 182 (44.3) |
| Current medications |
bTypical antipsychotics cAtypical antipsychotics |
222 (54.0) 189 (46.0) |
| variables | Category | Frequency (%) |
|---|---|---|
| Sleep disturbancea | Yes No |
293 (71.3) 118 (28.7) |
| Subjective sleep quality | Very good Fairly good Fairly bad Very bad |
112 (27.3) 147 (35.8) 94 (22.9) 58 (14.1) |
| Sleep latencyb | Very good Fairly good Fairly bad Very bad |
100 (24.3) 121 (29.4) 79 (19.2) 111 (27.0) |
| Sleep duration | >7 6-7 5-6 <5 |
156 (38.0) 140 (34.1) 65 (15.8) 50 (12.2) |
| Sleep efficiencyc | >85% 75-84% 65-74% <64% |
139 (33.8) 128 (31.1) 77 (18.7) 67 (16.3) |
| Sleep medication use | Used during last month Not used in the past month |
210 (51.1) 201 (48.9) |
| Daytime dysfunctiond | Yes No |
266 (64.7) 145 (35.3) |
On PANSS measure, the mean score for the negative scale was relatively lower than that of a positive scale and for the WHOQOL scale, the participants had scored lower on social and psychological domains and overall WHOQOL score.
On the chi-square test, both male and female had almost the same percentage for sleep disturbance, while subjects between the ages of 45-64 had a higher (74.9%) proportion than those between the ages of 18-44 (68.4%). On the other hand, the current place of residence had significantly associated with sleep disturbance and the proportion of urban participants who had reported sleep disturbance was higher than that of rural respondents and participants who reported greater than mean on a positive scale had a moderately strong association when Cramer’s V-test was considered. (Table 3).
For continuous variables, an independent samples t-test was conducted to compare the mean score of WHOQOL domains and PANSS score for participants with and without sleep disturbances. The magnitude of the difference in the mean was considered by calculating eta squared and interpreted as Cohen guidelines (0.01= small effect, 0.06 = moderate effect and 0.14 = strong effect) [48]. Accordingly, social domain (M±SD=3.92±2.51, t=8.46, p= <0.001, eta2=0.15) and overall WHOQOL (M±SD=57.60±16.87, t=9.24, p= < 0.001, eta2= 0.17) the score had a large difference in their means and about 15% and 17% of the variance in sleep disturbance have been explained by social domain and overall WHOQOL score, respectively. On the PANSS score, the positive scale showed a moderate effect in mean difference while negative and general psychopathology had little effects (Table 4).
| Variables | Category | Sleep Disturbance | X2 |
Asy. Sig (2-sided) |
V-test | |
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | |||||
| Age | 18-44 45-64 |
156 (68.4) 137 (74.9) |
72 (31.6) 46 (25.1) |
1.76 | 0.19 | 0.07 |
| Sex | Male Female |
206 (71.0) 87 (71.9) |
84 (29.0) 34 (28.1) |
0.003 | 0.95 | 0.01 |
| Educational status | No formal education Primary (1-8) Secondary (9-12) Above secondary |
61 (64.9) 105 (76.6) 82 (74.5) 45 (64.3) |
33 (35.1) 32 (23.4) 28 (25.5) 25 (35.7) |
6.04 | 0.11 | 0.12 |
| Occupation | Gov’t employee Self employed Jobless |
84 (69.4) 144 (75.0) 65 (66.3) |
37 (30.6) 48 (25.0) 33 (33.7) |
2.68 | 0.26 | 0.08 |
| Place of residence | Urban Rural |
167 (82.3) 126 (60.6) |
36 (17.7) 82 (39.4) |
22.56 | < 0.001 | 0.24 |
| Marital status | Never married Married Divorced Widowed |
69 (65.7) 116 (68.2) 69 (77.5) 39 (83.0) |
36 (34.3) 54 (31.8) 20 (22.5) 8 (17.0) |
7.20 | 0.07 | 0.13 |
| Family status | 1-5 ≥ 6 |
167 (72.9) 126 (69.2) |
62 (27.1) 56 (30.8) |
0.51 | 0.48 | 0.04 |
| Current medication | Typical antipsychotics Atypical antipsychotics |
163 (73.4) 130 (68.8) |
59 (26.6) 59 (31.2) |
0.86 | 0.35 | 0.05 |
| PANSS Positive | Less than mean Greater than mean |
149 (59.8) 144 (88.9) |
100 (40.2) 18 (11.1) |
39.10 | < 0.001 | 0.31 |
| PANSS negative | Less than mean Greater than mean |
148 (62.4) 145 (83.3) |
89 (37.6) 29 (16.7) |
20.38 | < 0.001 | 0.23 |
| PANSS general | Less than mean Greater than mean |
162 (63.3) 131 (84.5) |
94 (36.7) 24 (15.5) |
20.25 | < 0.001 | 0.23 |
| Variables | Sleep Disturbance | M±SD | t-value | p-value | 95% CI | Eta squared |
|---|---|---|---|---|---|---|
| WHOQOL Domains | ||||||
| Physical | Yes No |
22.02±4.00 22.10±4.32 |
0.17 | 0.87 | -0.80, 0.95 | < 0.001 |
| Psychological | Yes No |
13.72±4.32 17.30±3.55 |
7.97 | < 0.001 | 2.69, 4.45 | 0.13 |
| Social | Yes No |
3.92±2.51 6.34±2.90 |
8.46 | < 0.001 | 1.86, 2.98 | 0.15 |
| Environmental | Yes No |
22.97±3.47 23.43±3.71 |
1.21 | 0.23 | -0.29, 1.22 | 0.004 |
| WHOQOL-overall | Yes No |
57.60±16.87 73.62±13.24 |
9.24 | < 0.001 | 12.61, 19.43 | 0.17 |
| PANSS Scales | ||||||
| Positive | Yes No |
17.25±9.31 10.80±6.86 |
6.81 | < 0.001 | -8.31, -4.59 | 0.10 |
| Negative | Yes No |
11.45±4.40 9.75±4.34 |
3.55 | < 0.001 | -2.64, -0.76 | 0.03 |
| General | Yes No |
24.68±8.70 20.74±7.74 |
4.28 | < 0.001 | -5.74, -2.13 | 0.01 |
4. DISCUSSION
In the current cross-sectional study, the majority (71.3%) of the participants with 95% CI: [67.2, 75.9] had sleep disturbances in general. This finding was in line with the previous study [28] may be due to comparable sample size and the same study design. However, the current finding was lower than the reported results from different studies [4, 5, 23]. This may be reasoned by the difference in assessment tools, study settings, differences in study design and inclusion criteria.
In this study, the proportion of participants with sleep disturbance was significantly higher in urban residents than rural residents. This is in agreement with the previous study, which reported that the participants from the urban residence had a shorter sleep time, more sleep disturbance and poorer sleep quality [49].
In our study, on the WHOQOL score, the means of psychological and social domains significantly differ for participants with sleep disturbance and without sleep disturbance, which accounted for about 13% and 15% of the variance, respectively. This is in line with the study conducted previously in which participants with sleep disturbances had a lower score of QOL on all domains and accounted for 12.6% of the variance. It also reported that the overall score of QOL reduced for patients with sleep disturbance and accounts for about 24% of the variance [7, 35]. Another cross-sectional study revealed the same result in which participants with sleep disturbance like daytime dysfunction and insomnia had a lower score on psychological and social domains of WHOQO [14].
Like the current study, the previous studies reported that participants with sleep disturbances had significantly decreased HRQOL score on all domains and patients with sleep disturbances have shown overall lowered quality of life [36].
Cross-sectional nature of the current study was identified as one limitation as it cannot explain the cause-effect relationship. The other possible limitation of this study was that we did not perform an objective assessment of sleep parameters by actigraphy and sleep laboratory. Despite these limitations, the current findings will be used as a baseline for future studies and help in giving evidence-based care among people with schizophrenia.
CONCLUSION
Generally, in this study, we identified that the prevalence of sleep disturbance was high among schizophrenic patients and it affects different domains in a patient’s quality of life and need great attention from treating clinicians and administrative bodies.
LISTS OF ABBREVIATIONS
| G | = General |
| HRQOL | = Health-related Quality of Life |
| N | = Negative |
| P | = Positive |
| PANSS | = Positive and Negative Syndrome Scale |
| PSQI | = Pittsburgh Sleep Quality Index |
| WHOQOL-BREF | = WHO Quality of Life Brief Version |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical clearance was obtained from the ethical review board of Institute of Health, Jimma University, Ethiopia with reference number JHRPGD/608/2019.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Data were collected after written consent was obtained and any personal information was kept entirely confidential and participants were assessed in a private room to maintain their privacy.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings are available with the corresponding author and can be availed on a necessary request.
FUNDING
The financial need for data collection was fulfilled by the Mettu University.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding this project and they approved it for publication.
ACKNOWLEDGEMENTS
We would like to acknowledge the psychiatric clinic staff of Jimma University for their great cooperation, Mettu University, for providing financial support and all our study participants.


