All published articles of this journal are available on ScienceDirect.
Role of Age and Sex on Simple and Complex Carbohydrates Rich Foods Consumption and Thyroid Cancer Risk: Hospital Based Case - Control Study
Abstract
Background:
The risk of thyroid cancer has already been related to diet/carbohydrates rich foods, but the association has not been investigated in terms of age and sex implications.
Objective:
We studied the relationship between thyroid cancer and the consumption of simply and complex carbohydrates rich foods, mainly investigating the possible predictive role of age and sex.
Methods:
We analyzed data from a hospital case-control study conducted in Italy from 2015 to 2018, including 106 cases of thyroid cancer and 121 controls. The consumption of simple and complex carbohydrates rich foods was investigated through the validated Lifestyles Questionnaire using a 4-level scale (never, 1 time per week, 2–3 times a week, 6 times a week). Statistical data analysis was conducted using the IBM SPSS Statistics 21.0 program.
Results:
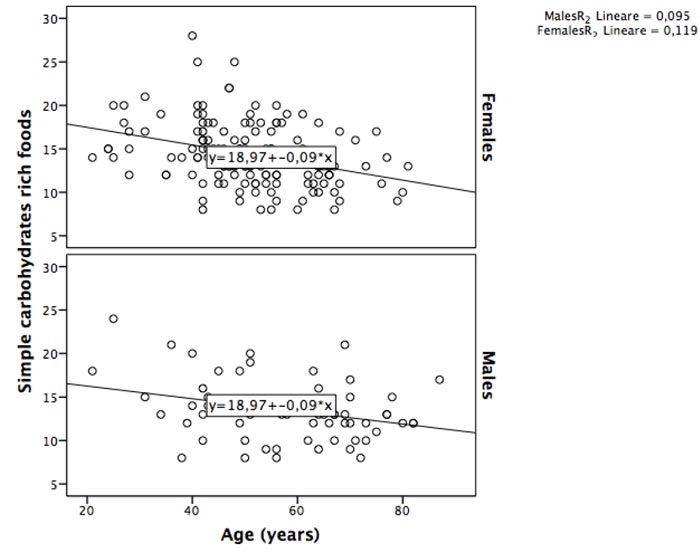
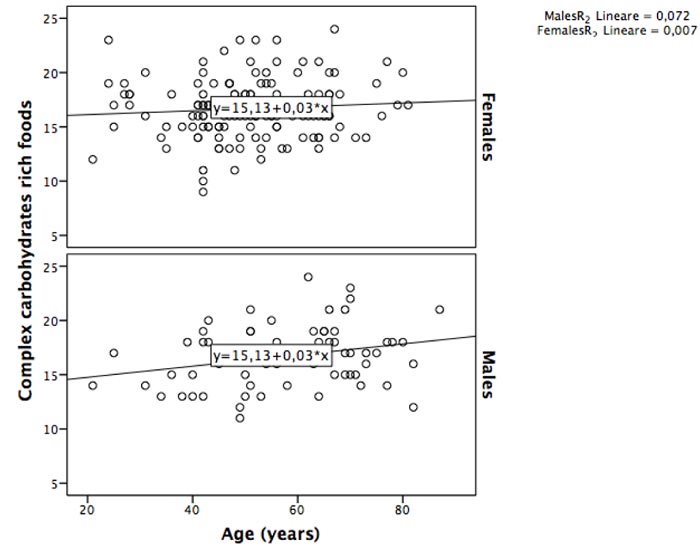
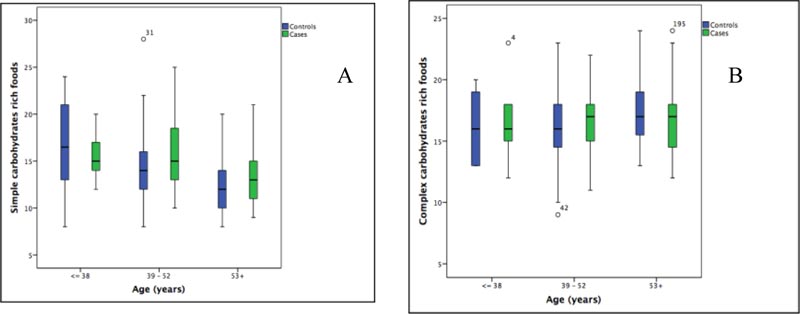
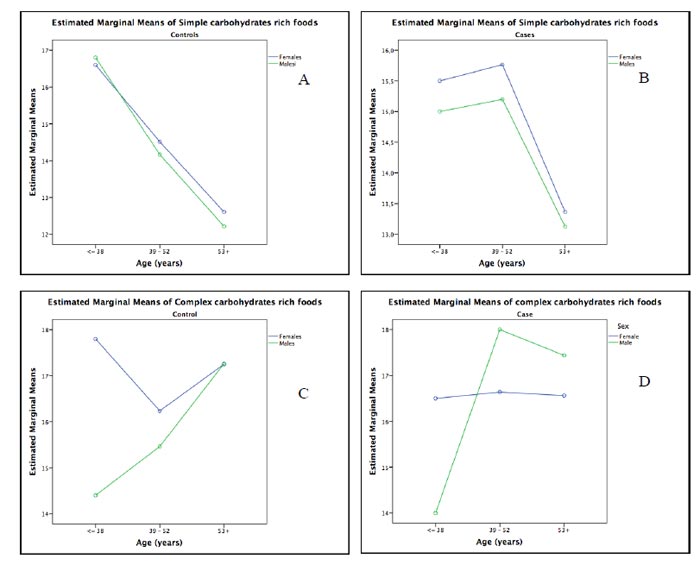
We found a moderate negative correlation between simple carbohydrates rich foods consumption and age (rho= -0.364, p:0.00), particularly in females (females: rho= -0.374, p:0.00; males: rho=-0.266, p=0.036). A weak positive correlation was found between age and complex carbohydrates-rich foods only in males (rho= 0.244, p=0.054). The Two-Way Analysis of Variance confirmed that, overall, simple carbohydrates rich foods consumption decreased with age categories (Case: F=2.59, p=0.032; Control: F=3.14, p=0.011), although it remains higher for female than male cases at all age categories (≤38, 39-52, 53+years). Conversely the complex carbohydrates rich foods intake had interaction with age categories only in controls group (Control: F=1.95, p=0.09; Case: F=0.744, p=0.592), where based on sex, it remains higher for female than male at ≤38 and 39-52 age categories. It should be emphasized that in category ≤38 we had only one male cases.
Conclusion:
Our study adds new and relevant information to support the hypothesis that age and sex could impact the thyroid cancer risk by their involvement in simple carbohydrates rich foods consumption. Therefore, future interventions are needed for an understanding of the pathophysiological associations between only carbohydrates rich foods intake and thyroid cancer, mainly for older and female populations, and also for the improvement of preventive public health policies and “Gender Medicine”.
1. INTRODUCTION
Thyroid cancer can occur about 3 times more often in women than in men, and the risk is higher in women at the age of 40 - 50 than in men, who are usually at risk of getting it in their 60s or 70s [1, 2]. Although diet is one of the most studied possible risk factors for thyroid cancer, aspects such as the risk associated with the consumption of carbohydrates rich foods remain unclear [3-5]. Moreover, intakes of total and added sugars are high in European countries [6]. Several studies have investigated the impact of a high-sugar diet on the risk of obesity and cancer, and most of them suggested that high postprandial glucose and insulin levels are involved in the etiology of colorectal and breast cancers [7-9]. Moreover, the hypothesis that diabetes, or daily-medicine especially the medicine which decrease the blood glucose level such as insulin, is associated with risk of thyroid cancer among postmenopausal women remains unclear [10].
The positive association between age, obesity, and thyroid cancer is well known [11, 12]. In addition, some authors have investigated age as a possible risk factor for a worse prognosis of thyroid cancer [13]. Unfortunately, age is a non-modifiable risk factor, so it is important to investigate the potentially modifiable risk factors associated with it, which could contribute to increasing the risk of thyroid cancer. For example, the frequency of physical activity often declines with age, particularly among the elderly, and the association between it and the risk of thyroid cancer is demonstrated [14]. The consumption of simple and complex carbohydrates rich foods also seems to be influenced by sex and gender [15]. Therefore, we have hypothesized a possible role of carbohydrates rich foods, which consumption differs according to age and sex, on the risk of thyroid cancer. Previously, we carried out a case-control study to investigate the relationship between dietary habits and thyroid cancer [16]. Our results highlighted an increased risk of thyroid cancer associated with a diet rich in starchy foods, products rich in salt and fat, sweets, caffeinated drinks and iodized salt. In the same case-control study, questions about simple and complex carbohydrates rich foods consumption were also included. The present paper aimed to investigate the relationship between thyroid cancer and the consumption of simple and complex carbohydrates rich foods, mainly investigating the possible predictive role of age and sex.
2. MATERIALS AND METHODS
From January 1st 2015 and July 31st 2018 a hospital-based case-control study on thyroid cancer was conducted in the Endocrine-Surgery operative unit of the University Hospital “Policlinico – Vittorio Emanuele” of Catania (Sicily, Italy) [14]. The study was approved by the Ethics Committee of the University Hospital “Policlinico – Vittorio Emanuele” of Catania (agreement no. 553). Written informed consent was obtained from all participants. Participation in the study was offered to all patients treated for thyroid cancer, diagnosed in the period between January 2009 and July 2018. Of 118 cases initially notified, 12 patients refused to participate, leaving 106 subjects eligible for the study. The response rate from the cases was 89.8%. Thyroid cancers were histologically confirmed. Controls were enrolled at the clinical analysis laboratory of the same hospital, with the following exclusion criteria: not being diagnosed with neoplasms, hormonal or gynecological diseases. Two trained interviewers recruited cases and controls and administered the questionnaire that included items on socio-demographic and clinical data; whereas the simply and complex carbohydrates-rich foods consumption was estimated using a validated questionnaire as reported in the following paragraph. Cases were 83 women and 23 men, aged 21-82 years (median age 52 years). Of these, 97 had papillary, 5 follicular and 4 medullary thyroid cancer type. Controls were 121 (78 women and 43 men, aged 21-87 years, median age 51 years).
2.1. Simply and Complex Carbohydrates Rich Foods Consumption Assessment
Simply (added sugar foods) and complex carbohydrates (starch rich foods) rich foods consumption during the year prior to enrolment were established using the Lifestyle Assessment Questionnaire of the Italian Health Institute to collect data on food items consumption Istituto Superiore di Sanità. Osservatorio Fumo, Alcol e Droga–OSSFAD. Available online: http://old.iss.it/binary/ofad/cont/questionario %20giovani%20in%20forma.1225957648.pdf (accessed on 20 February 2019). The question “Considering all daily meals, how often did you consume the following foods in the year before the first symptoms of the disease?” was used to inquire about the weekly frequency of simple and complex carbohydrates rich foods consumes. The frequency of consumption of each food item was reported on a 4-level scale (never=1, one times a week=2, two-three times a week=3, every day of the week=4), finally the food items were grouped according to the type of carbohydrates they are made of as follows reported: complex carbohydrates rich foods (bread, pasta, rice, pizza, rusk, crackers, breadsticks, potatoes, cereal flakes and cereals) and simple carbohydrates rich foods (biscuits, brioches, croissants, chocolate and others sweets). We added the values of the Likert scale assigned to each individual carbohydrate item to calculate an index of the simple and complex carbohydrates-rich foods consumption (higher number corresponded to higher consumption).
2.2. Statistical Analysis
Statistical analysis was performed using statistical software SPSS for Windows (Statistical Package for the Social Science, version 21.0; SPSS Inc., Chicago, IL, USA). Consumption of simply and complex carbohydrates rich foods was reported as median (IQR)/mean ± Standard Deviation (SD) and box-plot illustrating the median within the box from the first quartile (25th percentile) to the third quartile (75th percentile) and whiskers ranging from the 10th to the 90th percentiles (extreme values are marked outside). Qualitative variables were presented as absolute frequencies and percentages (%). Bivariate analysis was performed using the following age categories: ≤38, 39-52, 53 + years first because the thyroid cancer risk is higher in women at the age of 40 - 50, and second because the hormonal changes characteristic of women taking place after 50 years of age.
Spearman’s correlation analysis was performed to investigate the linear relationship between age (continuous variable) and simple/complex carbohydrates rich food consumption. We also carried out stratified analysis by sex as a potential effect modifier. The Two-Way Analysis of Variance (Two-Way ANOVA) was used to evaluate the influence of age and sex on simple and complex carbohydrates rich food consumption. When a significant F ratio was found, Bonferroni post-hoc test was used to locate the differences.
3. RESULTS
We found a moderate negative correlation between simple carbohydrates rich foods consumption and age (rho= -0.364, p:0.00), particularly in females (females: rho= -0.374, p:0.00; males: rho=-0.266, p=0.036) (Fig. 1). Conversely, a weak positive correlation was found between age and complex carbohydrates rich foods (rho: 0.147, p=0.033); the stratified analysis by sex confirmed a weak correlation only for males (females: rho= -0.075, p:0.369; males: rho= 0.244, p=0.054) (Fig. 2).
Tables 1 and 2 show the consumption of simply and complex carbohydrates rich foods of 106 cases and 121 controls, by age classes and sex, respectively. As expected, the frequency of cases increases with advancing age Table 1. In particular, Table 1 shows a slightly higher consumption of simple carbohydrates rich foods in female cases than in males between 39-52 years, while Table 2 shows the highest consumption of complex carbohydrates rich foods in male cases aged 39-52 years. The consumption of simple carbohydrates rich foods appears slightly higher in cases than in controls in 39-52 and 53+ years categories (Fig. 3A). The consumption of complex carbohydrates rich foods seems to be slightly higher in cases than in controls in 39-52 years category (Fig. 3B).
| Age (years) |
Case N=106 n (%) |
Control N=213 n (%) |
Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | |||
| ≤38 | 13 (12.3) | 13 (10.7) | na | na | 15 (14-18) |
16±3 | 18 (11-22) |
17±6 | 15 (14-21) |
17±4 |
| 39-52 | 43 (40.6) | 54 (44.6) | 14 (12-20) |
15±4 | 15 (13-18) |
16±4 | 14 (12-18) |
14±3 | 14 (12-16) |
15±4 |
| 53+ | 50 (47.2) | 54 (44.6) | 13 (11-14) |
13±3 | 13 (11-15) |
13±3 | 12 (10-14) |
12±3 | 13 (10-15) |
13±3 |
| Age (years) | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | |
| ≤38 | na | na | 17 (15-18) |
17±3 | 14 (13-16) |
14±2 | 19 (16-20) |
18±3 |
| 39-52 | 19 (17-19) |
18±2 | 17 (15-18) |
17±2 | 15 (13-18) |
15±3 | 16 (15-18) |
16±3 |
| 53+ | 18 (16-19 |
17±3 | 16 (14-18) |
17±3 | 17 (15-18) |
17±3 | 17 (16-20) |
17±2 |
The Two-Way Analysis of Variance (ANOVA) confirmed that, overall, simple carbohydrates rich foods consumption (SCC) decreased with age class (Case: F=2.59, p=0.03; Control: F=3.14, p=0.01), although SCC remains higher for female than male cases at all age classes (Fig. 4A and 4B). So, both sex and age are needed to explain the simple carbohydrates rich foods consumption. Post hoc tests suggested that SCC was higher in 39-52 years category than 53+ (p=0.05), and at lower compared to the higher age category (53+ years), both for female and male cases. However, in the overall age case, females appeared to have higher SCC than males (Fig. 4B).
The two-way analysis of variance (ANOVA) highlighted that complex carbohydrate rich foods consumption (CCC) had interaction with age class only in the controls group (Control: F=1.95, p=0.09; Case: F=0.744, p=0.592), and CCC remains higher for female than male controls at ≤38 and 39-52 age classes (Fig. 4C and 4D). It should be emphasized that in category ≤38 we had only one male case (Fig. 4D). Post hoc tests suggested that the CCC was higher in 53+ years category, both for female and male controls than 39-52 years category (p=0.06). Comparison for female and male cases was not reported because the category ≤38 had only one male case.
4. DISCUSSION
The main findings of our hospital-based case-control study lay in an interaction effect between age and sex; thus, simple carbohydrates rich foods seem to change with age depends on sex, and vice versa. In particular, overall simple carbohydrates-rich foods consumption decreased with age, although it remains higher for female than male cases at all age classes. Conversely, the complex carbohydrates rich foods consumption had interaction with age class only in controls group, and it remains higher for female than male controls at ≤ 38 and 39-52 age classes. Moreover, the results of our study seem to confirm the association, already noted, between the consumption of simple carbohydrates rich foods and thyroid cancer attributed to the fact that the simple carbohydrates rich foods have a high glycemic index, that is, they raise glycemia and then insulin fast. It’s established that high levels of insulin and insulin-like growth factor appear to increase the risk of thyroid, colon and breast cancer. Therefore, a diet rich in carbohydrates may play an important role in the etiology of these tumors [17-20]. Studies in the literature have investigated separately the relationship between thyroid cancer and obesity [21], whereas others have investigated the relationship between sugar consumption and sex or age [22]; the innovative aspect of our results is to investigate the relationship between thyroid cancer and the simply/complex carbohydrates rich foods consumption, focusing on the possible predictive role of age and sex.
Debras et al., found an association between total sugar intake and higher overall cancer risk (HR for quartile 4 compared with quartile 1: 1.17; 95% CI: 1.00, 1.37; Ptrend = 0.02); significant associations with cancer risk were also observed for added sugars, free sugars, sucrose, sugars from milk-based desserts, dairy products, and sugary drinks (Ptrend ≤ 0.01). These results suggest that sugars may represent a modifiable risk factor for cancer prevention [23].
The fact that rates of some cancer have risen along with increasing gender disparity lends itself to many hypotheses, but unfortunately, there are very few studies examining the causes of the disparity [24-26]. Rahbari et al., in a review on thyroid cancer and gender disparity, concluded that dietary and environmental factors do not appear to have a significant role in thyroid cancer gender disparity and that the application of high-throughput genomic and proteomic approaches to the study of thyroid cancer gender disparity could be helpful for better understanding the molecular basis for gender differences in thyroid and other cancers. Unfortunately, the review took in account only the role of fish (high in iodine) and cruciferous vegetables on thyroid cancers [27]. Kilfoy, et al, examined thyroid cancer incidence in the United States, stratified by gender and with a focus upon papillary lesions to develop etiological clues regarding gender-related differences. They concluded that gender was an age-specific effect modifier for papillary thyroid cancer incidence [28]. Therefore, the differences associated with the consumption of simple and complex carbohydrates-rich foods between males and females in different age groups probably represent the modifiable risk factor on which we must act differently by sex/gender and age to reduce the risk of cancer. These results should be applied in clinical practice considering the aspects associated with nutrition and focusing on both patients’ age and sex, in order to prevent overweight and obesity. As a matter of fact, it is known that more than half of all cancers diagnosed in women and nearly a quarter of all cancers diagnosed in men have been associated with overweight and obesity [29]. Moreover, the results of our study maybe useful for providing concrete suggestions to enrich the existing knowledge on patient care and so much for the prevention of thyroid cancer and all diseases associated with overweight and obesity in adults.
5. LIMITATION OF THE STUDY
Sample size may be the main limitation. Due to the small sample size, we could not perform the analysis by histological type of thyroid cancer, but most of the cases we recruited had papillary type cancer, known as the thyroid cancer type with higher incidence rates [2]. Another limitation of the present study is the dependence on a single assessment to estimate antecedent simple/complex carbohydrates rich foods consumption, with the potential for measurement error because of difficulties in recalling usual dietary habits and insensitivity to long-term dietary changes. However, the questionnaire used in this study is a validated tool specifically designed to assess long-term (1 y) usual consumption of different food items (Lifestyle Assessment Questionnaire of the Italian Health Institute to collect data on food items consumption [Istituto Superiore di Sanità. Osservatorio Fumo, Alcol e Droga –OSSFAD. Available online: http://old.iss.it/binary/ofad/ cont/questionario%20giovani%20in%20forma.1225957648.pdf (accessed on 20 February 2019).
CONCLUSION
Our findings confirm the contribution of simple carbohydrates-rich foods consumption increasing the thyroid cancer risk, supporting the hypothesis that age and sex can act as effect modifiers in disease outcome. Therefore, future interventions are needed for understanding of the pathophysiological associations between simply carbohydrates rich foods and thyroid cancer, mainly for older and female populations, and also for the improvement of preventive public health policies and “Gender Medicine”, because it is necessary to consider that eating habits are also determined by other factors that can be strongly influenced by gender differences, such as education, ethnicity, cultural traditions, religious and / or ideological motivations, education and status individual socio-economic.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol has been approved by the institutional Ethics Committee of the University Hospital “Policlinico-Vittorio Emanuele” of Catania, Italy (agreement n. 553).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from each participant prior to the study.
AVAILABILITY OF DATA AND MATERIALS
All data presented in the result but the raw data that supports the finding of this study are available from the corresponding author [F.M.] upon request.
FUNDING
Project funded by intradepartmental research plan 2016/2018 of Department of Medical, Surgical and Advanced Technologies, University of Catania (number 5C722012104, 2018).
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We sincerely appreciate the cooperation of the Dott. Roberto Parisio who helped the researchers to carry out this research.


