All published articles of this journal are available on ScienceDirect.
Seroprevalence of SARS-CoV-2 Antibodies and its Risk Factors in the North-West of Iran: A Population-Based Cross-Sectional Study
Abstract
Background:
The aim of this study is to determine the prevalence of SARS-CoV-2 seropositivity and to examine the risk factors for seropositivity among the people of Ardabil, in the northwestern part of Iran.
Methods:
A community-based survey was carried out involving 1013 participants (690 from urban and 323 from rural areas), who were selected based on the cluster sampling method. Iran’s FDA-approved Pishtaz Teb SARS-CoV-2 ELISA kits were used to assess the presence of SARS-CoV-2-specific immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in serum samples. Weighted seroprevalence, the number of infections, infection to case ratio (ICR), and infection fatality ratio (IFR) were estimated after adjusting for survey design and serial test performance. The factors associated with IgG/IgM positive were determined using logistic regression.
Results:
Between May 20 and June 7, out of 1013 survived people, 123 (12.11%) were IgG positive, 49 (4.8%) were IgM positive and 122 (12.04%) were having both IgG and IgM antibodies. The highest frequency of positive test for IgG and IgM antibodies was found in people with diabetes, followed by people with obesity and heart disease, respectively. Multivariate logistic regression showed old age (2.04, 95% CI: 1.02 to 11.74), male sex (1.52, 95% CI: 1.15 to 2.13), urbanization (1.40, 95% CI: 1.02 to 3.22), higher family number (9.44, 95% CI: 1.69 to 52.13), obesity (2.14, 95% CI: 1.11 to 5.86), NCDs (1.22, 95% CI: 1.01 to 2.16), having symptoms (3.02, 95% CI: 1.64 to 8.61), traveling (2.70, 95% CI: 1.76 to 10.8), history of contact with infected patients (2.38, 95% CI: 1.08 to 7.03), as factors associated with IgG/IgM positive test.
Conclusion:
Around the mid of May 2020, SARS-CoV-2 seroprevalence was low among Ardabil's adult population. Several factors have been found to be associated with SARS-CoV-2 seroprevalence, which should be considered by policymakers to set policies against the SARS-CoV-2 pandemic.
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a highly contagious disease caused by the acute respiratory syndrome of the coronavirus (SARS-CoV 2). The first case of the disease was detected in Wuhan, China, in December 2019, and then the virus spread rapidly around the world [1, 2]. The global mortality rate of coronavirus is 2.8%, but according to the Iranian Ministry of Health, the mortality rate in Iran is 4.3%, which is higher than the global average [3]. Following the outbreak of the global pandemic, countries have adopted various strategies to control the prevalence of COVID-19, including quarantine and herd immunity [4].
Herd immunity is a form of indirect protection against infectious agents and is employed when a large part of the population becomes immune to the infection, so it is considered a method of protection for people who lack immunity [5]. In a population that is largely immune, the transmission chain is disrupted, leading to cessation or reduction of the disease outbreak. There are two types of herd immunity, intrinsic and acquired. Intrinsic type is a natural occurrence that involves physiological changes and the production of antibodies or other defense mechanisms that are genetically determined in a population and do not depend on the population's previous exposure to infection; however, these physiological changes may be due to prolonged exposure to infection. Acquired type is in which a sufficient number of people are naturally or artificially exposed to infectious agents during their lifetime. This type of exposure to infection can occur early in life [6, 7].
In herd immunity, it does not matter if the immunity is caused by vaccination or by people who have the disease. The important thing is to create immunity [8]. According to a simple model proposed by Smith and Dietz, the herd immunity threshold depends on a single parameter, R0. R0 refers to the average number of secondary infections caused by an infected person entering a highly sensitive population. If we assume a hypothetical pathogen with an R0 of 4, this means that, on average, an infected host will infect four more people during the infectious period, assuming that there is no immunity in the population. Mathematically, the herd immunity threshold is defined by 1 – 1/R0 (e.g., if R0 = 4, the corresponding herd immunity threshold is 0.75) [9]. Therefore, in high transmissible diseases, R0 and the proportion of the population that must be immunized to prevent sustainable transmission, is high [8, 10].
The transmission of an infectious agent depends on many factors that affect its transmission dynamics, including population density, population structure, and differences in contact rates between the population groups. All of these factors directly or indirectly affect R0, and thus, the herd immunity threshold. For some pathogens, such as acute respiratory syndrome coronavirus (SARS-CoV-2), clinical manifestations are a poor indicator of transmission because asymptomatic hosts can be highly infectious and contribute to the spread of an epidemic [11].
Once the herd immunity threshold is reached, the effect of herd immunity largely depends on the strength and duration of immunity obtained. Herd immunity is very effective for pathogens that develop lifelong immunity, such as measles, as well as immunizations developed through vaccination, both of which can prevent the spread of the pathogen within the population. Because the immunity of many other infectious diseases, such as pertussis and rotavirus, decreases over time, herd immunity is less effective, and periodic outbreaks may occur [12].
The British government implemented a herd immunity strategy against the coronavirus. The UK government's initial strategy for minimizing the impact of COVID-19 was to allow the virus to spread throughout the population so that herd immunity is achieved. Because they believed that widespread contact with the coronavirus and creating herd immunity can protect people, even this transmission must be very slow and delayed [13]. They believed that the health system of their country has the capacity to not collapse due to overcrowding as most of the acute symptoms associated with the disease need the required medical services. A similar approach was first adopted in Sweden. One clear goal of their approach was to bridge the gap between pre-vaccination and post-vaccination (or no-vaccine) stages while having a functional society [14].
Currently, the detection of SARS-CoV2 RNA is a standard approach to the diagnosis of COVID-19. However, there is an urgent need for rapid and reliable serological diagnostic methods to detect people infected with SARS-CoV2, including those without obvious symptoms. The most recent studies have described serological tests based on the detection of SARS-CoV2-specific IgM and IgG antibodies [15, 16]. However, some publications have reported the detection of SARSCoV2-specific IgA in serum. Analysis of levels of IgA in a large number of COVID-19 patients is still lacking [17, 18].
In Iran, after the emergence and expansion of COVID-19, one of the biggest challenges in the field of disease control and treatment was the study of the consequences of the disease among people in the community. Therefore, the aim of this study was to determine the prevalence of herd immunity against COVID-19 in the Ardabil population.
2. MATERIALS AND METHODS
In this population-based cross-sectional study, we conducted serologic testing for SARS-CoV-2 antibodies to assess the prevalence of SARS-CoV-2 in Ardabil, Iran.
2.1. Study Population and Sampling Procedure
The present study was performed on 1000 people (including 30% probability of collective immunity, 95% confidence level and 5% error) living in urban and rural areas of Ardabil. According to the 68% urban and 32% rural population of Ardabil, 680 people from the city and 320 people from the village were selected; consent was obtained from the participants.
The sampling method was random clustering in urban and rural health centers, and for this purpose, the areas covered by Ardabil city health center were designated as the main clusters and from each center, proportional sample was randomly selected according to the population covered. Rural areas were divided into four areas: north, south, east and west, and according to the sample size, people were randomly selected from the relevant health center.
The data collection was done through face-to-face interviews by trained people and a two-part checklist that included clinical and demographic information, such as personal characteristics, history of exposure to risk factors in the last 14 days, clinical symptoms status, medical history as well as that of hospitalization, status of the individual in terms of underlying diseases, personal habits, history of referral to medical centers, history of possible contact, history of referral and use of social services of the participants. Also, a laboratory technician collected 5 mL of venous blood into an EDTA-coated microtainer. Iran’s FDA approved Pishtaz Teb SARS-CoV-2 ELISA kits were used to assess the presence of SARS-CoV-2-specific immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in serum samples. Details of sample collection and ELISA kits are provided in the supplementary materials.
2.2. ELISA Test Characteristics
According to the manufacturer, the test sensitivity was assessed by using serum samples of 34 patients who had COVID-19 clinical symptoms with positive RT-PCR. To assess test specificity, 111 serum samples collected and stored at -20°C prior to the SARS-CoV-2 infection were tested. The manufacturer reported sensitivity and specificity for SARS-CoV-2 IgG and IgM ELISA kits as 94·1% and 98·3%, and 79·4% and 97·3%, respectively. Pishtaz Teb SARS-CoV-2 ELISA kits (Available at http://pishtazteb.com/en/sars-cov-2- igg-elisa-kit/, https://pishtazteb.com/en/sars-cov-2-igm- elisa-kit/) authorized by the Iranian Food and Drug Administration were used to identify SARS-CoV-2-specific IgG and IgM antibodies in blood samples from participants. Seroprevalence was calculated using ELISA test results, with population weighting (by age, sex, and city population size) and test performance (as determined by our independent sensitivity and specificity validation) [19].
2.3. Statistical Analysis
Rapid test was used to measure the number of antibodies in the blood. The collected data were entered into SPSS 24 statistical software and analyzed. Parametric (non-Pearson) and non-parametric statistical tests (Chi-square and Fisher) were used depending on the type of variable. Individuals who were seropositive for SARS-CoV-2 infection were compared to those who were seronegative for SARS-CoV-2 infection using logistic regression analysis to find socio-demographic characteristics related with IgG/IgM positivity. Poisson regression was used to achieve the crude and adjusted prevalence, and their respective 95% confidence interval (CI). The demographic variables and chronic disease status were included in the model to calculate the odds ratio (OR) of COVID-19 infection by using multivariate logistic regression. The odds ratio (OR) with 95% CI was determined after each component was adjusted for any known confounders. P value less than 5% was considered significant. Using Stata software, version14.0.0 (Stata Corp, College Station, TX, USA), we analyzed the data.
3. RESULTS
The results of the present study showed that out of 1013 patients, 123 (12.11%) were IgG positive, 49 (4.8%) were IgM positive, and 122 (12.04%) were positive for both IgG and IgM antibodies. The results of Table 1 showed no significant difference between IgG and IgM and IgG/IgM antibodies in terms of dispersion among age groups. The highest frequency of positive IgG test was related to the age group of 36-45 years and the highest frequency of positive IgM test was related to the age group of 56-65 years. The same age group had the highest frequency of positive IgG and IgM antibodies.
| - | IgG | p-value | IgM | p-value | IgG/IgM | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| - | n | % | n | % | n | % | |||
| Age Categories | - | - | - | - | - | - | - | - | - |
| <15 | 1 | 0.73 | 0.056 | 0 | 0.00 | 0.122 | 0 | 0.00 | 0.146 |
| 16-25 | 5 | 3.64 | 1 | 2.57 | 1 | 3.15 | |||
| 26-35 | 13 | 8.73 | 4 | 7.71 | 3 | 6.30 | |||
| 36-45 | 37 | 25.48 | 11 | 23.13 | 11 | 28.34 | |||
| 46-55 | 24 | 16.74 | 13 | 25.70 | 8 | 18.89 | |||
| 56-65 | 26 | 17.47 | 14 | 28.27 | 13 | 31.49 | |||
| >65 | 17 | 11.65 | 6 | 12.85 | 5 | 12.59 | |||
| Sex | - | - | - | - | - | - | - | - | - |
| Female | 68 | 46.58 | 0.358 | 23 | 46.27 | 0.821 | 19 | 47.23 | 0.899 |
| Male | 87 | 59.69 | 26 | 53.98 | 21 | 53.53 | |||
| Inhabitation | - | - | - | - | - | - | - | - | - |
| Urban | 70 | 48.04 | 0.016 | 35 | 71.97 | 0.499 | 26 | 66.12 | 0.883 |
| Rural | 53 | 36.39 | 14 | 28.27 | 14 | 34.63 | |||
| Family Member | - | - | - | - | - | - | - | - | - |
| <5 | 108 | 88.13 | <0.001 | 44 | 89.72 | 0.085 | 35 | 88.16 | 0.039 |
| 5-8 | 7 | 6.05 | 3 | 5.13 | 3 | 6.30 | |||
| >8 | 7 | 6.05 | 3 | 5.13 | 3 | 6.30 | |||
| Smoking | - | - | - | - | - | - | - | - | - |
| Yes | 6 | 5.2 | 0.066 | 4 | 10.3 | 0.970 | 1 | 3.1 | 0.185 |
| No | 109 | 94.8 | 35 | 89.7 | 31 | 96.9 | |||
| Alcohol | - | - | - | - | - | - | - | - | - |
| Yes | 4 | 3.5 | 0.008 | 2 | 5.1 | 0.013 | 2 | 6.3 | 0.004 |
| No | 110 | 96.5 | 37 | 94.9 | 30 | 93.8 | |||
| BMI Categorized | - | - | - | - | - | - | - | - | - |
| Underweight | 1 | 0.9 | 0.048 | 0 | 0.0 | 0.045 | 0 | 0.0 | 0.010 |
| Normal weight | 27 | 24.5 | 7 | 18.9 | 4 | 12.9 | |||
| Overweight | 39 | 35.5 | 12 | 32.4 | 10 | 32.3 | |||
| Obesity | 43 | 39.1 | 18 | 48.6 | 17 | 54.8 | |||
| Non-Communicable Diseases | - | - | - | - | - | - | - | - | |
| Yes | 10 | 8.7 | 0.769 | 4 | 10.3 | 0.597 | 4 | 12.5 | <0.001 |
| No | 105 | 91.3 | 35 | 89.7 | 28 | 87.5 | |||
| Clinical Status at Admission | - | - | - | - | - | - | - | - | - |
| Cough | 37 | 31.9 | 0.008 | 9 | 23.1 | 0.896 | 6 | 18.8 | 0.903 |
| Shortness of breath | 19 | 16.4 | 0.065 | 3 | 7.7 | 0.804 | 3 | 9.4 | 0.935 |
| Diarrhea | 16 | 13.8 | 0.004 | 7 | 17.9 | 0.014 | 6 | 18.8 | 0.019 |
| Weakness | 22 | 19.0 | 0.047 | 8 | 20.5 | 0.252 | 8 | 25.0 | 0.076 |
| Fever | 15 | 12.9 | <0.001 | 3 | 7.7 | <0.001 | 3 | 9.4 | 0.592 |
| Headache | 31 | 26.7 | 0.637 | 11 | 28.2 | 0.763 | 9 | 28.1 | 0.806 |
| Nausea | 14 | 12.1 | 0.010 | 5 | 12.8 | 0.170 | 5 | 15.6 | 0.059 |
| Pneumonia | 1 | 0.9 | 0.644 | 0 | 0.0 | 0.834 | 0 | 0.0 | 0.863 |
| Runny nose | 12 | 10.3 | 0.088 | 2 | 5.1 | 0.134 | 1 | 3.1 | 0.057 |
| Vomiting | 6 | 5.2 | 0.051 | 2 | 5.1 | 0.484 | 2 | 6.3 | 0.324 |
| History of contact and traveling | - | - | - | - | - | - | - | - | |
| Traveling out of residence place | 9 | 7.8 | 0.501 | 4 | 10.3 | 0.424 | 4 | 12.5 | 0.222 |
| Contact with patients | 11 | 10.3 | 0.326 | 6 | 15.6 | 0.065 | 5 | 15.6 | 0.098 |
| - | Odds Ratio | ||
|---|---|---|---|
| IgG (95% CI) | IgM (95% CI) | IgG-IgM (95% CI) | |
| Age group | - | - | - |
| <15 | 1 | 1 | 1 |
| 16-25 | 0.80 (0.07 to 8.09) | 0.18 (0.01 to 2.43) | 0.17 (0.01 to 2.74) |
| 26-35 | 0.69 (0.07 to 6.21) | 0.26 (0.05 to 1.32) | 0.21 (0.03 to 1.33) |
| 36-45 | 1.30 (0.15 to 11.14) | 0.52 (0.14 to 1.87) | 0.61 (0.16 to 2.49) |
| 46-55 | 1.26 (0.14 to 11.08) | 1.07 (0.31 to 3.66) | 0.65 (0.16 to 2.49) |
| 56-65 | 1.86 (0.21 to 16.37) | 1.36 (0.40 to 4.36) | 1.28 (0.36 to 4.50) |
| >65 | 2.16 (1.23 to 19.68)* | - | 2.04 (1.02 to 11.74)* |
| Sex | - | - | - |
| Male | 1.27 (1.17 to 2.39)* | 1.05 (0.51 to 2.19) | 1.52 (1.15 to 2.13)* |
| Female | 1 | 1 | 1 |
| Inhabitation | - | - | - |
| Urban | 1.38 (1.08 to 2.15)* | 1.08 (0.24 to 2.59) | 1.40 (1.02 to 3.22)* |
| Rural | 1 | 1 | 1 |
| Family Member | - | - | - |
| <5 | 1 | 1 | 1 |
| 5-8 | 1.05 (0.44 to 2.55)* | 0.88 (0.20 to 3.87) | 1.15 (0.25 to 5.23) |
| >8 | 7.09 (2.12 to 23.72)* | 7.15 (1.34 to 38.01)* | 9.44 (1.69 to 52.4)* |
| Smoking status | - | - | - |
| Smoker | 2.43 (0.97 to 6.06) | 0.97 (0.29 to 3.14) | 5.06 (0.57 to 44.9) |
| Non-smoker | 1 | 1 | 1 |
| Alcohol | - | - | - |
| Yes | 0.24 (0.14 to 1.76) | 0.08 (0.01 to 1.55) | 0.06 (0.008 to 1.43) |
| No | 1 | 1 | 1 |
| BMI | - | - | - |
| Under weight | 1 | 1 | 1 |
| Normal weight | 2.52 (0.30 to 20.8) | 1.50 (0.29 to 2.29) | 3.57 (1.06 to 12.5)* |
| Over weight | 2.15 (1.14 to 7.78)* | 1.45 (0.20 to 1.98) | 2.43 (1.03 to 5.55)* |
| Obesity | 3.19 (1.38 to 6.59)* | - | 2.14 (1.11 to 5.86)* |
| Non-Communicable Diseases | - | - | |
| Yes | 1.38 (1.08 to 2.15)* | 2.61 (2.37 to 2.88)* | 1.22 (1.01 to 2.16)* |
| No | 1 | 1 | 1 |
| Having symptoms related to COVID-19 | - | - | |
| Yes | 4.05 (1.75 to 9.96)* | 2.88 (1.29 to 7.70)* | 3.02 (1.64 to 8.61)* |
| No | 1 | 1 | 1 |
| Traveling out of RP | - | - | - |
| Yes | 1.85 (0.24 to 2.96)* | 1.92 (1.74 to 6.66)* | 2.70 (1.76 to 10.8)* |
| No | 1 | 1 | 1 |
| Contact with Patients | - | - | - |
| Yes | 2.53 (1.46 to 6.23)* | 2.56 (1.04 to 7.14)* | 2.38 (1.08 to 7.03)* |
| No | 1 | 1 | 1 |
The highest frequency of positive test for IgG and IgM antibodies was related to people living in the city. According to the results, there was a significant difference between positive IgG and IgM antibodies and in terms of dispersion of both antibodies in participants according to the number of family members, alcohol consumption, body mass index, having coronary-related clinical symptoms, such as cough, shortness of breath, headache and weakness (p >05/0).
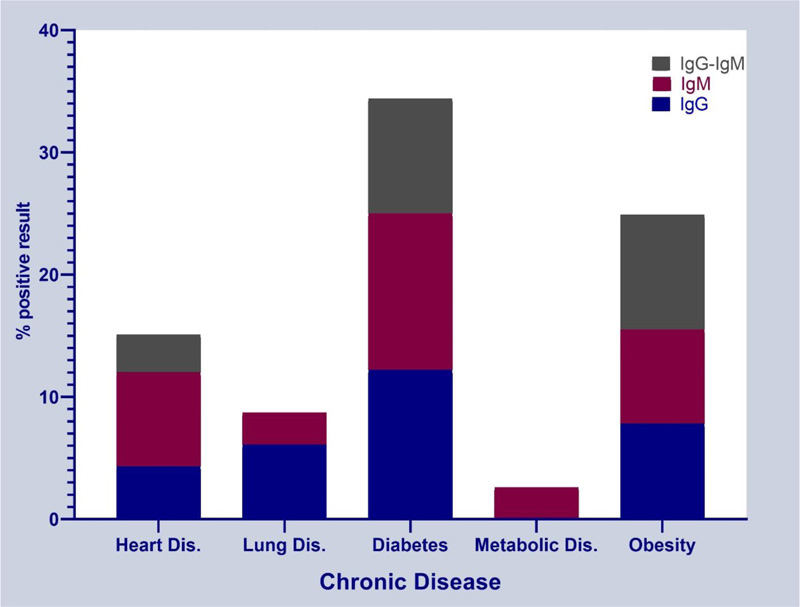
The highest frequency of positive test for IgG and IgM antibodies was observed in people with diabetes, followed by people with obesity and heart disease, respectively. Metabolic disease was seen only in people with a positive IgM test, and no person with a positive test for both IgG and IgM antibodies had lung disease. According to the results, there was no statistically significant difference between individuals with underlying diabetes, obesity and heart disease in terms of positive test for IgG and IgM antibodies and the distribution ratio of positive test for IgG and IgM antibodies was the same (Fig. 1).
The results of multivariate logistic regression in Table 2 showed that with increasing age, the chance of positive IgG test and IgG/IgM test increased significantly, so the age group over 65 years compared to the reference group, respectively for IgG and both IgG/IgM tests, had 2.16 times (1.23 to 19.68) and 2.04 times (1.02 to 11.74) higher chances of positive test, respectively.
This finding did not apply to the IgM test result. And age was not recognized as a factor influencing the positivity of this test. Also, the chance of positive IgG (1.27, 95% CI: 1.17 to 2.39) and combined IgG / IgM (1.52, 95% CI: 1.15 to 2.13) in men was significantly higher than women and also urban residents had significantly higher chances of infection. The urban residents were 1.38 times more likely to test positive for IgG and 1.40 times more likely to be tested positive for both IgG / IgM.
One of the important findings was the effect of the number of household members on the positive tests related to COVID-19. People with more than 8 family members were 7.09, 7.15 and 9.44 times more likely to test positive for IgG, IgM and IgG-IgM, respectively, indicating that with increasing number of household members, the risk of COVID-19 or active infection increased.
According to research, with weight gain and obesity, the chance of positive tests significantly increases; in this study, with regard to these factors, the positive rate for IgG was equal to 3.19 (95% CI: 1.38 to 6.59), and for IgG / IgM test, it was 2.14 (95% CI: 1.11 to 5.86). Having an underlying disease and having other symptoms of the infection were the factors influencing the positive test of individuals, so people with underlying disease had a higher chance of positive IgG test accounting for 1.38 (95% CI: 1.08 to 2.15) and an equal chance of getting a positive IgG / IgM test, i.e., 1.22 (95% CI: 1.01 to 2.16). Whereas people with symptoms had a comparatively equal chance of a positive IgG test, 4.5 (95% CI: 1.75 to 9.96), while a higher chance of IgM positive, 2.88 (95% CI: 1.29 to 7.70), and positive IgG / IgM test, 3.02 (95% CI: 1.64 to 8.61). Finally, traveling and contact with sick people were identified as two other factors increasing the chances of positive test.
4. DISCUSSION
The results of this population-based serosurvey, as the first assessment in northwestern part of Iran, revealed that 12.04 percent of adults in Ardabil were subjected to SARS-CoV-2 infection, amounting to 152500 infections in total by the mid of May 2020.
According to the results of our study, despite the peak time of the COVID-19 pandemic, total seroprevalence in Ardabil was low, with about 12% of the adult population infected with SARS-CoV-2 by mid-May to June 7, 2020. The low incidence of SARS-CoV-2 infection suggests Ardabil to still be in the early stages of the outbreak and the bulk of Ardabil population to still be vulnerable to infection.
In the present study, the results showed that among those who tested positive for antibodies, the prevalence of diabetes, obesity and cardiovascular disease was higher than other non-communicable underlying diseases, which is compatible with other studies [20, 21]. Bello-Chavolla et al. showed obesity to be responsible for 49.5 percent of the COVID-19 lethality caused by diabetes in Mexico. Early-onset diabetes was linked to a higher risk of hospitalization, while obesity was linked to a higher risk of ICU admission and intubation [22]. A research from the UK suggested that the mortality rate due to COVID-19 could be up to 2-3 times higher in people with diabetes [23]. Other reports have recorded a 2-fold elevated risk for referral to intensive care and an enhanced need for artificial ventilation compared with those who do not have diabetes [24, 25]. It has been proven that bacterial and fungal diseases are more common in diabetic patients, so COVID-19 is no exception [26].
Demographic characteristics, such as age group older than 65 years (OR: 2.04, 95% CI:1.02-11.74) and male sex (OR:1.52, 95% CI:1.15-2.13), were associated with an elevated risk of producing SARS-CoV-2 antibody positive test (IgG/IgM), according to univariate analyses (Table 2). Akinbami et al. discovered that women were less likely than men to be seropositive when controlling for other causes [27]. Women's lower risk could be attributed to their unequal representation in infection-prone professions. Other research found no age-related trend or a higher seroprevalence among the elderly. Xu et al., in China, found SARS-CoV-2 infection to be significantly higher in individuals older than 65 years [28]. In contrast, there are several studies which claim that the chance of SARS-CoV-2 infection significantly decreases with respect to age. The older people were revealed to less likely live with a household contact in a demographic survey conducted in Switzerland, which also showed lower seroconversion among them [27, 29]. The difference in the results of the studies may be due to different biological cultures and the policies of countries suggesting the isolation of the elderly in the COVID-19 pandemic [30].
Kim et al. found obese people (obesity class I, obesity class II, and obesity class III) to be more likely to require intrusive mechanical ventilation because of COVID-19 and suggested a statistical connection among underweight and obese groups II and III and death from COVID-19 [31]. The present study also showed the probability of SARS-CoV-2 infection positive test in overweight and obese people to be, respectively, 2.15 and 3.19 times higher than people with normal weight. This finding can be justified by the positive association of COVID-19 with metabolic disease and obesity, which has been proven in various studies [32].
According to the findings, the residents in urban areas had a high risk of contracting COVID-19 and the higher probability of SARS-CoV-2 infection positive test. Bijari et al. discovered comparatively strong associations between total urban population and total reported cases of COVID-19 in an analysis performed to expose the interrelationship between urban-related factors and the COVID-19 outbreak in Iran [33]. High population density, more activities and social interactions, and lack of open spaces can be the main reasons for the high incidence of COVID-19 in urban areas [34]. Much of the difference in strategies to combat COVID-19 is due to cultural and institutional differences across countries, and can be majorly attributed to social stability and the capacity to enforce inter-regional cooperation [35].
Caseload and morbidity have a significant correlation with noncommunicable disease DALYs and mortality globally [36]. Severity and associated death are more common in older adults and patients with comorbidities. Diabetes mellitus (DM), hypertension, cerebrovascular disease, coronary artery disease (CAD), and chronic obstructive pulmonary disease (COPD) are the most widely identified non-communicable diseases that have been shown to predict poor prognosis in patients with COVID-19 (COPD) [37]. In this study, the odds of a positive antibody test for COVID-19 were higher in people with diabetes and cardiovascular disease.
Close contact with infected patients was found to be a risk factor for positive antibody test in this study, where people with a history of contact with infected patients were 2.38 times more infected compared to people with no contact. Yu et al. revealed that in the case of confirmed outbreaks of COVID-19, a large number of nearby partner countries may be exposed to a high risk of infection. As a result, the handling of this demographic factor is critical in the COVID-19 campaign [38]. Controlling the spread of contagions also involves tracking close contacts across confirmed cases [39].
One of the main limitations of this study is the continuous changes in the COVID-19 epidemic in the form of periodic peaks, so in each peak, a large number of people are infected, and certainly over time, the number of people with positive antibodies to COVID-19 varies. Therefore, the results of the present study can be considered with reference to the study period, as of now, we may have a higher percentage of positive antibodies than reported in the present study. However, the factors influencing this infection and the positive antibody test can be a valuable finding for designing policies to combat the spread of this disease.
Reported serology tests focus on IgM, IgG and total immunoglobulins although IgA plays an important role in mucosal immunity [40]. One of the limitations of the present study is the lack of testing of IgA as SARS-CoV-2 antibody.
CONCLUSION
In early May 2020, the results of the serosurvey revealed a low prevalence of SARS-CoV-2 infection in Ardabil general population. Since the majority of the population is already vulnerable to illness, our public health policy must be devised with respect to a rise in transmission that is unavoidable. Based on this study results, policies to restrict COVID-19 should be designed by considering variables, such as old age, male sex, inhabitation in urban area, big family size, obese people with noncommunicable underlying disease. Also, protective behaviours, such as reducing social gatherings, not traveling to high-risk areas, and using personal protective equipment, should be pursued more vigorously.
Repeating the population-based serosurvey will help assess the possible effect of containment techniques over time in various parts of the world and further advise improvements in the degree and pace of implementation of containing strategies. Seroprevalence assessments taken later in the outbreak, or in areas where the prevalence is greater, would yield more reliable infection to case and infection to fatality ratios.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The project was approved by the Ethics Committee of Ardabil University of Medical Sciences with the ethics code IR.ARUMS.REC.1399.039.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. The study on humans was conducted in accordance with the ethical standards of the Helsinki Declaration and Good Clinical Practice.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available within the article.
STANDARDS OF REPORTING
STROBE guidelines were followed and methodologies were followed in this study.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


