All published articles of this journal are available on ScienceDirect.
Participation in Social Group Activities and Risk of Dementia: A Systematic Review
Abstract
Introduction:
This systematic review aimed to assess the association between social participation in group activities or associations and the risk of dementia based on longitudinal cohort studies.
Methods:
We searched the electronic database PubMed for relevant studies in English published up to April 13, 2021. The search strategy included a combination of terms related to (1) longitudinal cohort studies, (2) assessing the association between social participation in group activities or associations and the risk of dementia, and (3) the article must be published in English or Japanese.
Results:
Of the 1,881 identified studies, 7 were included in the current systematic review. Five of these seven studies indicated social participation in group activities or associations to be significantly associated with a decreased risk of dementia. Our search also revealed the following points: 1) four studies evaluated the association between the specific type of social participation and the risk of dementia; 2) two studies evaluated the association between the frequency of social participation and the risk of dementia, and 3) one study investigated the effects of changes in the state of social participation on the risk of dementia.
Conclusion:
To clarify the association between social participation in group activities or associations and the risk of dementia, future studies should: 1) evaluate the association between the specific type and frequency of social participation and the risk of dementia, and 2) investigate the effects of changes in the states of social participation on the risk of dementia.
1. INTRODUCTION
According to the World Health Organization, the number of people with dementia is increasing worldwide, and has been estimated to reach over 50 million and almost triple by 2050 [1]. Social isolation is considered an important modifiable risk factor for the incidence of dementia, and accounts for 3.5% of cases [1, 2]. Social isolation refers to a state with objectively low levels of contact with others and connection with the community [3]. Participation in social activities is an effective way to prevent social isolation [3].
Social participation is defined as participation in activities that provide interaction with others in society and the community [4]. A prior systematic review, which included studies published until September 17th, 2012, suggested that social participation is associated with a decreased risk of dementia [5]. Recently, a prospective cohort study that included 48,216 older Japanese participants revealed that engagement in leisure activities involving group-based interactions showed the strongest association with reduced mortality [6]. Therefore, this systematic review of prospective cohort studies examining the association between participation in social group activities or associations with the development of dementia aimed at summarizing the reported results and identifying future perspectives.
2. MATERIALS AND METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We searched the PubMed electronic database on April 13th, 2021. The search strategy included a combination of terms related to (1) longitudinal cohort studies, (2) assessing the association between social participation in group activities or associations with risk of dementia, and (3) an article published in English or Japanese. The search period involved articles published up to April 13th, 2021. Medical Subject Heading (MeSH) and free-text terms used were as follows: (“Human Activities”[Mesh] OR “Social Behavior”[Mesh]) AND “Dementia”[Mesh] AND (“cohort studies”[MeSH Terms] OR (“cohort”[All Fields] AND “studies”[All Fields]) OR “cohort studies”[All Fields] OR “cohort”[All Fields]) AND Humans[Mesh] AND (English[lang] OR Japanese[lang]).
The studies identified during the systematic review were screened by two researchers (RT and SU) for the titles, abstracts, and full texts for eligibility. Any discrepancies were resolved through discussion.
3. RESULTS
3.1. Identification of Studies
A total of 1,881 studies were identified in PubMed. We excluded 1,862 articles after assessing the titles/abstracts. Nineteen publications were screened in full text, and seven studies met the inclusion criteria [7-13]. A detailed flowchart of the systematic review is presented in Fig. (1).
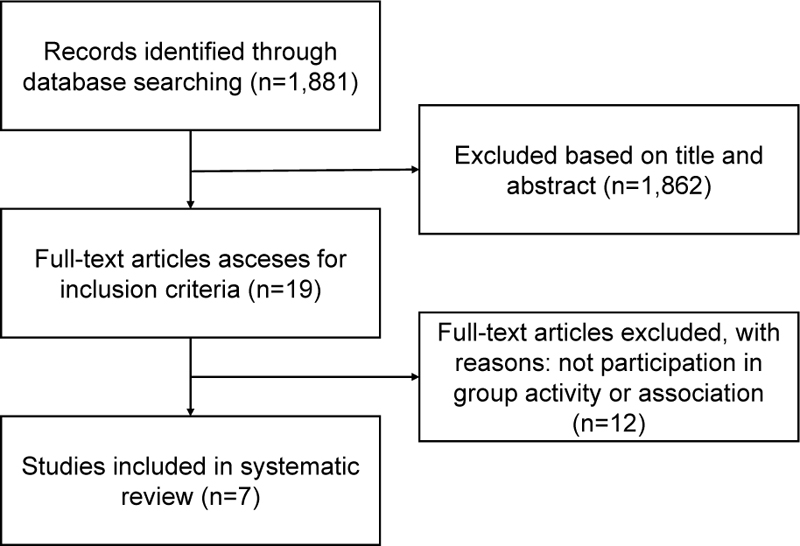
3.2. Study Characteristics
Table 1 summarizes the characteristics of the included studies. Two studies were published each in 2020 [8, 9] and 2017 [10, 11], and one study was published each in the years 2021 [7], 2016 [12], and 1995 [13]. Three studies were conducted in the United Kingdom [7-9]; one study each was conducted in Sweden [10], Japan [11], the United States [12], and France [13]. The sample size for each study ranged from 587 [12] to 851,307 [7]. The duration of follow-up varied from 3 [12, 13] to 18 years [8]. One of the seven studies included a person with dementia [10], whereas six were excluded [7-9, 11-13]. In addition, one study excluded people who received benefits from Japanese long-term care insurance and those who developed dementia within two years of baseline [11].
3.3. Types of Social Participation
Social participation in group activities or associations was measured using a self-administered questionnaire among all reviewed studies. Four studies evaluated the specific types of social participation [7, 9, 12, 13]. Participation in any activity was defined as a social group activity in three studies [9-11].
3.4. Scale
Participants were asked about the frequency of social participation in four studies [8, 9, 11, 12], whether they participated in group activities in two studies [7, 13], and average hours per week of participation in one study [10]. Of those, the authors of five studies categorized them into binary variables such as “participating” or ”no participating” [7, 11-13], two studies used the frequency of social participation [8, 9], and one study observed the changes in the state of social participation during the study period [10].
3.5. Definition of Onset of Dementia
International Diagnostic criteria were used to diagnose dementia in three studies [7, 8, 13]. Two studies used the International Classification of Disease 10th revision to assess dementia [7, 8], and one study used the Diagnostic and Statistical Manual of Mental Disorders (DSM), 3rd Edition, Revision criteria [14], to assess dementia [13].
An indirect measure was used to assess the onset of dementia in two studies [10, 11]. One study [10] used data on anti-dementia treatments from the Swedish National Prescribed Drug Register that contains data on dispensed out-patient prescriptions at all Swedish pharmacies, excluding drugs sold over-the-counter and inpatient use in hospitals [15]. The type of anti-dementia treatment involved Donepezil, Rivastigmine, Galantamine, and Memantine. One study [11] used incident functional disability with dementia under Japan’s long-term care insurance program. The degree of independence was evaluated according to eight levels: 0, I, IIa, IIb, IIIa, IIIb, IV, and M (0=Independent, M=individuals with severe mental or physical diseases and behavioral disorders that require specialized medical care), and commonly, a level of higher than IIa was defined as onset dementia [16].
Combinations were used to assess the onset of dementia in two studies [9, 12]. One study used an algorithm that combines self-reported or informant-reported physician diagnosis of dementia or Alzheimer’s disease or an informant-reported score on the 16-question Informant Questionnaire on Cognitive Decline in the Elderly [9]. One study [12] used two methods. A neurological exam and the Mini-Mental State Examination [17] were used for participants who allowed in-person evaluation. If participants did not allow a full in-person evaluation, the authors used the mixed algorithm from the Dementia Questionnaire (DQ) [18-20], the short version of the Cognitive Abilities Screening Instrument (CASI-short) [21], the Dementia Severity Rating Scale (DSRS) [22], the Functional Activities Questionnaire (FAQ) [23], and the index of independence in activities of daily living (ADL) [24].
3.6. Confounders
The following variables were adjusted in the multivariable analysis: age [7-13], education [7-12], physical activity [7-11], sex [8, 9, 11, 12], employment status [7-9, 11], depressive status [7, 9, 11], history of hypertension [7, 8, 11] and diabetes [7, 8, 11], marital status [7, 8, 11], alcohol consumption [7, 8, 11], smoking habit [7, 8, 11], history of cardiovascular conditions [8, 9, 11], residential status [7, 11], wealth/income [9, 10], self-rated health [7, 10], body mass index [7, 8], history of stroke [8, 11], cognition [8, 13], the proportion of deprivation in their residential area [7], history of menopausal hormones usage [7], ethnicity [7], occupational position based on grade of last employment [8], eyesight [8], hearing [9], prolonged sickness or disability [10], independence of activity of daily living [11], daily caffeine consumption [12], and/or supplemental vitamin intake [12].
Table 1.
| Author/Year/ Location |
Sample type | Sample size/Mean age (S.D)/range | Type of social participation | Scale | Dementia criteria | Confounders | Findings |
|---|---|---|---|---|---|---|---|
| Floud S et al, 2021, UK [7] | Person without dementia | 851,307 60(5) NA |
Voluntary work Group for art, craft or music |
Participating, or no participating | Hospital record [ICD-10 codes (F00-03, G30)] |
Age, education, frequency of strenuous physical activity, paid work, depression, hypertension, diabetes, currently married, alcohol consumption, smoking, living with a partner, self-rated health, BMI, area deprivation, use of menopausal hormones | Follow up less than 5 years, from 5 to 10 years, more than 10 years Not participate in voluntary work: HR = 1.27, 99%CI: 0.99-1.62, 1.10, 99%CI: 1.00-1.22, 0.96, 99%CI: 0.92-1.00 Not participate in artistic activity: HR = 1.37, 99%CI: 1.01-1.85, 1.19, 99%CI: 1.06-1.34, 1.04, 99%CI: 0.99-1.09 |
| Sommerlad A et al., 2020, UK [8] |
Person without dementia and who works in the London office of 20 Whitehall department | 8,280 55.9(6.0) 44.8-69.2 |
Involvement in clubs and organizations, voluntary or official Positions or office; school governor, councilor, etc. Religious activity or observance |
Never, less often, monthly, or weekly | National Health Service digital hospital episode statistics and mental health services data [ICD-10 codes (F00x-F03x, F05.1, and G30x-31.0)] | Age, education, hours per week of moderate or vigorous physical activity, sex, employment status, hypertension, type 1 or 2 diabetes mellitus, marital status, weekly alcohol consumption, smoking, coronary heart disease, BMI, acute stroke, cognition, ethnicity, occupational position based on grade of last employment | No specific type of leisure activity was associated with dementia risk (HR per 1 point increase on activity scale). |
| Fancourt D et al., 2020, UK [9] |
Person without dementia | 9,550 65.2(9.2) 50-99 |
Community group engagement [(1) meetings relating to a common interest such as a political party, trade union or environmental group, (2) tenants group, resident group, or neighborhood watch, (3) church or other religious group, (4) charitable association, (5) education class, arts or music group evening class, (6) social club or (7) any other organization, club or society] | Less than once a year, once or two times per year, or every few months or more | Combines self-reported or informant-reported physician diagnosis of dementia or Alzheimer disease or an informant-reported score above the threshold of 3.38 on the 16-question IQCODE | Age, education, physical activity, sex, employment status, depression, cardiovascular conditions, wealth, eyesight, hearing | No significant association was found between community group engagement and dementia. |
| GriepY et al., 2017, Sweden [10] |
Retired | 1,001 67.0(1.68) 65+ |
Voluntary work (a society, relief organization, religious organization, political party or non-profit organization) | Engaged, or not engaged in voluntary work | Prescribe for anti-dementia treatment from the Swedish National Prescribed Drug Register | Age, education, how much exercise they get in an average week, income, general self-rated health, prolonged sickness or disability | Continuously participated in volunteer work: HR = 2.44, 95%CI: 1.86-3.21 (3 years of follow up); 2.46, 95%CI: 1.89-3.24 (5 years) |
| Nemoto Y et al., 2017, Japan [11] |
Person in local community without dementia, not received public long-term care, and who didn’t developed dementia within two years of the baseline | 13,850 79(2.4) 65+ |
Organization (neighborhood association, senior citizen club/fire-fighting team, religious group, political organization or group, industrial or trade association, volunteer group, citizen or consumer group, hobby group, and sports group or club) |
Participate, or not participate in any social activities (neighborhood association, senior citizen club/fire-fighting team, religious group, political organization or group, industrial or trade association, volunteer group, citizen or consumer group, hobby group, and sports group or club) Had leadership positions, regular members, or non-member |
Incident functional disability with dementia | Age, education, walking time, sex, employment, depression, hypertension, diabetes, marital status, alcohol status, smoking status, heart disease, residential status, stroke, IADL | Among young-older people (65-74 years) Participants: HR = 0.75, 95%CI 1.02-1.46 Had leadership positions: HR = 0.81, 95%CI, 0.65-0.99 Among old-old people (more than 75 years) No significant association was observed. |
| Paganini-Hill A et al., 2016, USA [12] |
Person without dementia | 587 93(2.6) 90-103 |
Time spent religious activity (Going to church, synagogue, religious events) | Rarely/never, or more frequently | Neuropsychological test battery (MMSE, DQ, CASI-short, DSRS, FAQ, ADL) | Age, education, sex, caffeine intake, supplemental vitamin C intake, reading | Going to church, synagogue, religious events: HR=0.66, p < 0.05 |
| Fabrigoulre C et al., 1995, France [13] |
Person without dementia and live at home | 2,040 74.8(6.9) 65-101 |
Golden age clubs Associations |
Yes or no | Screenings according to the DSM-III-R criteria | Age, baseline cognition | No significant association was observed. |
3.7. Synthesis of Results
Five out of the seven studies indicated social participation in group activities or associations to be significantly associated with a decreased risk of dementia [7, 9-12]. A follow-up of less than 5 years, and from 5 to 10 years, and participation in artistic work were associated with dementia risk (HR, 1.37; 99% CI, 1.01-1.85; HR, 1.19; 99% CI: 1.06-1.34). During the second decade, participation in voluntary and artistic work showed little or no association with the development of dementia [7]. After excluding people who developed dementia within two years of the baseline, no specific type of leisure activity was associated with dementia risk [8]. Compared with community group engagement less than once a year, the hazard risk of dementia was lower once or twice per year (HR, 0.69; 95% CI: 0.53-0.89) [9]. Compared with discontinuous participants or non-participants, people who continuously volunteered were less likely to be prescribed an anti-dementia treatment (2012: HR, 2.44; 95% CI, 1.86-3.21; 2014, HR, 2.46; 95% CI, 1.89-3.24) [10]. Among the younger subgroups of the elderly, compared with non-participants, regular members and those with leadership positions had a lower risk of dementia (HR, 0.75; 95% CI: 0.64-0.88). Non-participants had a higher risk of dementia (HR, 1.22, 95%CI: 1.02-1.46), and those in leadership positions had a lower risk of dementia (HR, 0.81; 95% CI: 0.65-0.99). Among the older subgroups of the elderly (75 years or older), neither participant status nor role in an organization was significantly associated with the risk of dementia [11]. Compared with participants who participated in religious activities rarely or never, daily or almost daily participation in religious activities was associated with a lower risk of dementia (HR, 0.66, p<0.05) [12]. Participation in golden age clubs or associations was not significantly associated with the risk of dementia [13].
4. DISCUSSION
The majority of the studies in this systematic review indicated social participation with group activities or associations to be significantly associated with a decreased risk of dementia. In this study, we conducted a systematic review by limiting the literature to prospective cohort studies. Prospective cohort studies have the highest level of evidence among observational studies that only observe the presence or absence of exposure without intervention. The advantage of cohort studies is that the validity of exposure information is high because observations begin with exposure, and the temporal relationship between exposure and the occurrence of the outcome is relatively clear. In addition to cohort studies, cross-sectional studies are the most common observational studies. Exposure and outcome are assessed simultaneously in cross-sectional studies, so that information on exposures that are simple to conduct and invariant (e.g., sex, race) can be collected accurately. However, cross-sectional studies do not address exposures that may change with disease occurrence because it is difficult to assess the temporal relationship between exposure and disease occurrence [25].
Two potential mechanisms may be involved in the decreased risk of dementia through participation in group activities or associations. First, participation in social activity, including physical, cognitive, and social stimulation, helps build neural pathways and contributes to cognitive reserve [2, 26]. The physical, cognitive, social stimulation generated by social activities promotes brain and neural development [26]. These stimuli cause an increase in synaptic density [27] and activate unused areas of the brain, which seems to preserve cognitive function [26]. Experimental mouse studies have also shown that new neurons are generated in the hippocampus when they develop in a stimulating environment [28-30]. Differences in levels of baseline cognitive reserve might affect the subsequent cognitive and clinical trajectories. Although baseline cognition was controlled in the multivariate analysis in two studies [8, 13], cognitive reserve, which can be measured by questionnaires [31], should be taken into account in future studies as a confounder. Second, participation in social activity increases social support and helps reduce stress levels associated with a low risk of dementia incidence [2, 32]. Participation in social activities expands social networks and, consequently, increases social support [33]. Social support reduces the stressor itself and buffers the effects of stress on the body and mind by improving coping ability against stressors [34]. Buffering stress may reduce the onset of dementia [35].
One out of three studies [7, 8, 13], which measured dementia onset using the International Diagnostic criteria, did not find a statistically significant association between participation in social group activities and dementia risk [13]. However, unlike the other two studies, dementia was defined based on criteria provided in the DSM 3rd Edition, which is no longer in use [14]. The DSM 5th Edition, which was published by the American Psychiatric Association in 2013, is currently available [36]. The criteria for the diagnosis of dementia in the DSM 5th Edition are broader compared to the 3rd and 4th Edition. This has resulted in a 40% increase in the number of patients diagnosed with dementia [37]. Therefore, the inconsistency of results may have occurred due to the difference in the criteria for dementia used.
Older people in the United Kingdom, Sweden, Japan, the United States, and France participated in group activities or associations. According to the World Values Survey Wave 5 (2005-2009) [38], in these five countries, 37% of people participated in churches or religious organizations, 29% of people in sports or recreation organizations, 23% of people in art, music, or educational organizations, 21% of people in labor unions, 22% people in a political party, 14% of people in an environmental organization, 17% people in a professional organization, 25% of people in charitable/humanitarian organizations, 15% in consumer organizations, and 16% of people participated in any other organization. In addition, 56% of Japanese people older than 65 participated in group activities or associations in the World Values Survey Wave 7 (2017-2020) [39]. Accordingly, future studies that focus on the association between the specific type and frequency of social participation and the risk of dementia are needed. A study evaluating the effects of changes in social participation on the risk of dementia is also needed.
This study has some limitations, which reflect our positionality and differential proximity to relevant studies published in several languages (previous works need to be addressed for epistemological reflections on these issues [40-43]). First, potential publication bias was inevitable because of the unavailability of unpublished data. Second, as we included published peer-reviewed studies only, a review including grey literature may have led to different findings. Third, proximity operators and field codes differ between databases. Thus, to avoid inaccuracies that may result from translating a search strategy into different interfaces and search syntaxes, the studies were identified using a single free database, PubMed. However, using multiple paid databases such as PsycINFO and EMBASE for the literature search would make our results more robust [44-46]. Fourth, this review only considered articles written in English or Japanese.
CONCLUSION
This systematic review included articles related to prospective cohort studies that examined the association between social participation in group activities or associations and the risk of dementia. The incidence of dementia was significantly lower in those who participated in group activities or associations than in those who did not participate in five out of seven of the articles retrieved. Future studies should consider the following aspects: 1) the relationship between the specific type and frequency of social participation and the risk of dementia, and 2) the effect of changes in social participation on the risk of dementia.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
This study was funded by the Promotion of Science, KAKENHI; Grant Number: JP 20K02392 (CoBiA).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


