All published articles of this journal are available on ScienceDirect.
Diagnostic Value of Chest CT scan for COVID-19 Suspected Cases and Its Compatibility with RT-PCR Method: A Survey from Jiroft, Iran
Abstract
Introduction:
Accurate diagnosis of the COVID-19 disease is important. Currently, chest computed tomography (CT) and reverse polymerase chain reaction (RT-PCR) are being used for the diagnosis of the COVID-19 disease. This study was performed to evaluate the Chest computed tomography (CT) diagnostic value in comparison with the RT - PCR method among COVID-19 patients.
Methods:
This cross-sectional study was performed on suspected cases of COVID-19 in Imam Khomeini Hospital, Jiroft, Iran. Studied patients were evaluated via both a chest CT scan and nasopharyngeal swab for SARS-CoV-2 detection. Data was collected using a self-administered checklist, including demographic information, medical history, and symptoms of COVID-19, chest CT scan, and RT-PCR findings. Data were analyzed using SPSS-V21.
Results:
One thousand and ninety (1090) cases participated in the study; the mean age of the cases of COVID-19 was 48.20± 7.31 years old. The results of the RT-PCR test were 410 (37.6%) positive and 680 (62.4%) negative cases. According to the results of RT-PCR, which is the gold standard method, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive values of chest CT were 98.5%. (99.4-96.8 CI: 95%), 55.7% (59.5 – 51.9 CI: 95%), 71.5% (74.4 -69.0 CI: 95%), 57.3% (60.9 – 53.5 CI: 95%), and 98.4% (99.4% - 99.6 CI: 95%), respectively.
Discussion:
The results of the present study showed that a chest CT scan is highly sensitive for the diagnosis of the COVID-19 disease. Therefore, it can be used as a suitable method for screening and early detection, which requires knowledge of its common radiologic patterns. However, the results showed that the use of this method has low specificity, so it cannot be used for definitive diagnosis and should be used as a complementary method concomitant to the RT - PCR test.
1. INTRODUCTION
In late December 2019, the novel coronavirus (SARS-CoV-2 or COVID-19) triggered the worldwide spread of uncommon pneumonia emanating from Wuhan, China. Studies have shown that the origin of the disease was likely in the market of seafood, poultry, and live animals in this city [1, 2].
The new coronavirus (SARS-CoV-2) belongs to the beta-coronavirus classification and is the third coronavirus outbreak, after the SARS and MERS viruses in the two previous decades. Phylogenetic analysis shows that the new coronavirus is closely related to the SARS bat coronavirus. Regarding these findings, it is plausible that the new virus experienced zoonotic transmission in the Wuhan market [3].
There is a wide variety of clinical manifestations of COVID-19, including asymptomatic cases, upper respiratory tract infection, severe viral pneumonia, respiratory failure, and even death; however, hospital admission is needed for only a few cases [4]. Fever, cough, shortness of breath, fatigue, diarrhea, nausea, and sore throat represent the most common symptoms [5]. Imaging findings also include patchy bilateral shadow, interstitial abnormalities, and focal ground-glass opacity in chest computed tomography (CT) scans. Moreover, lymphopenia, neutropenia, and thrombocytopenia have also been reported among the laboratory criteria in COVID-19 patients, and lymphopenia was shown to be remarkable in severely ill cases [6].
As there is no definitive treatment or optimal vaccination method for this disease, early diagnosis of the patients and their isolation is essential to reduce the spread of the disease in society [7 ]. One of the rapid diagnostic methods for the disease is chest CT scan; however, using this method is controversial and may lead to a confusing interpretation in abnormal cases [8-10]. Reverse polymerase chain reaction, or RT-PCR, as a cellular-molecular diagnostic test is another diagnostic method for this purpose, it has sufficient sensitivity and specificity, so it is the most common method. On the other hand, the use of the CT scan method requires less time than the cellular molecular method [11, 12]. Indeed, detection of the cases in the early stages of the disease is an effective way to reduce its prevalence and mortality. This study was performed to evaluate and compare chest CT scan and RT-PCR for the diagnosis of suspected cases of COVID-19.
2. METHODS
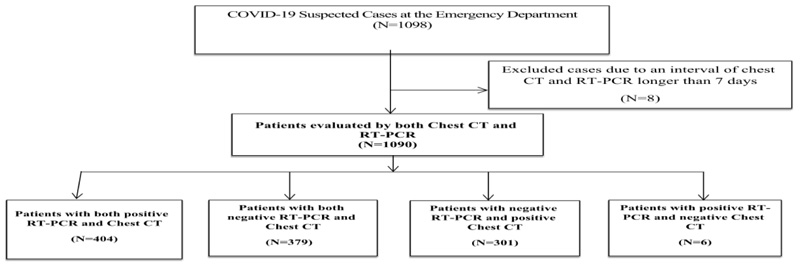
This cross-sectional study was performed from April to June 2020 to evaluate the diagnostic value of chest CT scan and RT-PCR nasopharyngeal swab specimens among COVID-19 suspected cases in the emergency department of Imam Khomeini Hospital in Jiroft, Iran (Fig. 1).
The study sample included COVID-19 suspected patients who were tested to for the diagnosis of COVID-19 via RT-PCR through a nasopharyngeal swab specimen and a chest CT scan, with an interval of less than a week. Individuals who had a CT scan and nasopharyngeal swab sample interval of more than seven days were excluded from the study. Data were collected using a self-administered checklist, including demographic information, history of common symptoms of COVID-19 such as (fever, cough, etc.), chest CT and RT-PCR test results that were obtained from the picture archive and communication systems (PACS), Radiology Information System (RIS) and Hospital Information System (HIS). After completing the checklist, the collected data were entered into SPSS-v21 (SPSS Inc., Chicago, IL, USA) software and analyzed using descriptive statistics. Chi-square, Fisher, and univariate logistic regression tests were also used to investigate the relationship between qualitative variables and the results of RT- PCR and chest CT scan tests. A value of p < .05 was considered to be significant. In addition, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy indices of chest CT were calculated via the following formulas:
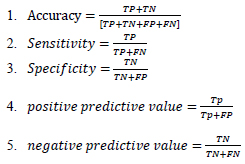
TP: The total number of patients with acute and moderate conditions, identified correctly by the system.
TN: The total number of patients with a mild condition, identified correctly by the system.
FP: The total number of patients with acute and moderate conditions, not identified correctly by the system.
FN: The total number of patients with a mild condition, not identified correctly by the system.
| Frequency (Percent) | Variables |
| 31/7 ± 20/48 | age (mean standard ± deviation) |
| - | Gender |
| 512 [47] | Male |
| 578 [53] | Female |
| - | Chest CT findings |
| 517 [47.4] | Ground glass opacity (GGO) |
| 129 [11.4] | Progressive release |
| 67 [6.1] | Consolidation |
| - | Background disease |
| 119 [10.9] | Hypertension |
| 91 [8.3] | Chronic obstructive pulmonary disease |
| 78 [7.1] | Diabetes Mellitus |
| 102 [9.3] | Chronic heart disease (CHD) |
| 25 [2.2] | Chronic kidney disease (CKD) |
| - | Symptoms associated with COVID-19 |
| 896 [82.2] | Fever |
| 764 [70] | Cough |
| 391 [35.8] | Dyspnea |
| 119 [10.9] | Chest discomfort |
| 61 [5.6] | Hemoptysis |
| 143 [13.1] | Sore throat |
3. RESULTS
From 1098 patients, suspected cases of COVID-19 were referred to the emergency department. Patients were excluded because of the time interval of more than 7 days between RT-PCR and chest CT scan. Therefore, 1090 patients were included in the study, of which 578 (53%) were female and 512 (47%) were male, and the mean age was 48.20 ± 7.31 years. As shown in Table 1, 82.2 % of patients had symptoms of fever (38 degrees), 70% cough, and 35.8% dyspnea at the emergency department of the hospital. The most common observed pattern in chest CT-Scan of these patients was Grand Glass Opacity (GGO), with a frequency of 47.4%. Moreover, the most common patterns were progressive diffusion patterns (11.4) and consolidation (6.1) (Table 1).
Table 2 shows the RT-PCR and chest CT scan function in the diagnosis of COVID-19. Out of a total of 1090 patients, 410 (37.6%) were positive for RT-PCR and 680 (62.4%) were negative. Out of 410 patients who were positive for RT-PCR, 404 were positive for chest CT evaluation. Also, out of 680 people who were negative for RT-PCR, 379 people were negative for chest CT scan. Overall, 705 patients (64.6%) were identified as having a high risk of COVID-19 based on CT-Scan findings. Based on the results of RT-PCR as gold standard, sensitivity, specificity, and accuracy of chest CT in COVID-19 were estimated at 98.5% (99.4-96.8: 95% CI), 55.7% (51.9 – 59.5: 95% CI), was 71.5% (74.4- 69.0: 95% CI), respectively. Also, the positive predictive value of chest CT was 57.3% (60.5 - 53.5: 95% CI) and the negative predictive value of chest CT was 98.4% (95.4% CI: 99.6-6.4.4).
Table 3 presents the relationship between the chest CT scan and RT-PCR results and qualitative variables. There was a statistically significant relationship between RT-PCR and chest CT scan findings in different groups based on previous medical history, CT scan findings, cough, sore throat, bloody sputum, chest discomfort, and dyspnea (P <0.05). However, there was no statistically significant relationship between RT-PCR and chest CT results according to sex (P = 0.35, P = 0.48) (Table 3).
| Frequency (Percentage) | Sensitivity CI 95% |
Specificity CI 95% |
PPV CI 95% |
NPV CI 95% |
Accuracy CI 95% |
||
| RT-PCR [Gold standard] |
Positive | 410 [37.6%] | _ | _ | _ | _ | _ |
| Negative | 680 [62.4%] | ||||||
| Chest CT scan |
Positive | 705 [64.6%] | 98.5 96.8 – 99.4 |
55.7 [51.9 – 59.5] |
57.3 [53.5 -60.9] |
98.4 [96.6 -94.4] |
71.8 [69.0 – 74.4] |
| Negative | 385 [35.1%] | ||||||
| P-value | PCR Results | P-value | CT-Scan results | Subgroup | Variable | ||
| Negative | Positive | Negative | Positive | ||||
| 0.35 | 316 | 196 | 0.43 | 179 | 333 | Male | Gender |
| 364 | 214 | 206 | 372 | Female | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 675 | 0 | <0.05 | 374 | 301 | Negative | Past Medical History | |
| 4 | 115 | 8 | 111 | Hypertension | |||
| 0 | 91 | 0 | 91 | Chronic obstructive pulmonary disease | |||
| 1 | 77 | 3 | 75 | Diabetes Mellitus | |||
| 0 | 102 | 0 | 102 | Chronic heart disease [CHD] | |||
| 0 | 25 | 0 | 25 | Chronic kidney disease [CKD] | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 40 | 164 | <0.05 | 40 | 164 | No | Fever | |
| 640 | 246 | 640 | 246 | Yes | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 371 | 6 | <0.05 | Negative | CT findings | |||
| 190 | 327 | Ground glass opacity [GGO] | |||||
| 77 | 56 | Progressive release | |||||
| 42 | 25 | Consolidation | |||||
| <0.05 | 680 | 410 | Total | ||||
| 67 | 259 | <0.05 | 42 | 284 | No | cough | |
| 613 | 151 | 343 | 421 | Yes | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 656 | 291 | <0.05 | 364 | 583 | No | Sore throat | |
| 24 | 119 | 21 | 122 | Yes | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 649 | 380 | <0.05 | 373 | 656 | Yes | Hemoptysis | |
| <0.05 | 31 | 30 | 12 | 49 | No | ||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 363 | 971 | <0.05 | 356 | 615 | Yes | Chest discomfort | |
| 72 | 47 | 29 | 90 | No | |||
| <0.05 | 680 | 410 | 385 | 705 | Total | ||
| 536 | 163 | <0.05 | 307 | 392 | Yes | dyspnea | |
| 144 | 247 | 78 | 313 | No | |||
| 680 | 410 | 385 | 705 | Total | |||
4. DISCUSSION
This study sought to evaluate the diagnostic value of chest CT and RT-PCR in suspected COVID-19 patients referred to the emergency room of Imam Khomeini Hospital, Jiroft in 2020. Imaging represents a good diagnostic tool for COVID-19 suspected cases, as it indicates the severity and progression of COVID-19 disease. Compared to RT-PCR, chest CT scan is a more reliable, easier, and faster tool to identify and evaluate cases of COVID-19 [ 13-16]. Therefore, being aware of the imaging manifestations of the emerging COVID-19 disease is essential for all radiologists and radiology departments around the world. These manifestations include the presence of GGO, Crazy-Paving Pattern, Consolidation, number of lobes involved in grand glass turbidity, the degree of involvement of each lobe, the presence of nodules, and the presence of pleural effusion [17-19].
Preliminary results have shown that a low-dose CT scan, which is a widely available and relatively inexpensive imaging technique in Iran, is effective for the diagnosis of COVID-19 in suspected patients [10]. In addition, the current pattern of chest CT scan reports can be used to classify and predict which patients should be treated and discharged in an outpatient setting, which patients need further evaluation and monitoring, and even identifying need for ICU admission if necessary [20].
The results of the present study showed that the sensitivity of chest CT scan was 98.5%, which is consistent with the results of the studies by Fang et al. [ 21 ], Lung et al. [ 22], Ai et al. [23], Caruso et al. [ 24], and Kim et al. [ 25], and confirms the high sensitivity of this method in the diagnosis of COVID -19. Due to the high sensitivity of CT-Scan, it is therefore suitable for screening the suspected COVID-19 cases.
This study showed that the specificity of CT scan was 55.7%. It has been reported as 53%, 26%, 26%, 97%, 97%, and 37% by Hemito et al. [26], Cheng et al. [27], Ai et al. [23], Caruso et al. [24], and Kim et al. [25] studies, respectively, which is concordant with the results of the present study and confirms that chest CT scan may not represent a suitable method for definite diagnosis due to the low specificity.
The findings of the present study demonstrated that the most common pattern observed in the CT scan of COVID-19 patients was Ground-glass opacity (GGO), with a frequency of 74.6%, which was consistent with the results of Caruso et al. [24] and Lung et al. [22].
Fang et al. [2020] conducted a descriptive-analytic cross-sectional study in China to evaluate changes in CT scan images of COVID-19 patients from the early stages of the disease to recovery and discharge from the hospital. The results of their study showed that, in the early stages (0-4 days after the first signs of the disease), GGO was the most obvious radiological sign on CT images, seen as subpleural in the lower lobes unilaterally or bilaterally. In four patients, CT scan was normal at this stage, but abnormal findings were also seen on their CT scan. Five to eight days after the onset of the first symptoms, the progressive stage of the disease was seen on CT scan images. At this stage, the infection intensified rapidly and spread bilaterally to the lung lobes. In the advanced stage, GGOs were released with a rapid release pattern in the lung lobes. In patients with COVID-19 who were in the recovery period [patients without respiratory distress syndrome], lung abnormalities on chest CT scans showed their highest severity on approximately the tenth day of the disease. Further, lung involvement increased rapidly within two weeks of the onset of COVID-19 disease, where the highest peak of lung involvement occurred on days 9 to 13 of the disease. At this stage, dense areas were seen at the involved parts of the lungs and large generalized dense GGOs that also produced parenchymal lesions. From the fourteenth day onwards, the GGOs were dissected, and the lesions of the lung infection gradually disappeared [21].
Therefore, due to the high infectivity of COVID-19 and the lack of specific proven treatment and fully effective vaccines against it, early diagnosis of this disease is so important. Also, due to the limited number of nucleic acid test kits, such as RT-PCR and the possibility of false-negative RT-PCR results, chest CT imaging as a non-invasive imaging technique can be useful for screening for early detection of COVID-19 suspected cases [18]. However, it requires knowing the common radiological findings in these patients. When a radiologist observes common chest imaging findings from this new strain of coronavirus, they can detect the cases based on epidemiological and demographic characteristics. The limitations of this study included the following; the short duration of the study, limited clinical and laboratory data due to the urgency of the situation, and limitations related to the lack of randomized clinical trial (RCT) research. Further surveys should be conducted more comprehensively to elucidate our findings.
CONCLUSION
Overall, the results of the present study showed that the use of chest CT scan to diagnose COVID-19 disease is very sensitive, and thus may be used as a method for screening and early diagnosis, which requires knowledge of common radiological patterns of the disease. However, the use of this method has low specificity, so it cannot be used for definitive diagnosis. Accordingly, complementary methods such as PCR testing should be used in addition.
LIST OF ABBREVIATIONS
| CT | = Computed Tomography |
| RT-PCR | = Polymerase Chain Reaction |
| RIS | = Radiology Information System |
| HIS | = Hospital Information System |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of niversity of Medical Sciences, Jiroft, Iran. Ethics code: IR.JMU.REC.1399.039.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All the human procedure ware performed in accordance with the helsinki declaration.
CONSENT FOR PUBLICATION
Informed consent was obtained from the participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


