All published articles of this journal are available on ScienceDirect.
COVID-19 and HIV-associated Nephropathies: Double Whammy
Abstract
The number of COVID-19-associated nephropathies (COVAN) rapidly increased before the fourth wave of the COVID-19 pandemic. Similarities and common lesions with the HIV-associated nephropathy (HIVAN) remarkably affect mostly African Americans positive for the APOL1 risk variants; therefore, these cases must be prioritized in new targeted clinical trials.
Dear Editor,
In 1984, a life-threatening, first-ever case with severe proteinuria and acquired immune deficiency syndrome (AIDS) accompanied by overnight loss of kidney function were observed and later published [1]. One of the significant causes of mortality and morbidity in the population suffering from infection by the Human immunodeficiency virus (HIV) is renal disease. The various symptoms associated with kidney diseases are HIV- associated nephropathy (HIVAN), proteinuria, tubular functional abnormalities, electrolyte disorders, progressive chronic renal dysfunction, and acute renal failure [2]. HIVAN causes high-grade proteinuria and progresses toward end-stage renal disease (ESRD) [3]. The pervasiveness of HIVAN varies; it depends mainly on the race, volunteer understudy, and type of medicine used to treat HIV infection [2]. In African Americans aged 20 to 64, HIVAN is currently the third leading cause of end-stage renal disease [4]. When data collected from the United States renal data system (USRDS) was analyzed, HIVAN was prevalent firmly in the black race; moreover, it was the primary cause of ESRD [5]. Intravenous drug use (IVDU) was the major cause of HIVAN because the black HIV population exhibited higher IVDU than the white HIV population. This was contradicted when a study on a cohort of HIVAN patients in London showed that none were IVDU patients [6].
Its associated diseases and symptoms characterize HIVAN, mainly occurring in the glomerular, interstitial, and tubular compartments (Fig 1). Disease and symptoms found in the glomerular compartment are glomerulosclerosis which affects the glomerular tuft. The Tubular compartment is dilated along with the flattening of the tubular cells and its atrophy. In the interstitial compartment, there is evident lymphocytic infiltration. Examination by electron microscopy before the highly active antiretroviral therapy (HAART) era, endothelial tubule-reticular inclusions were commonly observed. On the other hand, Tubular-reticular inclusions are becoming less common, possibly due to antiretroviral therapy's success in lowering plasma interferon levels [7]. Focal segmental glomerulosclerosis (arrowhead) with the collapse of the glomerular tuft (arrow), tubular microcystic disease (asterisks), interstitial lymphocytic infiltration, and interstitial fibrosis are all visible with periodic acid–Schiff staining, are typical HIVAN histopathologic features [8].
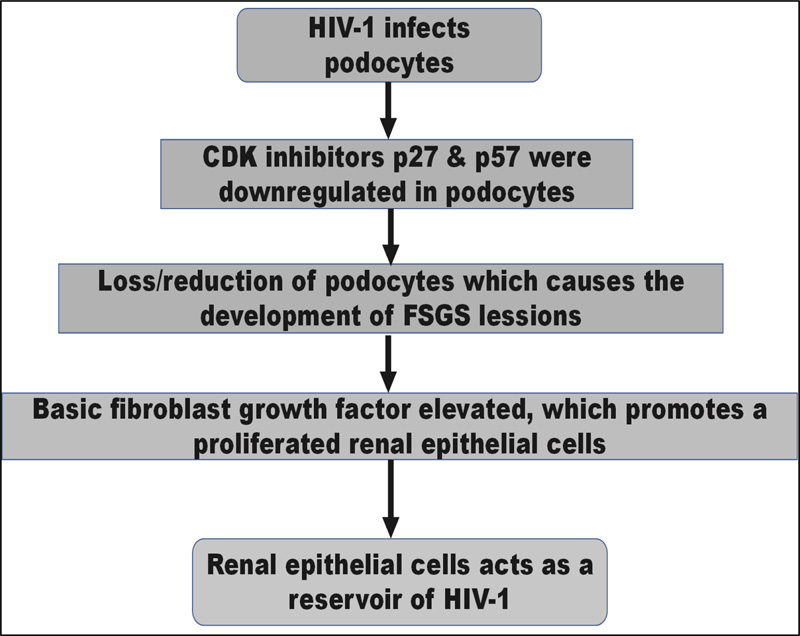
Evidence supports that in HIVAN, podocytes or renal epithelial cells are infected with HIV-1. Collapsing [focal segmental glomerular sclerosis] FSGS is one of the most prominent pathological symptoms of HIVAN. The loss or reduction of essential podocyte differentiation markers, most notably in collapsed glomeruli, is associated with developing the FSGS lesion in HIVAN [9]. In addition, evidence suggests that HIV-1 causes HIVAN to progress through the cell cycle [9]. The cyclin-dependent kinase (CDK) inhibitors p27 and p57 were downregulated in podocytes in HIVAN biopsies, but p21 was increased, according to Shankland et al. Basic fibroblast growth factor is elevated in HIVAN and has previously been demonstrated to promote renal epithelial cell proliferation in vitro [6].
HIVAN is also linked to a rise in transforming growth factor-β, which increases renal fibrosis and apoptosis [9]. Renal epithelial cells can act as a reservoir for HIV-1 in people who have no detectable HIV-1 in their blood [7].
Many teams have been working since the COVID-19 pandemic began. The infection has been found to have renal tropism. Proteinuria, hematuria, and acute renal failure were found in 44, 27 percent, and 14 percent of cases, respectively, in a prospective Chinese cohort of 707 hospitalized patients. Renal involvement was also a risk factor for poor prognosis [4]. The majority of the histopathological lesions were acute tubular necrosis. However, few authors have documented glomerular involvement in the form of collapsing glomerulopathy [9]. Due to the similarities between these lesions and those seen in HIV-associated nephropathy (HIVAN), the term 'COVAN' for 'COVID-19 associated nephropathy' has been proposed [10]. Most patients had severe acute renal failure, which necessitated dialysis in many cases and resulted in recurrent failure to regain kidney function.
Only 40 COVAN cases have been reported in the literature, mostly in men aged 30 to 80 and almost exclusively of African descent. More than half of all case reports were tested for APOL1: almost all were of African descent, all positive for the APOL1 risk variant [10]. APOL 1 in African descent increases the risk of collapsing for focal segmental glomerulosclerosis (cFSGS) [11]. In reality, heterozygous carriers of G1 and G2 polymorphisms have a selective advantage against African trypanosomiasis, at the cost of a 17-fold increased risk of developing FSGS or HIVAN [29-fold increased risk] for homozygous or compound heterozygous patients. These variations were quite common in an African American population with proven FSGS (23 and 13 percent, respectively). APOL1 risk variants operate as a 'first hit' in a recessive experimental model, causing podocyte distress and collapsing glomerular lesions in the context of type 1 interferon release to ('second hit') [9, 10]. Also, the anti-aging gene plays a key role in the kidney, heart, lungs, and pancreas [12]. Zinc and Sirtuin 1 play a close relation in regulating anti-aging genes. Anti-aging genes are closely related to insulin resistance [13], and in chronic nephropathies, insulin resistance is a notable clinical feature [14]. Zinc is closely related to hormonal activity, Sirt 1 activity, and IGF 1 activity. Higher serum IGF 1 activity is also associated with chronic kidney diseases [15]. Hence, anti-aging factors have a close relationship with renal functions. Before SARS-CoV-2 infection was recently added to the list, the primary acknowledged 'second hit' triggers were HIV, lupus erythematosus, and interferon-based therapy [10]. Currently, the extrapulmonary manifestations of CARS Cov-2 are well known, and nephropathies are also a part of this, but more research and clinical trials are needed in this field. [16]
The frequency of APOL1 risk haplotypes in an African American population varies according to their clinical condition: healthy donors, FSGS, HIVAN, and COVAN. Wild type (G0), G1GM (with two missense risk alleles), G1G+ (with one missense risk allele), and G2D6 are the four primary APOL1 haplotypes (with 6-bp deletion risk allele) FSGS, HIVAN, and COVAN haplotype frequencies among African Americans. The Kopp et al. study provided information on FSGS, HIVAN, and healthy donors [10].
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Athanasios Alexiou is the Associate Editorial Board Member of the The Open Public Health Journal.
ACKNOWLEDGEMENTS
Declared none.


