All published articles of this journal are available on ScienceDirect.
Dietary Status of Women who Gave Birth with Congenital Anomalies in Bale Zone Hospitals, Southeast Ethiopia
Abstract
Objective:
Congenital anomalies (CAs) are structural or functional anomalies that develop during intrauterine life and are present at birth. There has been very little knowledge on various forms of CAs as well as dietary status of women who gave birth with CAs in Southeast Ethiopia. This study, therefore, examined the types of CAs diagnosed at birth as well as the dietary status of women who gave birth with CAs in Southeast Ethiopia.
Methods:
An institutional-based cross-sectional study was conducted in Bale zone hospitals, Southeast Ethiopia. All women who gave birth with any form of CAs were included in the study. All births were examined by either obstetricians or emergency obstetric surgeons. Data were entered into SPSS version 21 for analysis. Descriptive statistics were computed to summarize the data.
Results:
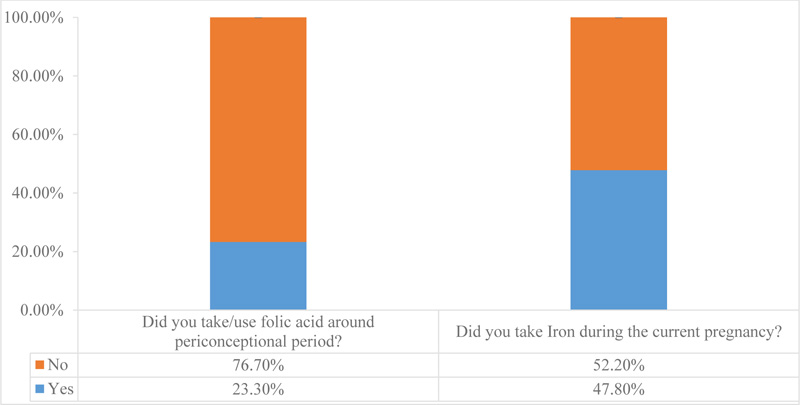
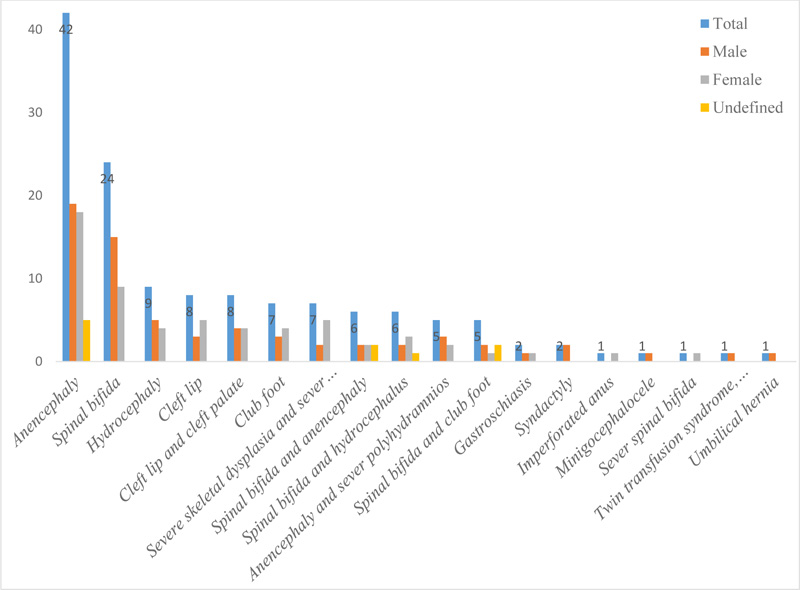
In this study, anencephaly (30.9%) and spinal bifida (17.6%) were the most commonly reported form of CAs. A single case of the imperforated anus, minigocephalocele, severe spinal bifida, twin transfusion syndrome, polyhydramnios and the umbilical hernia was also diagnosed during the study period. Nearly half percent (48.5%) of women who gave birth with CAs consumed less than one meal of meat per week, and 33% of women consumed 1-3 meals of fresh vegetables per week during their pregnancy. Furthermore, the reported consumption of folic acid and intake of iron during the current pregnancy was 23.3% (95% CI: 17.2, 33.7) and 47.8% (95% CI: 32.6, 61.9), respectively.
Conclusion:
The incidence of CAs in the study area is high. Consumption of a range of vegetables and fruits may reduce the development of Cas; hence, educational interventions that improve women's healthy diet practices must be considered. We also recommend further studies to better understand the relationships between the dietary status of women and the incidence of CAs.
1. INTRODUCTION
Congenital anomalies (CAs) are defined as structural or functional anomalies that develop during the organogenesis period and can be identified prenatally, at birth, or detected later in life [1]. Approximately 295 thousand newborns die during the neonatal period annually due to CAs, and these can contribute to substantial neonatal and child morbidity and long-term disability worldwide [2]. CAs also pose psychological and economic costs to the families and may have significant impacts on healthcare systems since many infants with CAs require more than one corrective surgery, causing hospitalization [3, 4].
Even though CAs can be caused by genetic, chromosomal, micronutrient deficiencies, and environmental factors, pinpointing the exact etiologic agents that produce anomalies can often be challenging [2]. A strong relationship has been reported between maternal pre-pregnancy dietary habits and certain congenital malformations [5, 6]. Environmental risk factors (such as drug use, chemical exposure, and alcohol consumption) have been shown to have an adverse and disruptive effect on embryonic life [7, 8]. Environmental risk factors also have mechanical effects on vascular and amnion interruptions by increasing the risk of deformity [9, 10]. Pre-pregnancy infections with syphilis and rubella are also reported as the risk of CAs in developing countries [11, 12].
The magnitude of congenital anomalies in developing countries remains very high and continues to be a significant public health problem in Africa [3, 9]. It is estimated to account for 21 births per 10,000 live births in Africa [3]. In Ethiopia, the incidence of CAs was reported to be 61 births per 10,000 live births, and the magnitude varies widely from region to region, i.e., 2% in northern Ethiopia [13], 1.9% in central and northwest Ethiopia [14, 15], and 3.1% in southeast Ethiopia [16].
This is not surprising given the evidence that suggests that in low-income countries, like Ethiopia, women of reproductive age are exposed to potential teratogenic risks, such as infectious agents and environmental pollutants [17, 18]. For example, a study conducted in central Ethiopia found that women were exposed to high levels of heavy metals, nitrates, and other teratogens that could lead to CAs [19]. Similarly, findings from southeast Ethiopia revealed that pesticide-exposed pregnant women were more likely to have malformed infants than non-exposed women [20]. Intake of herbal medicine and drinking alcohol during pregnancy were also reported as risks of birth defects in northwest Ethiopia [21].
Even though limited studies have described the incidence and the risks of CAs in some parts of Ethiopia, there has still been very little knowledge on various forms of CAs in Southeast Ethiopia, and its incidence could be significantly different from other parts of the country because of unique cultural and societal issues among populations that are mostly pastoralist. Pastoralist women have a low educational background and poor utilization of antenatal care services, which can lead to a lack of folic acid understanding and healthy dietary practices [20]. Although modifiable risk of CAs has been identified and reported in our previous publication [22], the dietary status of women who give birth with CAs has not been well-described. This study, therefore, examined the types of CAs diagnosed at birth as well as the dietary status of women who gave birth with CAs in Southeast Ethiopia, an area where there is a high rate of neonatal mortality due to adverse birth outcomes, including CAs. Given the high frequency of CAs in various parts of Ethiopia, it is vital to examine the forms of CAs by prospectively following delivery outcomes. Suggesting plausible recommendations of preventive public health interventions against these disorders is also important.
2. MATERIALS AND METHODS
2.1. Study Area and Period
A cross-sectional study was conducted from February 2018 to February 2019 in Bale zone hospitals, Southeast Ethiopia. The Bale zone is located in the southeastern part of Ethiopia and has four functional hospitals, namely Goba referral hospital, Robe general hospital, Ginnir and Dellomenna primary hospital. The aforementioned hospitals have been giving healthcare services to a catchment population of more than 1.4 million people. The four hospitals currently provide almost all types of obstetric and gynecologic care, and with more than 10,000 deliveries per year.
Before being discharged from the hospitals, all live births were regularly screened for CAs. Even though the actual number of newborns with CMs is unknown, on average, five infants with congenital malformation are seen in these hospitals every month.
2.2. Study Population
All live births delivered from February 2018 to February 2019 were the source population. All births with any type of CAs were included in the study. All newborns were examined by either obstetricians or emergency obstetric surgeons. Minor anomalies were omitted from the study because of the difficulties in ascertaining such abnormalities. A purposive sampling technique was employed, and a total of 136 deliveries that have occurred during the study period were included.
2.3. Data Collection
An interviewer-administered questionnaire was used to collect the data. The questionnaire was designed first in English and then translated into Amharic and Afan Oromo (local languages). The questions were taken from previously published peer-reviewed articles [9, 10, 14, 21], and the reliability and validity of questions were cheeked first in 5% of the sample. Some questions were modified based on the results of the pre-test. Exit interviews were applied by trained enumerators. All midwives and clinical nurses were told to notify the data collectors whenever suspected cases of CAs were delivered or medically terminated. Data completeness was checked by the investigators.
2.4. Variables
The main outcome variable was forms of congenital anomalies. The explanatory variables were maternal socio-demographic and obstetric characteristics, maternal toxic or environment exposure status, and dietary status of pregnant women with CAs. The details of categorizing explanatory variables are available in Tables 1-4.
2.5. Data Processing and Analysis
Completeness and inconsistencies of data were checked by the investigators. Data were entered into Statistical Package for the Social Sciences (SPSS) version 21 for analysis. Descriptive statistics, such as frequency and cross-tabulation, were computed to summarize the data. The findings were presented in text, tables and figures.
| Characteristics | Category | N (%) |
|---|---|---|
| Age of respondents (in years) | ≤20 | 16(11.8) |
| 21-25 | 40(29.4) | |
| 26-30 | 37(27.2) | |
| 31-35 | 25(18.4) | |
| ≥36 | 18(13.2) | |
| Marital status of respondents | Married | 131(96.3) |
| Single/separated | 5(3.7) | |
| Religion of respondents | Muslim | 87(64.0) |
| Orthodox | 42(30.9) | |
| Protestant | 5(3.7) | |
| Catholic | 2(1.4) | |
| The educational level of respondents | Illiterate | 51(37.5) |
| Primary education | 43(31.6) | |
| Secondary education | 23(17.0) | |
| College education | 19(13.9) | |
| Occupation of respondents | Housewife | 89(65.4) |
| Employed | 18(13.2) | |
| Farmer | 15(11.1) | |
| Merchant | 11(8.1) | |
| Others (daily laborer and student) | 3(2.2) | |
| Residence of respondents | Urban | 71(52.2) |
| Rural | 65(47.8) |
| Obstetric Characteristics | Category | N (%) |
|---|---|---|
| Number of gravida | Primigravida | 39(28.7) |
| Multigravida | 61(44.8) | |
| Grand multigravida | 36(26.5) | |
| Gestational age in weeks | <28 weeks | 19(14.0) |
| ≥28-37 weeks | 70(51.5) | |
| ≥37 weeks | 47(34.5) | |
| Number of infants delivered | Single | 111(81.6) |
| Twin and above | 25(18.4) | |
| Fetal sex | Female | 65(48.4) |
| Male | 64(47.1) | |
| Undefined | 7(5.5) | |
| Did you have previous pregnancy or neonatal death? | Yes | 5(5.2) |
| No | 92(94.8) | |
| Did you have an abortion history/pregnancy loss before 28 weeks? | Yes | 20(21.6) |
| No | 77(79.4) | |
| Did you have a family history of anomalies? | Yes | 4(4.1) |
| No | 93(95.9) | |
| Did you use contraceptives before the current pregnancy? | Yes | 40(41.3) |
| No | 57(58.7) |
2.6. Ethical Consideration
Ethical clearance was obtained from the research review committee of Madawalabu University, and a letter of support was submitted to the office of respective hospitals (ref. RCVP/170/7A). Written informed consent was taken from the study participant. All information gained from the study participant was kept confidential throughout the study. Withdrawing from the study at any point if they wished was guaranteed.
| Toxic or Environmental Exposure Status | Category | N (%) |
|---|---|---|
| Have you been exposed to pesticides during pregnancy? | Yes | 18(13.2) |
| No | 118(86.8) | |
| Did you smoke a cigarette during your current pregnancy? | Yes | 4(2.9) |
| No | 132(97.1) | |
| Did you chew khat during the periconceptional period? | Yes | 28(21.1) |
| No | 108(79.4) | |
| Have you consumed/drank alcohol during the current pregnancy? | Yes | 16(11.2) |
| No | 120(88.8) | |
| Have you been exposed to heavy metals (such as mercury, lead, arsenic, aluminium) during the current pregnancy? | Yes | 3(2.2) |
| No | 133(97.8) | |
| Have you undergone X-ray/radiation therapy during pregnancy? | Yes | 2(1.4) |
| No | 134(97.6) | |
| Did you use a separate cooking kitchen? | Yes | 65(47.8) |
| No | 71(52.2) | |
| Was there any ventilation during heating/cooking? | Yes | 67(49.3) |
| No | 69(50.9) | |
| Have you used a coal stove for heating? | Yes | 98(72.1) |
| No | 38(27.9) |
| DietaRy Status of Pregnant Women | Category | N (%) |
|---|---|---|
| How much meat did you consume during the current pregnancy? | Less than one meal/week | 66(48.5) |
| 1-3 meals/week | 39(28.7) | |
| Greater than four meals/week | 20(14.7) | |
| Do not know | 11(8.1) | |
| How much egg/milk did you consume during the current pregnancy? | Less than one meal/week | 50(36.8) |
| 1-3 meals/week | 61(44.8) | |
| Greater than four meals/week | 18(13.2) | |
| Do not know | 7(5.2) | |
| How much fresh vegetables did you consume during the current pregnancy? | Less than one meal/week | 29(21.3) |
| 1-3 meals/week | 46(33.8) | |
| Greater than four meals/week | 34(25.0) | |
| Do not know | 27(19.9) | |
| How much fresh fruit did you consume during the current pregnancy? | Less than one meal/week | 40(29.4) |
| 1-3 meals/week | 48(35.3) | |
| Greater than four meals/week | 20(14.7) | |
| Do not know | 28(20.6) | |
| How much legume (such as beans, chickpeas, peas, and lentils) did you consume during the current pregnancy? | Less than one meal/week | 33(24.3) |
| 1-3 meals/week | 53(38.9) | |
| Greater than four meals/week | 22(16.2) | |
| Do not know | 28(20.6) |
3. RESULTS
3.1. Maternal Socio-demographic Characteristics
One hundred and thirty-six women were interviewed successfully, and seven women rejected to participate in the study because they were uncomfortable being interviewed. Nearly one-third of respondents (29.4%) were in the age group of 21-25 years. The majority of respondents (96.3%) were married. Around 64% of women were Muslim. The majority of women (65.4%) were housewives. 37% of the study participants did not attend formal education, and only a small percentage of participants completed higher education (13.9%). Nearly half (52.2%) of respondents were urban residents. Table 1 shows the distribution of sociodemographic characteristics of respondents. Since this study is the continuation of our previous study, the sociodemographic characteristics have also been described elsewhere [22].
3.2. Obstetric Characteristics of Respondents
Out of the total respondents, less than half of them (44.1%) were multigravidas. 21% of study participants reported a history of pregnancy loss before viability (28 weeks of gestation). 4% of respondents reported that they had a family history of CAs (Table 2). Since this report is the continuation of our previous study, the detailed obstetric characteristics of respondents have been described in the previous publication [22].
3.3. Environmental Exposure Status of Respondents
Concerning the exposure status of respondents, 13% of pregnant women have been exposed to pesticides. 21% of women reported chewing khat during the periconceptional period (three months before and after conception). The reported consumption of alcohol during the current pregnancy was 11.2%. More than half (52.2%) of study participants did not have a separate cooking kitchen (Table 3).
3.4. Dietary Status of Pregnant Women
As can be seen from Table 4, nearly half of women (48.5%) consumed less than one meal/week of meat during the current pregnancy. 33% of women consumed 1-3meal/week of fresh vegetables during their pregnancy. About 39% of women reported that they ate 1-3meal/week of legumes during the current pregnancy (Table 4). Additionally, the reported consumption of folic acid and intake of iron during the current pregnancy were 23.3% (95% CI: 17.2, 33.7) and 47.8% (95% CI: 32.6, 61.9), respectively (Fig. 1).
3.5. Forms of congenital anomalies
As can be seen in Fig. (2), various forms of CAs were diagnosed during the study period. Of the total CAs diagnosed during the study period, 42(30.9%) of newborns (19 male, 18 female and 5 undefined) were anencephaly and 24 (17.6%) were spinal bifida. Besides, single cases of the imperforated anus, minigocephalocele, severe spinal bifida, twin transfusion syndrome, polyhydramnios and the umbilical hernia were diagnosed during the study period (Fig. 2).
4. DISCUSSION
This study is the continuation of our previous publication that investigated the risk factors of congenital malformations in the same hospitals [22, 23]. Unlike the previous publication, dietary patterns of women who gave birth with congenital anomalies were described in this study. Also, it is one of the few studies that can provide evidence about various forms of CAs and the dietary status of women who gave birth with CAs in Southeast Ethiopia. Accordingly, anencephaly (30.9%) and spinal bifida (17.6%) were the most commonly reported forms of CAs in the study area. A single case of the imperforated anus, minigocephalocele, severe spinal bifida, twin transfusion syndrome, polyhydramnios and the umbilical hernia, was also diagnosed during the study period. Similarly, neural tube defects (which are defined as cases of anencephaly, spina bifida, and encephalocele based on the international classification of diseases (ICD-10) criteria), minigoceomphalocoele, umbilical hernia, and anorectal malformation were reported from Nigerian [24] and Indian [25] studies. Neural tube defects and musculoskeletal defects were also reported from studies conducted in central [17] and northern Ethiopia [23]. This study shows a window of opportunity for CAs' peri-conceptional preventative efforts in areas where environmental risk factors are more problematic. In Addis Ababa, Ethiopia, for example, women were exposed to high levels of metals, nitrates, coliform, and other infections that could cause birth abnormalities [26].
Although genetic factors contribute to the risks of CAs, environmental teratogenic factors also play a role in the formation of CAs, especially in low-income countries like Ethiopia, where a large number of women have been exposed to teratogenic environments that may lead to a high rate of CAs [23, 27]. In this study, 13% of women were exposed to pesticides during their current pregnancy, and more than half of the participants did not have their own kitchen for cooking. As clearly stated by previous studies, toxic chemicals are dangerous to intrauterine fetal development and this is the most common public health challenge that healthcare providers are facing worldwide [3, 28]. Hence, health professionals alone may have a limited ability to avoid the occurrence of CAs. Instead, more coordinated efforts by all those involved in maternal healthcare and early pregnancy, including the governments, might be required to prevent environmental risk exposure of women during pregnancy.
In terms of the dietary status of women who gave birth with CAs, 48.5% of our study participants consumed less than one meal of meat per week during their current pregnancy. Similarly, 33% of women consumed 1-3 meals of fresh vegetables per week during their pregnancy. Furthermore, the reported intake of folic acid from health facility during the current pregnancy was 23.3%. These findings are in agreement with the study conducted in northwestern Ethiopia; 47% of pregnant women ate fewer than three veggie meals each week during their current pregnancy [29]. This indirectly shows that pregnant women in the study area may be deficient in folic acid, which is found in green vegetables. Consumption of a range of vegetables and fruits may reduce the development of CAs; hence, interventions that improve women's healthy diet practices must be considered [30].
Even though the world health organization recommends periconceptional folate supplementation, the highest proportion of women still do not follow the recommendations, particularly those women who are from low socioeconomic backgrounds [28, 20]. This was also true in our study, in which less than half (47.8%) of the study participants took iron during the current pregnancy. This inadequate intake of vital nutrients may contribute to the high rate of birth defects, which has also been linked to an increased risk of CAs [31, 32]. Healthcare providers should advise women to eat folic acid-fortified meals because folate is an essential nutrient that acts as a cofactor for enzymes involved in biosynthesis, preventing the development of CAs [21, 33].
5. LIMITATIONS
This study may have limitations that must be acknowledged. We were unable to consider some CAs that can be identified later in life. There were no investigations among women who gave normal births. Furthermore, the diagnosis of CAs relied only on clinical examinations, and cytogenetic and metabolic analyses were not done because these procedures were not available in the study area.
CONCLUSION
The incidence of CAs in the study area is high. Consumption of a range of vegetables and fruits may reduce the development of CAs, hence educational interventions that improve women's healthy diet practices must be considered. We also recommend further studies to better understand the relationships between the dietary status of women and the incidence of CAs.
LIST OF ABBREVIATIONS
| CAs | = Congenital Anomalies |
| SPSS | = Statistical Package for the Social Sciences |
AUTHORS' CONTRIBUTIONS
AGM and TTK participated in the design of the study, supervised the data collection, and analyzed and interpreted the data. AGM drafted and edited the manuscript. Both authors reviewed and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical clearance was obtained from the research review committee of Madawalabu University, and a letter of support was submitted to the office of respective hospitals (ref. RCVP/170/7A).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written consent was taken from the study participant.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
FUNDING
This study was funded by grant support from Maddawalabu University (protocol No: RCSVP/113/2018).
AVAILABILITY OF DATA AND MATERIALS
All data generated/analyzed during this study are included in this published article. Besides, part of the row datasets will be available from the corresponding author [A.G.M] upon a reasonable request.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
The authors would like to thank Maddawalabu University for its financial support. Theyare also grateful to the study participants, data collectors, and heads/directors of hospitals for their contribution to data collection.


