All published articles of this journal are available on ScienceDirect.
The Role of the World Health Organization in Increasing the Capacity of COVID-19 Laboratories in Indonesia
Abstract
Objective:
The aim of this research to explore the role of WHO in increasing the capacity of the COVID-19 laboratories in Indonesia.
Methods:
Explorative research, assesses deeply about issues or social phenomena that is not widely understood, new cases that have not been handled optimally. This study explores various data and information regarding the development of Corona cases and WHO program to solve the health crisis through data, literature review, and interview.
Results:
The role of WHO in increasing the capacity of COVID-19 laboratories in Indonesia can be seen in two sectors, namely surveillance activation and improvement of national laboratory diagnostics. To activate and strengthen the surveillance, several actions were taken by WHO, such as introducing the COVID-19 surveillance system to the Indonesian Government, supporting surveillance activities on various platforms, utilizing the Influenza Surveillance System to track COVID-19 cases, anticipating the increase of COVID-19 cases due to religious event, broadening the contact tracing network, developing an application to track close contact, and facilitating regular training for contact tracing officer. In the second sector, improvement of national laboratory diagnostic, WHO is actively supplying COVID-19 testing kits to Indonesia, conducting capacity building for laboratory staff, creating guidelines for COVID-19 testing, activating the existing Influenza Laboratory as COVID-19 Laboratory, and monitoring the quality of COVID-19 diagnostic.
Conclusion:
In order to increase the capacity of COVID-19 Laboartory in Indonesia, WHO actively contributes in strengthening the surveillance system and improving national laboratories' diagnostic.
1. INTRODUCTION
Since the post-cold war, there has been a shift in the study of international security. Previously, scholars tended to focus on national security as the main object in international security, but after the cold war, scholars started to give attention to human (individual) security [1]. Disease outbreaks, such as Coronavirus Disease 2019 (COVID-19), which is currently being faced by the international community, are one of the major threats to human security. The World Health Organization (WHO) -an international organization that is responsible for global health programs- is making efforts to formulate strategies to eradicate COVID-19 both at the global and national levels. WHO actively assists its members to respond to the COVID-19 pandemic, including Indonesia.
A new type of Coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was discovered at the end of 2019. This virus became the main cause of Coronavirus Disease 2019 (COVID-19). SARS-CoV-2 was first identified in Wuhan, Hubei, China, then began to spread to various regions of the world [2]. The spread of the Coronavirus has encouraged WHO to declare COVID-19 as a global health emergency or Public Health Emergency of International Concern (PHEIC) on January 30, 2020 [3]. COVID-19 has spread rapidly through human-to-human transmission. The number of COVID-19 cases in the world continues to increase every day [4]. WHO then declared COVID-19 as a global pandemic, an epidemic that spreads in a very wide area, crosses national borders, and causes large numbers of victims [5].
COVID-19 transmission occurs in almost all parts of the world. One of the countries that could not escape from the transmission of Coronavirus is Indonesia. The spread of the Coronavirus in Indonesia can be said “quite late” compared to the other countries. While COVID-19 has spread to various countries around the world since January 2020. In the January-February period, no COVID-19 cases were detected in Indonesia. This had raised many questions from many parties, such as the Australian Government and researchers from Harvard University. They criticized that Indonesia is not capable of accurately detecting people who are exposed to the Coronavirus [6], considering that Indonesia is surrounded by countries that have already been affected by the pandemic [7].
The first and second cases of COVID-19 in Indonesia were identified in two Depok residents, namely a 31-year-old woman and her 64-year-old mother. A few days before being tested positive for Corona, the first COVID-19 patient attended a dance event in South Jakarta. During the event, she made contact with a Japanese citizen who, after being investigated, also confirmed positive for Corona in Malaysia. Upon the report, the Indonesian government immediately established an investigation team to trace people who attended the dance event on February 14, 2020. From the tracking activities, the investigation team managed to collect 113 specimens and 11 of them were proven to be infected with COVID-19 [8].
To deal with COVID-19 at the national level, the Indonesian government immediately issued a series of policies. Several policies were issued, such as appointing 100 referral hospitals for COVID-19 and the formation of the Task Force for the Acceleration of Handling COVID-19 on March 13, 2020. The formation of this task force was based on Presidential Decree (Keppres) Republic of Indonesia I No. 7 of 2020, which was later updated through Presidential Decree No. 9 of 2020. Another important policy of the Government of Indonesia is Presidential Instruction No. 4 of 2020, which specifically instructs that development activities, government budgets, procurement of goods and services are aimed at handling COVID-19. The President's instruction is then followed up with policies at the Ministerial level. However, the policies issued by the Indonesian government have not been effective enough to deal with COVID-19 [7].
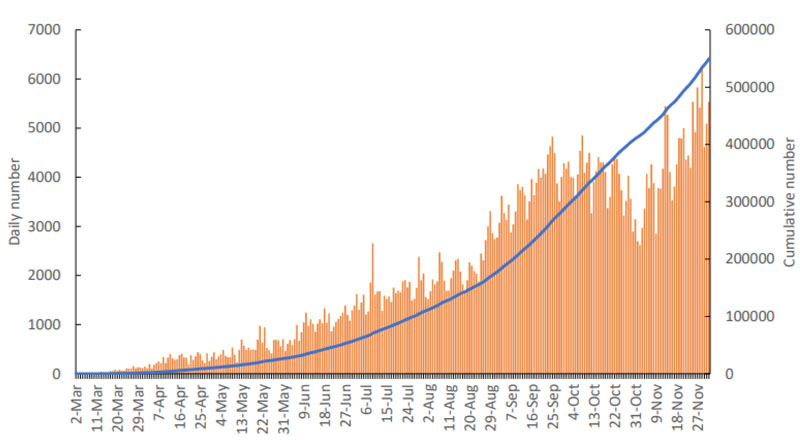
This is evidence of the number of COVID-19 cases in Indonesia, which was still relatively high at that time. It can be seen in Fig. (1) that the number of COVID-19 cases continued to increase from March to November 2020. The daily number of confirmed cases in Indonesia was still in the range of 4,000 to 5,000 cases per day. On December 16, 2020, COVID-19 cases in Indonesia reached 629,429 cases and the total death toll was 19,111 cases [9].
Compared to the United States, India, and countries in Europe, the number of COVID-19 cases in Indonesia is lower than in these countries. However, at the regional (Southeast Asia) level, Indonesia is in the first place as the country with the highest number of confirmed cases and a high death rate due to Corona. The Case Fatality Rate (CFR) due to COVID-19 in Indonesia has reached 8.9%. This percentage is much higher than the global CFR which is only 5.85% [10].
The Indonesian government responds to the global pandemic through collaboration between countries or international organizations. In this case, WHO also contributes to Indonesia's efforts to fight COVID-19. Indonesia has been a member of WHO since May 23, 1950 [11]. In the case of COVID-19, WHO has the responsibility to assist its members in responding to the health crisis. This is based on Article 2 of the WHO's constitution in 1946, which explains that WHO's functions are to assist its members in improving health services, providing technical assistance in emergencies, and coordinating between countries in efforts to eradicate disease outbreaks [12]. Based on the Indonesian WHO Country Cooperation Strategy 2014-2020, it was also explained that WHO has five strategic priorities in Indonesia. The WHO's strategy to deal with COVID-19 transmission in Indonesia is by collaborating with the Government to increase national capacity in responding to health crises or disasters [13].
This study is intended to discuss the role of WHO in increasing the capacity of the national COVID-19 laboratory. This study also discusses the results and challenges faced by WHO in increasing the capacity of COVID-19 laboratory in Indonesia.
2. MATERIALS AND METHODS
This study is categorized as exploratory research. Exploratory research is a part of qualitative research that aims to inform and analyze social issues or phenomena that have not been widely understood or new problems that have not been faced before [14]. The authors used an exploratory study because the spread of COVID-19 is a new phenomenon. Nevertheless, few academics have studied the role of WHO in increasing laboratory capacity in infectious disease outbreaks. In this study, the authors will explore various data and information related to the WHO's strategies for dealing with the limitations of the national laboratory to handle the health crisis that is being faced by Indonesia either in the present or in the future. The authors collected primary data from interviews with the Head of the COVID-19 Integrated Laboratory, Faculty of Medicine, Islamic State University Syarif Hidayatullah Jakarta. The authors also conducted a literature review from secondary data such as books, journal articles, reports from the Indonesian government, and WHO to obtain relevant information.
The selection of WHO as the main subject of our research was based on several considerations, namely: First, WHO as an international organization that focuses on global health, has a responsibility to assist its members in dealing with health crises. This is based on article 2 of the WHO constitution, which mentioned that the WHO is responsible for helping the governments of its members in improving health services, providing technical assistance in emergencies, and facilitating a collaborative effort to eradicate disease [12]. Furthermore, the responsibility of WHO to assist Indonesia in handling health crises is also aligned with the previous WHO commitment to Indonesia, the WHO Country Cooperation Strategy 2014-2020. One of WHO's commitments in Indonesia is to collaborate with the Indonesian Government to improve national capacity in response to health crises or disasters.
Second, WHO has been actively assisting Indonesia in tackling a number of diseases in Indonesia such as tuberculosis, AIDS, malaria, mental health, malnutrition, non-communicable diseases, and increasing the number of preventable diseases immunization. In fact, WHO already has experience in assisting Indonesia dealt with the newly emerging infectious diseases. Prior to the COVID-19 pandemic, WHO assisted the Indonesian government in tackling Avian Influenza (H5N1) in 2005 [15].
Third, the involvement of WHO in handling COVID-19 in Indonesia also shows the collaboration between WHO and the Government in pandemic preparedness and response. Since 2017, WHO and Indonesia have collaborated on global pandemic preparedness and response. Even in 2019, WHO and the government have also designed a pandemic preparedness contingency plan. WHO's commitment to assist Indonesia in responding to the pandemic is driven by the fact that Indonesia is extremely vulnerable to infection and the spread of epidemics, due to its biodiversity, as well as its geographical location between two continents [15].
The purpose of this study is to describe the role and contribution of WHO in increasing the capacity of the COVID-19 laboratory in Indonesia 2020 and identify the limitations faced by WHO. Academically, this research is expected to contribute to the development of International Relations, particularly related to the role of international organizations in dealing with global health crises that threaten health security. Practically, this research is expected to provide information or knowledge to the public and future researchers regarding the role of WHO in increasing the capacity of the COVID-19 laboratory in Indonesia.
3. RESULTS
3.1. Role of WHO in Increasing Indonesia Laboratory Capacity
WHO carries out its role as an international actor which provides technical and operational assistance to tackle COVID-19 in Indonesia. Throughout 2020, WHO actively assisted the Indonesian Government in responding to the global pandemic, by providing input for national COVID-19 guidelines; recommending policies; facilitating meetings between policymakers; socialization and educating their strategies to fight the Coronavirus. This technical assistance is divided into nine areas which include planning and risk assessment; surveillance; national laboratory diagnostics; case management; risk communication and community engagement; infection prevention and control; essential health services; mental health and psychosocial support; and vaccination programs. WHO also collaborates with other actors to provide funds and health logistics to eradicate COVID-19 in Indonesia.
The role of WHO as an international actor who assists the Indonesian Government in eradicating COVID-19 is aligned with health security. The Coronavirus is a serious threat to health security. If we do not take action immediately, the COVID-19 pandemic will continue to take its toll. Tackling disease outbreaks such as COVID-19 could not be done independently by the government, but it also needs collaboration with various actors. Moreover, COVID-19 is an imported case, where the needs of health and laboratory logistics could not be fulfilled by the government alone, but it also needs to be imported from abroad. This is where WHO plays its role to fill the limitations of the Indonesian government on handling COVID-19. Furthermore, WHO involvement is driven by the fact that Indonesia is a developing country that is very vulnerable to the threat of disease outbreaks, which furthermore threaten health security [10].
Indonesia is one of the countries extremely vulnerable to infection and has the potential to become the center of the outbreak, due to its biodiversity and socio-cultural practices and its geographical location between two continents. WHO's involvement in handling COVID-19 in Indonesia also demonstrates the sustainability of the collaboration between WHO and the Indonesian Government in pandemic preparedness and response. Since 2017, WHO and Indonesia have collaborated on global pandemic preparedness and response. Even in 2019, WHO and the Indonesian government have also designed a pandemic preparedness contingency plan [15].
WHO assists Indonesia in expanding the national COVID-19 laboratory network, by facilitating training for laboratory staff and creating guidelines for PCR tests. WHO is aware that broadening the laboratory network not only require adequate tools and materials, but also requires qualified laboratory staff who are familiar with the COVID-19 testing mechanism. In April 2020, for the first time WHO together with the National Health Research and Development Agency (Balitbangkes) conducted a Real-Time Polymerase Chain Reaction (RT-PCR) training for 18 staff from 9 regional laboratories. Representatives from the WHO Collaborating Center for Infectious Diseases Reference Laboratory in Australia also attended the training and presented the procedure for detecting SARS-CoV-2 in the specimens [16]. Increasing the capacity of the laboratory is achieved through the activation of surveillance and improvement of national diagnostics capacity.
3.1.1. Activating and Strengthening the Surveillance Systems
Surveillance is the main key in efforts to deal with COVID-19. Every country in the world, including Indonesia, is required to have a strong surveillance system so that it can track the transmission of the Coronavirus quickly and produce accurate data that can be used to design a national response. Surveillance is part of efforts to increase the capacity of COVID-19 laboratory capacity. With the activation of the surveillance system, there will be more people who will be encouraged to take COVID-19 cases. Therefore, the activation of COVID-19 surveillance system in Indonesia should coincide with the improvement and expansion of the COVID-19 laboratories.
The increasing need for COVID-19 data in various regions has led to an increase in the number and quality of standard laboratories. WHO encourages each region to have a standardized and validated laboratory, so that surveillance results can be trusted for accuracy. During the first three months of COVID-19 transmission in Indonesia, several strategies were implemented by WHO to strengthen the surveillance system at the city, provincial and national levels:
3.1.1.1. Introducing the COVID-19 Surveillance Systems
WHO introduces Go.Data and the Early Warning and Alert Response System (EWARS) as the main system for COVID-19 surveillance. The Go.Data program is an outbreak investigation tool designed to collect data during health emergencies. Go.Data is then implemented in the DKI Jakarta surveillance system [17]. Meanwhile, EWARS is a system designed to increase the capability of detecting outbreaks in an emergency. On 23 April 2020, WHO socialized EWARS to representatives of the Directorate of Health Surveillance and Quarantine, Ministry of Health (MoH) of the Republic of Indonesia, and regional surveillance officers. WHO together with representatives from Indonesia also agreed on the consistent use of EWARS and conducted weekly meetings to discuss surveillance challenges in the field [18].
3.1.1.2. Supporting Surveillance Activities in Various Platforms
WHO supports surveillance activities on Halodoc, GOJEK, and Tokopedia. The three online applications provide screening and health consultation regarding COVID-19 symptoms. Users of the three applications who have symptoms of COVID-19 will be directed to take the COVID-19 test and get further treatment [19].
3.1.1.3. Encouraging the Indonesian Government to use Influenza Surveillance System to track COVID-19 Cases
The Global Influenza Surveillance and Response System (GISRS) is usually used to monitor cases of Influenza-Like Illness (ILI) and Severe Acute Respiratory Infections (SARI). By using GISRS, the tracking of COVID-19 cases will be even tighter. Suspected cases with mild symptoms, which are often not identified through the COVID-19 surveillance system, will still be identified through the ILI and SARI surveillance sentinels [20]. To strengthen the use of GISRS in Indonesia, WHO held refresher training for Influenza surveillance sentinel officers. The refresher training is divided into two sessions. The first session was conducted on 25-26 June 2020 and was attended by 133 officers from 26 ILI surveillance sentinels. Meanwhile, the second refresher training session was held on 29-30 June 2020 and was attended by 35 officers from 6 SARI surveillance sentinels [21].
3.1.1.4. Assisting Indonesian Government to Anticipate the Increased Number of COVID-19 Cases Due to Religious Ceremonies and Homecoming Tradition
In Indonesia, there is a homecoming tradition, where Indonesian people go back to their hometown to celebrate Eid al-Fitr with their families. With this tradition, there will be high mobilization than can lead to an increasing number of COVID-19 cases. Therefore, WHO assisted the Indonesian Government to anticipate the rising number of COVID-19 cases due to this tradition. WHO coordinated with the International Federation of Red Cross and Red Crescent Societies (IFRC) to activate Community Based Surveillance (CBS). Thereafter, the public can participate directly in the process of tracking the Coronavirus and the local Local Health Center (Puskesmas) can conduct COVID-19 testing for travelers [22].
3.1.1.5. Spreading the Contact Tracing Officer
WHO encouraged MoH to carry out field investigations by disseminating tracking officers throughout the Indonesian territory. With the assistance of the Food Agriculture Organization (FAO), WHO, and MoH succeeded in compiling and publishing a guidebook for contact tracing officers at the provincial level [23]. On July 6, 2020, WHO, FAO, and the Indonesian MoH also agreed to establish a Contact Tracing Center at the national and regional levels. This establishment is expected to improve and simplify the supervision of contact tracing [24].
3.1.1.6. Developing Close Contact Tracking Aplication
WHO, FAO, and MoH collaborated to develop an application to trace close contacts, called “Silacak”. The quality of this application continues to be improved. Recently, Silacak is already available in the mobile version and it has features to analyze data and monitor the daily COVID-19 reports from various Puskesmas. The purpose of developing the Silacak application is to make tracking officers easily trace close contacts at the provincial or district level, monitor close contact's activities, and analyze, and report data [25].
3.1.1.7. Facilitating Regular Training for Contact Tracing Officer
The contribution of WHO to strengthen Indonesia's COVID-19 surveillance was also seen in the refresher training and evaluation meetings for contact tracing officers. The refresher training was held online on 10-11 September 2020 and attended by at least 700 contact tracing officers from 514 regions of Indonesia [26]. Then, it was continued by facilitating refresher training for 300 contact tracing officers from 51 cities in 10 priority provinces: Aceh, Bali, Central Java, DKI Jakarta, East Java, North Sumatra, South Sumatra, South Kalimantan, Papua, and West Java on 3rd November 2020 [27].
The development of contact tracing in ten priority provinces was then evaluated in a meeting conducted on November 21-22 2020. This evaluation meeting became a forum for field officers from various regions of Indonesia to exchange information, experiences, and challenges encountered during the contact tracing process [28]. In addition to facilitating training for regional contact tracing officers, WHO were also paying attention to tracking COVID-19 cases at the country's Point of Entry (POE). Because COVID-19 is an imported case, the country's PEO can be the initial location for the transmission of the Coronavirus. If the COVID-19 surveillance system at the POE is weak, the virus will spread widely in the territory of Indonesia. To anticipate the worst scenario at the country's entrance, since March 2020, WHO has supported the implementation of training on contact tracing for 80 field officers and health workers at ports [19].
3.1.2. Improving the Capacity of National Laboratory Diagnostic
To face the global pandemic, every country including Indonesia is required to increase the capacity of its laboratories. So, they can carry out COVID-19 tests on a large scale. However, increasing laboratory capacity is not an easy task. Not all laboratory equipment and materials can be fulfilled domestically. Several tools and materials for COVID-19 diagnostic purposes need to be imported from abroad.
3.1.2.1. Supplying COVID-19 Dignostic Equipments
WHO has assisted Indonesia in providing COVID-19 testing tools and materials, such as testing kits, reagents, PCR equipment, extraction tools, Viral Transport Medium (VTM), and others. Starting from Quarter-I of 2020, WHO sent 150 reagents with a capacity of 15,000 reactions for screening and 45 reagents with a capacity of 4,500 reactions for COVID-19 confirmation tests to the Indonesia National Health Research and Development Agency (Balitbangkes) [17]. Then, in Quarter-II of 2020, WHO distributed 1,000 VTMs to the Directorate of Health Surveillance and Quarantine of the Indonesian MoH [22]. This was followed by the submission of 40,000 primers and probes for screening; 12,000 primers and probes for COVID-19 confirmatory testing; 20 PCR kits with a testing capacity of up to 108,900 people; 128 nucleic acids and extraction apparatus for sample preparation; and 3 sets of centrifuges to process the extraction with reagents, to Balitbangkes [29]. In the third quarter of 2020, WHO provided 253,800 VTM and swab kits, which is equivalent to US$ 532 thousand, to the Directorate of Health Surveillance and Quarantine of the Indonesian MoH. The laboratory equipment was then distributed to 34 provinces of Indonesia [30]. In addition, WHO also provided 140 antibody test kits and Enzyme-Linked Immunosorbent Assay (ELISA), worth US$ 80 thousand, which will be used for seroepidemiological research at Balitbangkes and selected laboratories [31]. In September 2020, WHO provided 140 sets of reagents that can be used for 13,440 COVID-19 tests in Indonesia.
3.1.2.2. Conducting Capacity Building for Laboratory Staff
WHO also contributes to the expansion of the national COVID-19 laboratory network, namely by facilitating training for laboratory staff and formulating guidelines for PCR procedures. WHO is aware that expanding the laboratory network does not only require adequate tools and materials but also requires qualified human resources who are familiar with the COVID-19 screening mechanism. In April 2020, for the first time, WHO together with Balitbangkes held training for 18 experts from 9 regional laboratories related to the use of Real-Time Polymerase Chain Reaction (RT-PCR). Representatives from the WHO Collaborating Center for Infectious Diseases Reference Laboratory in Australia also attended the training and presented the procedure for detecting SARS-CoV-2 in specimens [16].
WHO and Balitbangkes continue to socialize the procedures of using PCR as a COVID-19 diagnostic tool through several webinars. As in the webinar held on April 24, 2020, WHO and Balitbangkes explained risk management in handling the Coronavirus and procedures for using PCR tests in front of 135 participants from the Food and Drug Supervisory Agency (BPOM). The next day, WHO and Balitbangkes again held a webinar about the character and pathogens of the Coronavirus. In the webinar which was attended by 181 participants, WHO together with Balitbangkes also presented the importance of applying biosafety and biosecurity in COVID-19 laboratories [19].
In the same month, WHO, Balitbangkes, US Centers for Disease Control and Prevention (US CDC), and the Association of Public Health Laboratories (APHL) held a meeting to prepare two PCR training sessions for experts from 100 laboratories, which will be held on 28 April-2 May 2020 and 5-9 May 2020. This training will cover the discussion of procedures for specimen collection, testing, recording, interpretation of results, components of biosafety, and biosecurity [32], to fill the examination capacity gap and speed up test results, WHO introduced the use of PCR tools of the Abbott m2000 and Cepheid GeneXpert® types to the TB Sub-Directorate, HIV Sub-Directorate, and Emerging Infectious Diseases Sub-Directorate of the Indonesian Ministry of Health [18].
In May 2020, there were two online PCR training sessions conducted by WHO and Balitbangkes. This PCR training was attended by 366 participants from 52 Indonesian laboratories who met the qualifications for conducting PCR. WHO presented the latest laboratory examination guidelines, distributed laboratory capacity assessment tools, and identified problems or challenges encountered during the Corona assessment process. As of May 6, 2020, there were already 711 laboratory experts trained to use PCR [33]. The PCR examination training facilitated by WHO would continue until mid-2020. On 15-19 June 2020, WHO participated in two PCR training sessions organized by the Indonesian Ministry of Health, the Center for Health Human Resources Development and Empowerment (PPSDM), and APHL. In front of 200 training participants, WHO explained the development of COVID-19 cases and guidelines for identifying the new type of Coronavirus [21] Training for Indonesian laboratory experts is not limited to a national scope. WHO Country Office Indonesia always informed and invited Indonesian laboratory staff to participate in training about COVID-19 testing organized by the WHO South East-Asia Region (SEARO) or other regions.
The purpose of conducting various laboratory training is that the COVID-19 test in Indonesia has the same standards as WHO interim guidance. This was delivered by a spokesperson of the COVID-19 Laboratory of the Medical Faculty of Islamic State University Syarif Hidayatullah Jakarta, dr. Erike Anggraini explained that WHO encourages laboratories in Indonesia to comply with the principles of “True Positive” and “True Negative”. National laboratories have to deliver the results of the COVID-19 testing honestly and transparently. If there is an indication of SARS-CoV-2 in a specimen, the laboratory has to report the specimen as a positive case. Vice versa, if there is no indication of SARS-CoV-2 in a specimen, the laboratory has to categorize the specimen as a negative case [34].
3.1.2.3. Developing Guidelines for COVID-19 Diagnostic Procedure
Regarding the design of national diagnostic guidelines, WHO has participated in making video tutorials of PCR mechanisms for COVID-19 tests with the Tuberculosis Sub-Directorate of the Indonesian MoH and the Department of Microbiology, University of Indonesia. On the video-making process, WHO provided input regarding sampling, examination, and reporting of COVID-19.22 In September, WHO, Balitbangkes, US CDC, APHL, and the PPSDM Agency agreed to design a guide for PCR equipment which will be the main guidelines for laboratory staff on detecting Coronavirus. In the next meeting which was held on October 9, 2020, WHO and partners began to finish out the PCR guidelines [35]. WHO Country Office in Indonesia actively informs the laboratory diagnostic guidelines that have been updated to national laboratory officers. Shortly after the emergence of a new variant of COVID-19, WHO Indonesia immediately translated and distributed guidelines for detecting the new variant of COVID-19 to all reference laboratories [34].
3.1.2.4. Using the Existing Influenza laboratory as part of COVID-19 Laboratories
The role of WHO in expanding the COVID-19 laboratory network is also carried out by using laboratories under Influenza sentinel surveillance. WHO recommends testing samples from the ILI and SARI laboratories twice. The purpose of testing the samples twice is to confirm the indication of Influenza and COVID-19. The use of the Influenza laboratory is aligned with the WHO surveillance strategy in Indonesia, which is related to the activation of GISRS [36].
3.1.2.5. Ensuring the Quality of COVID-19 Diagnostic
The role of WHO in the national diagnostic laboratory is not limited to facilitating training and designing guidelines for Coronavirus tests. However, WHO observes the national laboratories directly to ensure the quality of the COVID-19 laboratory in Indonesia. Quality assurance is measured by conducting a proficiency test, whereas WHO sends an examination panel containing specimens that must be tested and answered by the laboratory staff. After examining the samples, the relevant laboratory staff needs to report which specimens are positive and which are negative in an application provided by WHO. If all the answers submitted are correct, the relevant laboratory will get a certificate of passing the proficiency test, 170 COVID-19 laboratories have passed the WHO proficiency test, and if they have not passed, they will be trained again [34].
4. DISCUSSION
Based on the result of this research, it shows that the WHO contributes to two sectors in an effort to increase the capacity of COVID-19 laboratories in Indonesia. The two sectors are surveillance and national laboratory diagnostics. From the results of this study, it can be seen that WHO played a role as an “international actor” who provides technical and operational assistance in an effort to increase the capacity of COVID-19 laboratories in Indonesia. WHO actively provides input and recommends activities to the Indonesian Government to stop the transmission of the Coronavirus. WHO also facilitates meetings between various parties to coordinate COVID-19 surveillance and diagnostics.
This research also illustrates that the role of WHO is needed by member countries to help increase the capacity of laboratories in order to respond to the health crisis. Furthermore, WHO's actions in world politics have often been underestimated. This is because WHO is categorized as an international organization that deals with technical and functional cooperation. In fact, the role and contribution of organizations such as WHO are very useful for people's daily lives. Especially in the midst of a health crisis like now.
Handling disease outbreaks such as COVID-19 can not be done independently by the government, but collaboration with various actors is needed. Moreover, COVID-19 is an imported case, where the needs of health and laboratory logistics can not be fulfilled by the government alone and it needs to be imported from abroad. This is where WHO plays its role to fill the limitations of the Indonesian government in handling COVID-19, especially related to laboratory capacity. Increasing laboratory capacity is the most important part of dealing with the COVID-19 pandemic. Furthermore, WHO's involvement is driven by the fact that Indonesia is a developing country that is extremely vulnerable to the threat of disease outbreaks that threaten health security.
In this study, the authors found two achievements of WHO in efforts to increase the capacity of COVID-19 laboratories in Indonesia:
4.1. The Surveillance System Can Track a Large Number of Suspected Cases and Close Contacts
A country can be claimed to succeed in handling the COVID-19 outbreak if it has a surveillance system and a good quality COVID-19 laboratory. Another indication is that the surveillance system can detect, analyze and report in less than 24 hours; the surveillance system that has been implemented has reached vulnerable groups and closed places such as prisons, nursing homes, orphanages, and others; 90% of suspected cases were detected, examined, and isolated within 48 hours; the results of the COVID-19 examination can be released in a maximum of 3x24 hours; 80% of close contacts of confirmed cases can be identified and quarantined in less than 72 hours; surveillance of close contacts for 14 days; and regions have a well-functioning data management system to manage data on COVID-19 cases.
Based on the evaluation, surveillance activities have been successfully implemented by the Government of Indonesia through several policies, such as the creation of the All records TC-19 application so that reporting and recording of all healthcare facilities, agencies, and laboratories are integrated; implementation of supervision through 27 ILI websites and 6 SARI websites; increase the power to detect and bind close contacts; and the creation of applications such as “Peduli Lindungi” which contains data on developments, case distribution, and close contacts.
4.2. COVID-19 Laboratories are Expanding throughout Indonesia with Qualified Laboratory Staff
Laboratory tests are progressing. In the early emergence of COVID-19 in Indonesia, Coronavirus tests were conducted centrally at the MoH's Health Research and Development Agency (Balitbangkes). However, recently, the network of national COVID-19 laboratories has been broadened. A total of 737 laboratories spread across 34 provinces of Indonesia have been prepared for COVID-19 testing locations. In addition, 430 laboratory staff and volunteers received online training related to the COVID-19 testing procedure. The government also ensures the availability of materials for COVID-19 testing such as PCR reagents, extraction tools, Viral Transport Medium (VTM), and others.
The author also found that WHO was facing challenges in increasing the capacity of the COVID-19 laboratory in Indonesia. The main challenge comes from internal, namely limited funds. Funds for WHO Indonesia are still in a deficit of US$ 28 million (Fig. 2) [37]. Although several countries have contributed, these donations are not enough to cover the total budget needed by WHO in dealing with COVID-19. Due to a lack of funds, WHO has limitations in providing laboratory or diagnostic logistics and technical assistance to Indonesia. WHO needs to collaborate with other actors to provide health logistics in Indonesia.
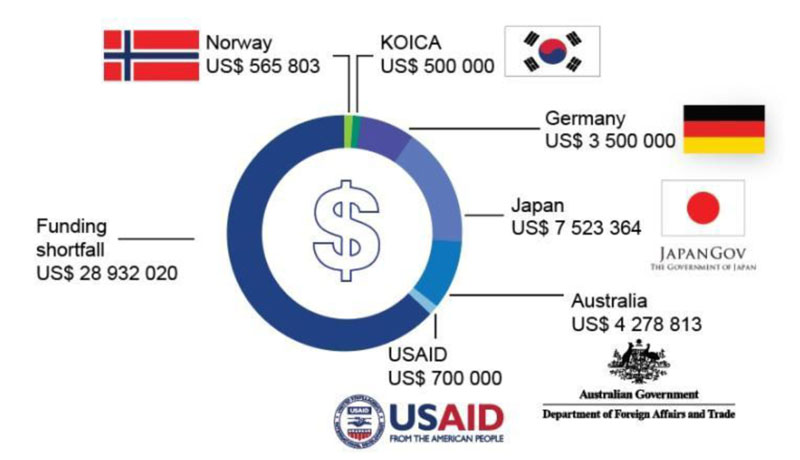
This is not the first time WHO has ecountered budget limitations when helping its members to tackle a health crisis. Since the establishment of WHO, this organization has depended its operational funding on mandatory contributions from its members, voluntary contributions from developed countries, charitable foundations, community institutions, and other actors. This financial dependence continues to the global level of the COVID-19 response program. The sudden emergence of the Coronavirus has made WHO feel overwhelmed, especially in terms of funding. So far, the WHO budget has been more allocated for the operations of the WHO Country Office and the sustainability of existing global health programs. Meanwhile, the budget for anticipating emergencies has not been prioritized [38].
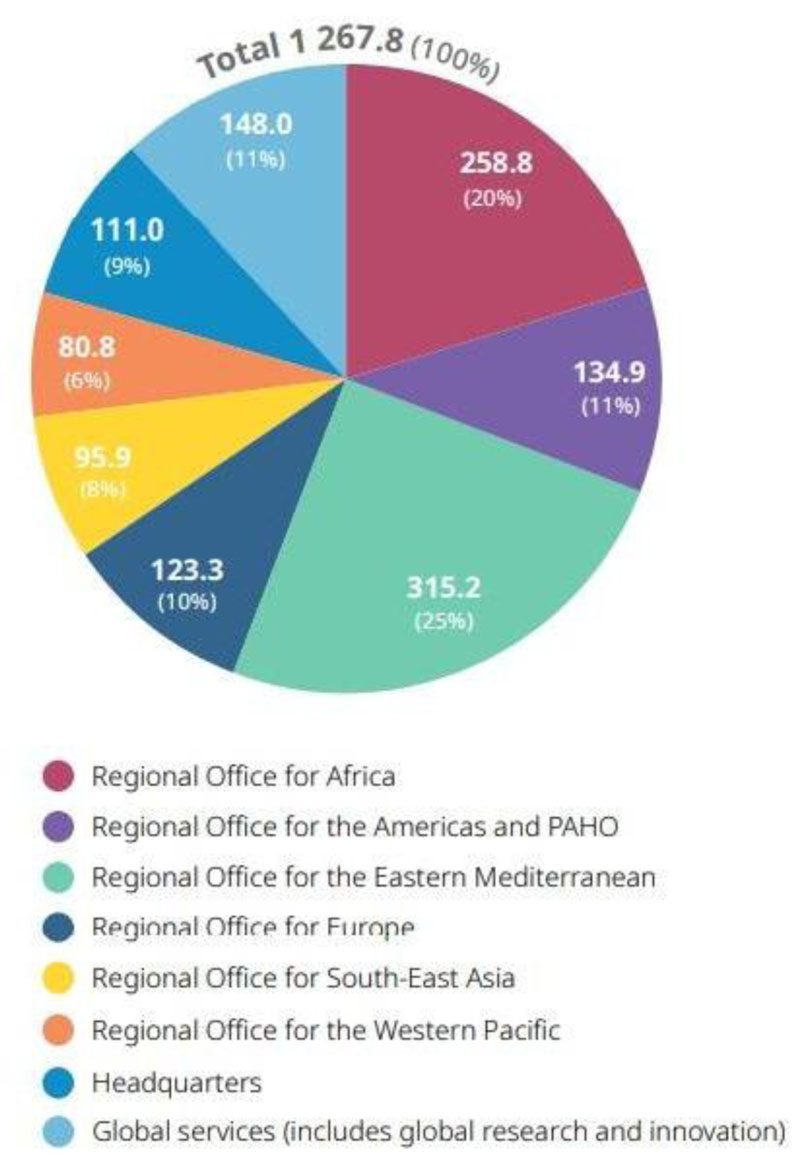
At the beginning of 2020, WHO estimated the budget needed for handling COVID-10 at the global level would be US$ 1.7 billion. Until the end of the year, WHO was only able to gain funds up to US$ 1.5 billion, most of the funds came from voluntary contributions and the COVID-19 Solidarity Response Fund. The funds collected were still able to support WHO activities in dealing with COVID-19 globally. This is because the actual budget used is only US$ 1.2 billion. Although funds are still surplus, the budget allocation for handling COVID-19 in the Southeast Asia region is very low. As seen in Fig. (3), WHO only allocated 8% of its budget or around US$ 95 billion for the prevention of COVID-19 in Southeast Asia. This number is very small when compared to the other regions and global services. The budget obtained by WHO SEARO has to be divided equally among 11 members in the Southeast Asia Region before it can be given to the WHO Country Office Indonesia [39].
CONCLUSION
WHO played a role as an “International Actor” who assist Indonesia to increase the capacity of national COVID-19 Laboratories. The role of WHO in Indonesia is aligned with the concept of health security, because the health crisis like COVID-19 can not be solved independently by a country, but it needs a collaboration with other countries. To increase the capacity of COVID-19 laboratory in Indonesia, WHO is actively providing technical and operational assistance in two sectors, namely surveillance and national laboratory diagnostic. The technical assistances that were given by WHO, such as introducing the COVID-19 surveillance system, supplying the COVID-19 testing kits, developing guidelines for COVID-19 diagnostic, facilitating capacity building for tracking officer or laboratory staff, etc. The contribution of WHO in increasing the capacity of the COVID-19 laboratory in Indonesia has brought good results. Now, Indonesia is able to quickly track COVID-19 cases and also the COVID-19 laboraries can be found in all of Indonesia regions.
LIST OF ABBREVIATIONS
| PHEIC | = Public Health Emergency of International Concern |
| WHO | = World Health Organization |
| CFR | = Case Fatality Rate |
| H5N1 | = Avian Influenza |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the finding of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


