All published articles of this journal are available on ScienceDirect.
Prevalence of Chronic Kidney Disease Among Diabetes and Hypertensive Patients in a Teaching Hospital in Ekiti State, Southwest Nigeria
Abstract
Introduction:
Chronic kidney disease (CKD) is a growing public health problem associated with enormous economic burdens, reduced quality of life, and untimely deaths, predominantly in developing countries. Aims: The study determines the prevalence and risk factors for CKD among diabetes and hypertensive patients in a teaching hospital in Ekiti State.
Methods:
Descriptive and cross-sectional research designs were employed using a quantitative strategy. Two hundred (200) randomly selected participants participated in the study. Socio-demographic data, awareness, and risk factors for CKD were determined using a standardized questionnaire, while CKD prevalence was investigated with biophysical measurements and laboratory investigations. Descriptive analyses were used to answer the research questions, while inferential statistics were used to test hypotheses at a significant level of p < 0.05.
Results:
Findings revealed that 50% and 57.1% of the diabetics and hypertensives were above 60 years, 36.7% of the people with diabetes had comorbidity, while only 2% and 3.1% of diabetics and hypertensives participants had a family history of CKD. The study revealed that the respondents' level of awareness of CKD was inadequate. Major risk factors of CKD identified among the respondents were already diagnosed with diabetes and hypertension, age above 60 years (50% and 57.1%), herbal concoction (77.7% and 73.5%), and NSAID (74.5% and 78.6%). The prevalence of CKD for people with diabetes was 39.8%, while 57.1% for hypertensives. There was a significant relationship between respondents’ level of education and awareness of CKD (X2 =44.20, p=<0.001). The prevalence of CKD among the studied population was high.
Conclusion:
Efforts should be intensified by nurses and all other stakeholders on awareness and prevention programs for CKD. Furthermore, the promotion of patients’ satisfaction with the quality of healthcare services should be the goal to promote positive health outcomes.
1. INTRODUCTION
According to Li et al. [1] and Fraser and Maarten [2], chronic kidney disease (CKD) is a serious public health challenge due to its growing burden. Chronic kidney disease is defined as the progressive loss of kidney function that can occur over a period of time ranging from several months to years; the condition occurs due to the gradual change in the normal structure of the kidney with glomerular filtration rate (GFR) of <60 mL/min per 1.73m2 for over 3 months [3, 4]. Thus, resulting in a continuous reduction in the ability of the kidney to process waste in the blood and perform other functions [4].
Diabetes mellitus (DM) stated a chronic, metabolic disease characterized by an increase in blood glucose levels that can result in severe damage to several organs in the body, such as the heart, eyes, kidneys, and nerves, with diabetes nephropathy if not controlled [5]. There are two major DM types: Type 1 diabetes (insulin-dependent) and Type 2 diabetes. According to Tannor et al. [6], DM is the principal risk factor and foremost cause of CKD, as diabetic nephropathy occurs in about 40% of persons diagnosed with Type 2 DM [1, 6]. Diabetes nephropathy is also known as diabetic kidney disease. It is clinically diagnosed clinically based on the value of the projected albuminuria and albumin creatinine ratio (UACR) ≥30 mg/g (or ≥ 3.4 mg/mol) with or without a progressive decrease in eGFR below 60 mL/min per 1.73 m2 caused by damage to the small renal blood vessels [6].
Hypertension is the persistent increase of blood pressure (BP) which can be diagnosed based on elevated systolic and diastolic BP or either [7]. There is a bidirectional link between hypertension and CKD; also, hypertension has been stated to be a major contributor to the progression of CKD as it accounts for about 28% of CKD causes among hypertensive patients [3]. A study conducted in Nigeria by Olanrewaju et al. (2020) identified hypertension as a traditional cause of CKD.
Previous studies by Asmelash et al. [8], Jitraknatee et al. [9], Bahrey et al. [10], and Kumela et al. [4] showed that hypertension and diabetes are the two major causes of CKD globally. Predictors associated with the development of CKD among DM and hypertensive patients include; advanced age, obesity, diabetes nephropathy, uncontrolled DM, poor knowledge of CKD, first-degree relatives and dyslipidemia, preexisting hypertension, long duration of DM, and hypertension. A Nigerian study conducted by Akokuwebe et al. [11] stated that some factors contributing to the burden of CKD include poor literacy level, inadequate knowledge of risk factors, dearth of CKD prevention programs, poor access to healthcare, late presentation, limited renal replacement therapy, and its unaffordability, as well as harmful socio-cultural practices, some lifestyle risk factors such as age, elevated blood pressure (BP), presence of diabetes mellitus, obesity, habitual intake of analgesics and herbs are associated with CKD development or progression.
Fiseha and Tamir [12], Miller et al. [13], and Kumela et al. [4] stated that timely detection and treatment of CKD can prevent or minimize its complications. Yet, most cases of CKD were not clinically recognized early due to a lack of patients’ awareness about CKD and associated risk factors, as CKD is usually silent till the advanced stages. Screening patients at risk of CKD with objective measures revealed that 29% had CKD, with only 7% having prior knowledge that they were suffering from kidney disease [4]. Studies have established that patients’ awareness and understanding of CKD are persistently low, ranging from 23% to 40% [4, 12, 14, 15]. The global burden of CKD is increasing and is projected to become the 5th most common cause of death worldwide by 2040 [1].
Previous studies conducted by Olanrewaju et al. [16], Fiseha and Tamir [12], and Tegegne et al. [3] have identified diabetes and hypertension as the leading causes of CKD globally. Furthermore, a recent study conducted in Ekiti State, which is the study setting, also confirmed that the leading risk factors for CKD are diabetes and hypertension [17]. Unfortunately, CKD in individuals with these identified risk factors is usually a subtle disease in the early stages, which becomes severe as it progresses. Patients seek care only when severe symptoms are usually manifestations of serious complications. Chronic kidney disease increases mortality risk by 31.1% in patients with diabetes, with the increase based on the severity of the disease, which ultimately imposes a colossal reduction in quality of life and financial burden on the patient, the family, and the society at large. Investigating the factors of CKD in patients with diabetes and hypertension is, therefore, an important initial step in understanding the disease burden and developing additional research priorities, which few studies have done. Thus, the study investigated the prevalence and risk factors of chronic kidney disease among diabetes and hypertensive patients in a tertiary hospital in Ado-Ekiti, Ekiti State.
2. METHODOLOGY
2.1. Study Design and Setting
The study adopted a descriptive research design with a quantitative strategy. The study setting was a Teaching Hospital in Ado Ekiti, Ekiti State, Nigeria.
2.2. Population, Sampling, and Sample Size
The target population for the study was diabetes and hypertensive patients receiving follow-up care at the Teaching Hospital that met the eligibility criteria. The inclusion criteria for this study were diabetes and hypertensive patients who were at least 18 years old and willingly volunteered to participate in the study. Diabetic and hypertensive patients who were already diagnosed with CKD, seriously ill, and pregnant patients with diabetes or hypertension were excluded from the study.
Purposive and convenience sampling techniques were used for the study. A purposive sampling method was used to select the subjects, which were diabetes and hypertensive patients receiving follow-up care at the hospital. At the same time, patients who met the inclusion criteria were conveniently recruited during their clinic follow-up care on Tuesdays for cardiology and Wednesdays for an endocrine clinic.
According to the hospital records of December 2021, approximately 30- 35 and 40-45 patients attended the endocrinology and cardiology clinics weekly, respectively. Based on the prevalence of 14.2% of chronic kidney disease (CKD) reported for the study area by a related study [18], a sample size of 200 was calculated for the study.
2.3. Data Collection
An adapted questionnaire with biophysical measurements and laboratory investigations was used for data collection. The questionnaire was adapted from Ngedahayo et al. [19], Kumela et al. [4], and Katuti [20].
The questionnaire consisted of three sections. Section A derived information on the demographical status of the participants, while Section B included items that assessed the level of awareness of CKD among the participants, while Sections C unveiled the risk factors of CKD. Awareness level of chronic kidney disease (CKD) based on the cumulative score of a participant on awareness-related questions. Participants with scores between 1-10 had inadequate awareness, 11-15 had moderate awareness, and 16-22 as adequate awareness. Similarly, the risk level of CKD was categorized based on cumulative scores on risk-related questions. Scores of 1-10, 11-15, and 16-22 were categorized as low, moderate, and high, respectively.
The biophysical measurements were blood pressure, weight, height, and body mass index (BMI). The following laboratory investigations were also carried out; fasting blood sugar, total cholesterol, low- and high-density lipoproteins, albuminuria, serum creatinine, and glomerular filtration rate estimation.
2.4. Anthropometric Measurements
Body mass index (BMI) was calculated as follows:
 |
Categorization of BMI was as classified: underweight (<18.5kg/m2), ideal weight (18.5- 24.9kg/m2), overweight (25.0-29.9 kg/m2), and obese (≥30 kg/m2).
Participants’ heights and weights were measured using a medical giraffe height measuring stadiometer (Model HMS PL) analog flat weighing scale. Measurements were taken to the nearest 0.1 m and 0.1 kg for height and weight, respectively.
Blood pressure (BP) was measured using a digital sphygmomanometer (Omron M2 ECO) while the participant was in a sitting position after a rest of about 5-10 min was ensured. The BP recordings were recorded to the nearest whole number. Systolic BP of ≤140 mmHg was regarded as normal, readings>140 mmHg were regarded as abnormal, while diastolic BP of ≤90 mmHg and > 90 mmHg were recorded as abnormal, respectively.
2.5. Laboratory Investigations
Fasting blood sugar was measured using the Accu-Chek Active blood glucose monitoring machine after an overnight fast of at least 8 hours was ensured. The patients were gently pricked with a lancet on their left thumb, and a drop of blood was put on the blood film of the test strip after it gave the drop signal. The fasting blood glucose level was taken in milligrams/deciliter to the nearest 0.1.
For serum creatinine, 5 mL of venous blood was collected in lithium heparin sample bottles. The blood sample was centrifuged to separate the plasma from the serum. The serum creatinine values were determined by Jaffe’s method after mixing the serum with alkaline picrate and were read at 520nm using the spectrophotometer machine (SpectroScan 60DV Spectrophotometer) by biotech following the manufacturer’s protocol. Participants’ age, gender, and serum creatinine results were used to calculate the GFR.
Serum creatinine results obtained were used to calculate the Glomerular filtration rate (GFR) using the chronic kidney disease- Epidemiology Creatinine Equation (CKD-EPI Creatinine Equation) according to National Kidney Foundation [13]. The glomerular filtration rate (GFR) was estimated as follows:
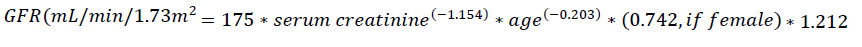 |
Total cholesterol and triglycerides were determined using an enzymatic method, measured at 500 nm using the spectrophotometer machine by biotech following the manufacturer’s protocol. Total cholesterol values of ≤5.18 mmol/L and >5.18 mmol/L were taken as normal and abnormal while triglycerides values of ≤1.69 mmol/L and >1.69 mmol/L were regarded as normal and abnormal, respectively
Low-density lipoprotein was calculated using the Friedewald equation. For High-density lipoprotein, precipitation was done first to remove the non-high-density lipoprotein, which was then subjected to the enzymatic method. Values of ≤ 2.59mmol/L and >2.59mmol/L were taken as normal and abnormal for LDP, while values of between 1-1.6mmol/L and <1mmol/L were normal and abnormal for HDL, respectively
For albuminuria, 10 mL of midstream spot urine was collected in a universal specimen container and was tested using M2 (Microalbumin Urine Strip URS-2). The concentration of the color produced determined the level of albumin in the urine. The test strip container has a color code that includes 10. 30, 50, and 150mg/L. A positive result for albuminuria was taken from 30mg/L.
Glomerular filtration rate (GFR) was categorized as follows: values ≥ 90 as stage 1, 60-89 stage 2, 45-59 as stage 3a, 30-44 as stage 3b, 15-29 as stage 4, and < 15 as stage 5.
2.6. Data and Statistical Analysis
Data were presented in the form of descriptive statistics and inferential statistics. Participants’ responses to the questionnaire were summarized in frequencies and proportions, while the clinical characteristics of respondents were calculated in the form of means and standard deviations. The One-Way Analysis of variance (ANOVA) was used to calculate the significance of means, while the Chi-Square analysis was used to test for relationships. All inferential statistics were carried out at a 95% confidence interval. All data were analyzed using the Statistical Package for Social Statistics (SPSS), version 23.0.
2.7. Ethical Consideration
Before the commencement of the study, approval was obtained from the Ethics Committee of the Ekiti State University Teaching with protocol number EKSUTH/A67/ 2022/01/001. In addition, participants’ autonomy was respected, as the study respondents were adequately informed on the study's process, purposes and objectives. Willing respondents were used for the study without coercing anyone to take part in the research. Participants' physical, mental, and social well-being was safeguarded, and the study did not cause any emotional/mental, or social harm to the respondents. The physical discomfort that characterizes needle pricks during blood sample collection was minimized as samples were drawn by skilled personnel, and asepsis was maintained to prevent the risk of infection. Furthermore, confidentiality was ensured by handling all information cautiously during data collection and processing. For all categories of participants, an informed consent form was filled out and duly signed.
3. RESULTS
Of the 200 participants recruited for the study, a response rate of 98% was attained; thus, 198 participants were used for analysis.
3.1. Socio-demographic and Clinical Profiles of the Study Participants
Most of the study participants, 50% (49) and 57.1% (56) with diabetes and hypertension were above 60 years of age. Most were females (63% for diabetes and 64.3% for hypertension), while over 80% were Christians. Furthermore, 42.9% (diabetes) and 56.1% (hypertension) were educated only up to the secondary school level. With respect to the length of diagnosis of the ailment, 38.8% of patients with diabetes had been diagnosed with the disease for more than 10 years, while 19.4% of participants with hypertension had been diagnosed with the ailment for more than 10 years. More than 90% of the participants had no family history of chronic kidney disease (Table 1).
With the clinical profile of the participants, the average weight of those with diabetes and hypertension was 71.89 kg and 77.76 kg, respectively. Averagely the participants with diabetes had a height of 1.56 m, body mass index (BMI) of 29.64 kg/m2, albuminuria of 24.88 mg/g, creatinine level of 108.96 mg/dL, and glomerular filtration rate (GFR) of 63.85 mL/min. For the participants with hypertension, an average height of 1.58 m, BMI of 30.25 kg/m2, albuminuria of 10.14 mg/g, creatinine of 110.55 mg/d, and GFR of 61.27 mL/min were recorded (Table 2).
| Demographic Variable | - | Patients with Diabetes (n=98) | Patients with Hypertension (n= 98) | ||
|---|---|---|---|---|---|
| - | - | Frequency | % | Frequency | % |
| Age (years) | 25-39 | 14 | 14.3 | 10 | 10.2 |
| 40-60 | 35 | 35.7 | 32 | 32.7 | |
| Above 60 | 49 | 50.0 | 56 | 57.1 | |
| Gender | Male | 36 | 36.7 | 35 | 35.7 |
| Female | 62 | 63.3 | 63 | 64.3 | |
| Level of Education | Primary | 11 | 11.2 | 26 | 26.5 |
| Secondary | 42 | 42.9 | 14 | 14.3 | |
| Tertiary | 38 | 38.8 | 55 | 56.1 | |
| No Formal Education | 7 | 7.1 | 3 | 3.1 | |
| Employment Status | Employed | 12 | 12.2 | 22 | 22.4 |
| Business/Private | 50 | 51.0 | 34 | 34.7 | |
| Not Employed | 9 | 9.2 | 11 | 11.2 | |
| Retired | 27 | 27.6 | 31 | 31.6 | |
| Average Monthly Income |
20,000-40,000 | 32 | 32.7 | 24 | 24.5 |
| 41,000-60,000 | 44 | 44.9 | 43 | 43.9 | |
| 61,000-80,000 | 3 | 3.1 | 14 | 14.3 | |
| 81,0000-100,000 | - | - | 3 | 3.1 | |
| Above 100,000 | 19 | 19.4 | 14 | 14.3 | |
| Religion | Islam | 12 | 12.2 | 17 | 17.3 |
| Christianity | 86 | 87.8 | 81 | 82.7 | |
| Tribe | Yoruba | 96 | 98.0 | 91 | 92.9 |
| Igbo | - | - | 7 | 7.1 | |
| Hausa | 2 | 2.0 | - | - | |
| Others | - | - | - | - | |
| Marital Status | Married | 98 | 100 | 74 | 75.5 |
| Widowed | - | - | 24 | 24.5 | |
| Health Insurance Status | No Health Insurance | 71 | 72.4 | 76 | 77.6 |
| Private Insurance | 18 | 18.4 | - | - | |
| NHIS | 9 | 9.2 | 22 | 22.4 | |
| Duration of Diabetes |
1-2years | 9 | 9.2 | 3 | 3.1 |
| 3-4 years | 7 | 7.1 | 6 | 6.1 | |
| 5-6 years | 34 | 34.7 | 4 | 4.1 | |
| 7-9 years | 10 | 10.2 | 4 | 4.1 | |
| Above 10 years | 38 | 38.8 | - | - | |
| Not Diabetic | - | - | 81 | 82.7 | |
| Duration of Hypertension | 1-2years | 7 | 7.1 | 3 | 3.1 |
| 3-4 years | 10 | 10.2 | 35 | 35.7 | |
| 5-6 years | 3 | 3.1 | 25 | 25.5 | |
| 7-9 years | 16 | 16.3 | 16 | 16.3 | |
| Above 10 years | - | - | 19 | 19.4 | |
| Not Hypertensive | 62 | 63.3 | - | - | |
| Family History of CKD | Yes | 2 | 2 | 3 | 3.1 |
| No | 96 | 98 | 95 | 96.9 | |
| Variable | Patients with Diabetes | Patients with Hypertension | Sig | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | SD | Mean | Min | Max | SD | ||
| Weight (kg) | 71.80 | 54 | 110 | 10.8 | 77.76 | 47 | 110 | 15.3 | 0.002 |
| Height (m) | 1.56 | 1.40 | 1.7 | 0.1 | 1.50 | 1.1 | 1.72 | 0.7 | < 0.001 |
| BMI kg/m2 | 29.64 | 20.80 | 45.2 | 4.4 | 30.25 | 18.7 | 39 | 5.5 | 0.392 |
| Systolic blood pressure mm/Hg | 137.20 | 110 | 180 | 17.4 | 142.49 | 107 | 180 | 20.1 | 0.050 |
| Diastolic blood pressure mm/Hg | 83.22 | 60 | 110 | 7.9 | 87.50 | 63 | 112 | 12.7 | 0.005 |
| Albuminuria mmol/L | 24.88 | 1 | 100 | 40.3 | 10.14 | 1 | 100 | 26.1 | 0.003 |
| Creatinine mmol/L | 108.96 | 65.50 | 378.6 | 46.8 | 110.55 | 50 | 305 | 40.9 | 0.801 |
| GFR (mL/min) | 63.85 | 15 | 110 | 20.0 | 61.27 | 14 | 114 | 19.3 | 0.359 |
| Triglycerides mmol/L | 1.80 | 0.22 | 72 | 7.2 | 1.41 | 0.46 | 2.80 | 0.6 | 0.595 |
| High density lipoprotein mmol/L |
1.15 | 0.80 | 1.7 | 0.25 | 1.20 | 0.75 | 1.81 | 0.23 | 0.203 |
| Low density lipoprotein mmol/L |
2.05 | 1.00 | 4.63 | 0.87 | 2.39 | 1.06 | 5.56 | 1.03 | 0.015 |
| Total cholesterol mmol/L |
3.68 | 2.33 | 6.73 | 1.21 | 4.21 | 2.30 | 7.34 | 1.21 | 0.002 |
Generally, weight, BMI, BP, LDL, and total cholesterol values were significantly higher in the participants with hypertension than in those with diabetes. However, albuminuria and height values were significantly higher among participants with diabetes than those with hypertension. Serum creatinine, HDL, triglyceride, and GFR values did not differ significantly between the two categories of participants (Table 2).
3.2. Prevalence of CKD
Among the participants with diabetes, the GFR rating revealed that 11.2%, 49.0%, 29.6%, 2.0%, and 8.2% were observed to be in stage 1, stage 2, stage 3a, stage 3b, and stage 4, respectively. None of the participants was observed to be in stage 5. None of the participants with diabetes was observed to have ESRF. At the same time, 39.8% of them showed moderate or severe CKD; all the clinical characteristics investigated were significantly associated with the GFR ratings of the participants (Table 3).
In the case of the participants with hypertension, their GFR revealed that 12.2% were in stage 1, 30.6% in stage 2, 45.9% in stage 3a, 8.2% in stage 3b, 2.0% in stage 4, and 1.0% in stage 5. Only 1% of the participants with hypertension were observed to have ESRF, while 42.8% showed moderate or severe CKD. Among the clinical characteristics of the participants, only systolic blood pressure (X2= 11.99, p= 0.035), albuminuria (X2= 25.91, p< 0.001), LDL (X2= 13.07, p= 0.0-23) were observed to be significantly associated with GFR rating of the participants (Table 4).
| - | GFR RATING | X2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | ||||
| BMI | Ideal weight | 1 | 7 | 0 | 0 | 0 | 0 | 28.31 | <0.001 |
| Overweight | 10 | 13 | 21 | 2 | 4 | 0 | |||
| Obese | 0 | 28 | 8 | 0 | 4 | 0 | |||
| Systolic blood pressure | Abnormal | 0 | 24 | 1 | 0 | 0 | 0 | 29.77 | <0.001 |
| Normal | 11 | 24 | 28 | 2 | 8 | 0 | |||
| Diastolic blood pressure | Abnormal | 0 | 11 | 0 | 0 | 0 | 0 | 12.91 | 0.012 |
| Normal | 11 | 37 | 29 | 2 | 8 | 0 | |||
| Albuminuria | Stage A1 | 4 | 36 | 28 | 0 | 0 | 0 | 39.10 | <0.001 |
| Stage A2 | 7 | 12 | 1 | 2 | 8 | 0 | |||
| Fasting blood sugar | Abnormal | 3 | 38 | 17 | 2 | 8 | 0 | 17.34 | 0.002 |
| Normal | 8 | 10 | 12 | 0 | 0 | 0 | |||
| Triglyceride | Abnormal | 6 | 7 | 2 | 2 | 8 | 0 | 42.38 | <0.001 |
| Normal | 5 | 41 | 27 | 0 | 0 | 0 | |||
| High-density lipoprotein | Abnormal | 0 | 20 | 11 | 0 | 0 | 0 | 12.48 | 0.014 |
| Normal | 11 | 28 | 18 | 2 | 8 | 0 | |||
| Low-density lipoprotein | Abnormal | 7 | 2 | 10 | 0 | 0 | 0 | 27.53 | <0.001 |
| Normal | 4 | 46 | 19 | 2 | 8 | 0 | |||
| Total cholesterol | Abnormal | 7 | 0 | 3 | 0 | 0 | 0 | 40.87 | <0.001 |
| Normal | 4 | 48 | 26 | 2 | 8 | 0 | |||
| CKD prevalence and stages (%) | 11.2 | 49.0 | 29.6 | 2.0 | 8.2 | 0 | - | - | |
| Participants without CKD | 60.2% | ||||||||
| Participants with moderate or severe CKD | 39.8% | ||||||||
| Participants with ESRF | 0% | ||||||||
| - | GFR | X2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | ||||
| BMI | Ideal weight | 0 | 6 | 8 | 0 | 0 | 1 | 16.33 | 0.091 |
| Overweight | 4 | 4 | 17 | 3 | 2 | 0 | |||
| Obese | 8 | 20 | 20 | 5 | 0 | 1 | |||
| Systolic blood pressure | Abnormal | 11 | 13 | 24 | 5 | 0 | 0 | 11.99 | 0.035 |
| Normal | 1 | 17 | 21 | 3 | 2 | 1 | |||
| Diastolic blood pressure | Abnormal | 4 | 17 | 31 | 4 | 0 | 0 | 9.48 | 0.091 |
| Normal | 8 | 13 | 14 | 4 | 2 | 1 | |||
| Albuminuria | Stage A1 | 9 | 30 | 37 | 8 | 0 | 0 | 25.91 | <0.001 |
| Stage A2 | 3 | 0 | 8 | 0 | 2 | 1 | |||
| Triglyceride | Abnormal | 0 | 8 | 20 | 2 | 0 | 0 | 11.01 | 0.051 |
| Normal | 12 | 22 | 25 | 6 | 2 | 1 | |||
| High-density lipoprotein | Abnormal | 0 | 4 | 3 | 0 | 0 | 0 | 3.52 | 0.621 |
| Normal | 12 | 26 | 42 | 8 | 2 | 1 | |||
| Low-density lipoprotein | Abnormal | 8 | 8 | 12 | 0 | 0 | 0 | 13.07 | 0.023 |
| Normal | 4 | 22 | 33 | 8 | 2 | 1 | |||
| Total cholesterol | Abnormal | 1 | 8 | 3 | 0 | 0 | 0 | 8.82 | 0.117 |
| Normal | 11 | 22 | 42 | 8 | 2 | 1 | |||
| GRF stages (%) | 12.2 | 30.6 | 45.9 | 8.2 | 2.0 | 1.0 | - | - | |
| Participants without CKD | 42.8% | ||||||||
| Participants with moderate or severe CKD | 56.1% | ||||||||
| Participants with ESRF | 1.0% | ||||||||
Apart from the family history of CKD (X2= 2.18, p= 0.712), which showed no significant relationship with GFR rating, all the socio-demographic characteristics of the participants with diabetics were significantly related. With respect to the participants with hypertension, only gender (X2= 14.83, p= 0.011) and duration of hypertension (X2= 36.58, p= 0.008) showed a significant association with the GFR rating (Table 5).
3.3. Participants’ Levels of Awareness and Involvement in Risk Factors of CKD
The level of awareness of CKD among the participants with diabetes revealed that 57.1% had inadequate awareness, 29.1% had moderate awareness, and 13.2% had adequate awareness of CKD. In the case of the participants with hypertension, 40.8% showed inadequate awareness, 32% showed moderate awareness, and 23.5% showed adequate. Apart from the family history of CKD (X2= 4.86, p= 0.09), all the factors investigated showed significant association with the level of awareness of CKD among the participants with diabetes. In the case of participants with hypertension, only age (X2= 437.18, p< 0.001), level of education (X2= 44.20, p< 0.001), and health insurance status (X2= 3.87, p< 0.001) showed significant association with the level of awareness of CKD (Table 6).
| Variables | GFR Rating | X2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| - | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | - | - | |
| Participants with Diabetes | |||||||||
| Age (years) | 25-39 | 0 | 14 | 0 | 0 | 0 | - | 36.15 | <0.001 |
| 40-60 | 10 | 15 | 6 | 1 | 3 | - | - | - | |
| > 60 | 1 | 19 | 23 | 1 | 5 | - | - | - | |
| Gender | Male | 3 | 9 | 14 | 2 | 8 | - | 35.99 | <0.001 |
| Female | 8 | 39 | 15 | 0 | 0 | - | - | - | |
| Level of Education | No Formal Education | 0 | 0 | 7 | 0 | 0 | - | 30.40 | 0.002 |
| Primary | 1 | 5 | 3 | 0 | 2 | - | - | - | |
| Secondary | 7 | 19 | 8 | 2 | 6 | - | - | - | |
| Tertiary | 3 | 24 | 11 | 0 | 0 | - | - | - | |
| Health insurance | No | 11 | 24 | 26 | 2 | 8 | - | 25.31 | 0.001 |
| Yes | 0 | 24 | 3 | 0 | 0 | - | - | - | |
| Duration of Diabetes (years) | 1-2 | 0 | 1 | 8 | 0 | 0 | - | 48.83 | <0.001 |
| 3-4 | 0 | 7 | 0 | 0 | 0 | - | - | - | |
| 5-6 | 8 | 18 | 6 | 0 | 2 | - | - | - | |
| 7-9 | 0 | 10 | 0 | 0 | 0 | - | - | - | |
| ≥ 10 | 3 | 12 | 15 | 2 | 6 | - | - | - | |
| Family history of CKD | Yes | 0 | 2 | 0 | 0 | 0 | - | 2.18 | 0.712 |
| No | 11 | 46 | 29 | 2 | 8 | - | - | - | |
| Participants with Hypertension | |||||||||
| Age | 25-39 | 0 | 4 | 6 | 0 | 0 | 0 | 15.78 | 0.106 |
| 40-60 | 8 | 10 | 14 | 0 | 0 | 0 | - | - | |
| > 60 | 4 | 16 | 25 | 8 | 2 | 1 | - | - | |
| Gender | Male | 1 | 12 | 19 | 0 | 2 | 1 | 14.83 | 0.011 |
| Female | 11 | 18 | 26 | 8 | 0 | 0 | - | - | |
| Level of Education | No Formal Education | 3 | 8 | 10 | 5 | 0 | 0 | 18.76 | 0.225 |
| Primary | 0 | 3 | 11 | 0 | 0 | 0 | - | - | |
| Secondary | 9 | 19 | 21 | 3 | 2 | 1 | - | - | |
| Tertiary | 0 | 0 | 3 | 0 | 0 | 0 | - | - | |
| Health Insurance Status | No | 8 | 23 | 34 | 8 | 2 | 1 | 4.12 | 0.533 |
| Yes | 4 | 7 | 11 | 0 | 0 | 0 | - | - | |
| Duration of hypertension | 1-2 | 0 | 0 | 3 | 0 | 0 | 0 | 36.58 | 0.008 |
| 3-4 | 9 | 9 | 16 | 1 | 0 | 0 | - | - | |
| 5-6 | 0 | 7 | 11 | 4 | 2 | 1 | - | - | |
| 7-9 | 0 | 4 | 12 | 0 | 0 | 0 | - | - | |
| ≥ 10 | 3 | 10 | 3 | 3 | 0 | 0 | - | - | |
| Family history of CKD | Yes | 0 | 0 | 3 | 0 | 0 | 0 | 3.65 | 0.602 |
| No | 12 | 30 | 42 | 8 | 2 | 1 | - | - | |
| Variable | Awareness Level | X2 | p | |||
|---|---|---|---|---|---|---|
| - | - | Inadequate | Moderate | Adequate | - | |
| Participants with Diabetes | ||||||
| Age (years) | 25-39 | 0 | 7 | 7 | 29.23 | <0.001 |
| 40-60 | 24 | 10 | 1 | - | - | |
| > 60 | 32 | 12 | 5 | - | - | |
| Gender | Male | 29 | 6 | 1 | 13.39 | 0.001 |
| Female | 27 | 23 | 12 | - | - | |
| Level of education | No formal education | 0 | 3 | 2 | 28.73 | <0.001 |
| Primary | 6 | 4 | 3 | - | - | |
| Secondary | 15 | 18 | 5 | - | - | |
| Tertiary | 35 | 4 | 3 | - | - | |
| Health insurance | No | 46 | 20 | 5 | 19.89 | 0.001 |
| Yes | 10 | 9 | 8 | - | - | |
| Duration of diabetes (years) | 1-2 | 8 | 1 | 0 | 25.94 | 0.001 |
| 3-4 | 0 | 4 | 3 | - | - | |
| 5-6 | 15 | 15 | 4 | - | - | |
| 7-9 | 10 | 0 | 0 | - | - | |
| ≥ 10 | 23 | 9 | 6 | - | - | |
| Family history of CKD | Yes | 0 | 2 | 0 | 4.86 | 0.09 |
| No | 56 | 27 | 13 | - | - | |
| % Awareness level | 57.1 | 29.1 | 13.2 | - | - | |
| Participants with Hypertension | ||||||
| Age (years) | 25-39 | 7 | 3 | 0 | 37.18 | <0.001 |
| 40-60 | 0 | 16 | 16 | - | - | |
| > 60 | 33 | 16 | 7 | - | - | |
| Gender | Male | 17 | 14 | 4 | 4.45 | 0.11 |
| Female | 23 | 21 | 19 | - | - | |
| Level of education | No formal education | 23 | 2 | 1 | 44.20 | <0.001 |
| Primary | 5 | 7 | 2 | - | - | |
| Secondary | 9 | 26 | 20 | - | - | |
| Tertiary | 3 | 0 | 0 | - | - | |
| Health insurance | No | 29 | 31 | 16 | 3.87 | 0.14 |
| Yes | 11 | 4 | 7 | - | - | |
| Duration of hypertension (years) | 1-2 | 3 | 0 | 0 | - | - |
| 3-4 | 2 | 17 | 16 | 42.90 | <0.001 | |
| 5-6 | 17 | 8 | 0 | - | - | |
| 7-9 | 12 | 2 | 2 | - | - | |
| ≥ 10 | 6 | 8 | 5 | - | - | |
| Family history of CKD | Yes | 0 | 3 | 0 | 5.571 | 0.06 |
| No | 40 | 32 | 23 | - | - | |
| % Awareness level | 40.8 | 35.7 | 23.5 | - | - | |
Generally, the participants’ level of involvement in risk factors of CKD was generally low or moderate. Among participants with diabetes, 27.6% were observed to have low-risk involvement risk factors of CKD, while 72.4% had moderate risk. For the participants with hypertension, 28.6% and 71.4% showed low and moderate risk to risk factors for CKD, respectively. For the participants with diabetes, only gender (X2= 18.75, p< 0.001) was observed to be significantly associated with risk factor involvement levels, while among participants with hypertension, only age (X2= 14.64, p< 0.001) showed significant association (Table 7).
A comparison of the CKD awareness and risk factor scores of the two categories of participants revealed no significant difference. However, a higher average awareness score was observed among the participants with hypertension, while higher risk factor scores were observed among participants with diabetes (Table 8).
| Variables | - | Risk Involvement Levels | X2 | p | ||
|---|---|---|---|---|---|---|
| - | - | Low | Moderate | High | - | - |
| Participants with Diabetes | ||||||
| Age (years) | 25-39 | 4 | 10 | 0 | 5.11 | 0.28 |
| 40-60 | 12 | 23 | 0 | - | - | |
| > 60 | 5 | 34 | 0 | - | - | |
| Gender | Male | 2 | 30 | 0 | 18.75 | <0.001 |
| Female | 29 | 37 | 0 | - | - | |
| Level of education | No formal education | 3 | 6 | 0 | 12.31 | 0.06 |
| Primary | 7 | 33 | 0 | - | - | |
| Secondary | 19 | 23 | 0 | - | - | |
| Tertiary | 2 | 5 | 0 | - | - | |
| Health insurance | No | 21 | 50 | 0 | 2.99 | 0.56 |
| Yes | 10 | 17 | 0 | - | - | |
| Duration of diabetes | 1-2 | 1 | 6 | 0 | 11.38 | 0.18 |
| 3-4 | 2 | 5 | 0 | - | - | |
| 5-6 | 11 | 21 | 0 | - | - | |
| 7-9 | 6 | 7 | 0 | - | - | |
| ≥ 10 | 11 | 28 | 0 | - | - | |
| Family history of CKD | Yes | 2 | 4 | 0 | 5.37 | 0.07 |
| No | 25 | 67 | 0 | - | - | |
| % Risk Involvement Levels | 27.6 | 72.4 | 0 | - | - | |
| Participants with Hypertension | ||||||
| Age (years) | 25-39 | 0 | 10 | 0 | 15.64 | <0.001 |
| 40-60 | 17 | 15 | 0 | - | - | |
| > 60 | 11 | 45 | 0 | - | - | |
| Gender | Male | 6 | 29 | 0 | 3.48 | 0.07 |
| Female | 22 | 41 | 0 | - | - | |
| Level of education | No formal education | 0 | 3 | 0 | 6.20 | 0.10 |
| Primary | 5 | 21 | 0 | - | - | |
| Secondary | 2 | 12 | 0 | - | - | |
| Tertiary | 21 | 34 | 0 | - | - | |
| Health insurance | No | 20 | 56 | 0 | 0.84 | 0.42 |
| Yes | 8 | 14 | 0 | - | - | |
| Duration of hypertension (years) | 1-2 | 1 | 2 | 0 | 9.24 | 0.06 |
| 3-4 | 16 | 19 | 0 | - | - | |
| 5-6 | 3 | 22 | 0 | - | - | |
| 7-9 | 3 | 13 | 0 | - | - | |
| ≥ 10 | 5 | 14 | 0 | - | - | |
| Family history of CKD | Yes | 1 | 2 | 0 | 0.03 | 0.85 |
| No | 27 | 68 | 0 | - | - | |
| % Risk involvement level | 28.6 | 71.4 | 0 | - | - | |
| - | Mean | Std. Deviation | 95% Confidence Interval for Mean | Min. | Max. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||||||
| Awareness | Hypertension | 12.1837 | 5.81918 | 11.0170 | 13.3503 | 2.00 | 22.00 | |||||||
| Diabetes | 10.8163 | 4.67356 | 9.8793 | 11.7533 | 2.00 | 22.00 | ||||||||
| Risk level | Hypertension | 10.5102 | 2.91899 | 9.9250 | 11.0954 | 3.00 | 15.00 | |||||||
| Diabetes | 10.9796 | 2.64372 | 10.4496 | 11.5096 | 4.00 | 16.00 | ||||||||
| ANOVA | ||||||||||||||
| - | Sum of Squares | Df | Mean Square | F | Sig. | |||||||||
| Awareness | Between Groups | 91.612 | 1 | 91.612 | 3.289 | .071 | ||||||||
| Within Groups | 5403.388 | 194 | 27.853 | - | - | |||||||||
| Risk level | Between Groups | 10.796 | 1 | 10.796 | 1.392 | .239 | ||||||||
| Within Groups | 1504.449 | 194 | 7.755 | - | - | |||||||||
4. DISCUSSION
The socio-demographic characteristics of the participants revealed that with respect to age, 50% and 57.1% of diabetes and hypertensive participants were above 60 years; this result is similar to findings of a similar study conducted in Northern Thailand by Jitraknatee et al. [9] where the mean age of the study population was 61.6. The majority of the participants were females and married; this is similar to the findings of Tegegne et al. [3] and Bahrey et al. [10]. Furthermore, 36.7% of the diabetes participants have hypertension as a comorbidity; this is consistent with the findings of Asmelash et al. [8] in a study conducted in Ethiopia, where 66.8% of participants had a comorbidity. Only 2% and 3.1% of diabetes and hypertensive participants have a family history of CKD, which is lower than the findings of 19.2% obtained by Fiseha and Tamir [12] in a similar study conducted in Ethiopia.
Participants with GFR below 60mls/min and albuminuria of >30mg/g were considered to have CKD, as mentioned by Tegegne et al. [3] and Kumela et al. [4]. In this study, 39.8% of people with diabetes have CKD, while 56.1% of hypertensives have CKD using CKD epidemiology collaboration (CKD-EPI) to calculate the eGFR. This might be a sequel to the fact that they are already diagnosed with diabetes and hypertension, with some having both disease conditions at 68% and 42%, respectively, with an associated increase in the prevalence of some of the major risk factors identified in herbal concoction use 77.6% and 73.5%, respectively and use of NSAID 74.5% and 78.6%, respectively. Conversely, a higher prevalence of 61.3% was obtained among diabetes patients in Northern Thailand [9].
The study findings were at variance with the prevalence of 18.7% obtained by Fiseha and Tamir [12] among diabetes participants and 26% obtained by Kumela et al. [4] among both DM and hypertensive participants. Correspondingly, a study conducted by Abd ElHafeez et al. [21] in Egypt among high-risk groups obtained a prevalence of 24.7%. The difference observed in the prevalence rates might be related to the variance in study subjects and setting, the formula used to calculate the estimated glomerular filtration rate, the difference in the sample size as well as the fact that CKD staging was determined by both eGFR and albuminuria of greater than 30mg/g. The study was conducted in an outpatient department, and the eGFR was calculated using CKD epidemiology collaboration (CKD-EPI).
Inadequate awareness and knowledge of CKD is the leading barrier to the timely diagnosis and prevention of disease progression [22]. As revealed in the study, the level of CKD awareness among the study participants was inadequate, 57.1% and 40.8% among diabetes and hypertensives patients, respectively. This is consistent with the study conducted in Rwanda by Ngendahayo et al. [19], where low awareness (55%) of CKD was reported among university students. Similarly, a hospital-based study conducted by Kumela et al. [4]) in Ethiopia reported an awareness level of 36.5%; however, the study reported a high level of awareness of 75% among diabetes participants at stage 4 of CKD. Additionally, a systematic study and meta-analysis study conducted by Chu et al. [23] in the USA established a low level of awareness of 19.2%. Furthermore, Asmelash et al. [8], in their community-based study conducted in North West Ethiopia, reported 68.7% good knowledge of CKD. The differences in the results are probably due to the different study settings, sample sizes, and populations. It is expected that hospital-based participants who might have had better access to health education than the general community may have better knowledge than others.
The study participants were diabetes and hypertension patients, the leading risk factors of CKD. In this study, most diabetes patients were also hypertensive, while almost half of the hypertensive participants also had diabetes. These findings corroborate the findings of Ibitoba et al. [17] in a study conducted in Ado-Ekiti, Ekiti State, Southwestern Nigeria, where diabetes (41.9%) and hypertension (64.7%) were identified as the leading risk factors for CKD. This contrasts with another similar study conducted in the Northcentral, Nigeria, by Olanrewaju et al. [16], where only 4.1% and 23.7% of diabetes and hypertension were identified as risk factors for CKD, respectively [24].
In addition, advanced age has been identified as another risk factor for CKD; in this study, 50% and 57.1% of diabetics and hypertensives, respectively, were above the age of 60 years. This is in line with a study conducted in Ethiopia by Bahrey et al. [10], where it was established that an age greater than 60 years is a risk factor for CKD. Additionally, participants in the study were using herbal concoctions and non-steroidal anti-inflammatory drugs (NSAIDs), risk factors for CKD. These findings are similar to the results of Ibitoba et al. [17] and Shiferaw et al. [25], which showed that habitual use of NSAIDS is two times more likely to result in CKD.
The study showed that the average mean score and standard deviation of the identified clinical risk factors include BMI 29.64±4.39 and 30.25±5.49 among diabetes and hypertensive participants, respectively. This is at par with a systematic review and meta-analysis study conducted in Ethiopia by Shiferaw et al. [25], which showed that patients with BMI ≥ 30 kg/m2 were 2.51 times more likely to develop CKD than those with BMI 18.5-24.9 kg/m2. The study also identified fasting blood sugar as a risk factor with a mean and standard deviation of 156.87±51.10 for diabetes participants. This is consistent with another similar study conducted by Kumela et al. [4], which showed that FBS of >150mg/dl was independently associated with CKD, likewise two of the studies reviewed in the systematic review and meta-analysis study conducted in Ethiopia by Shiferaw et al. [25] which showed that people with FBS >150mg/dl were statistically associated with CKD in patients with diabetes. Likewise, the study showed a mean score and standard deviation of systolic blood pressure for hypertensive participants as 142.49±20.12. This agrees with a study by Kumela et al. [4], which found that 83% of hypertensive participants with a long duration of hypertension and uncontrolled hypertension are independent predictors of CKD. Likewise, four studies reviewed in the systematic review and meta-analysis study conducted in Ethiopia by Shiferaw et al. [25] showed that people with SBP >140mmHg were 3.26 likely to develop CKD.
The relationship between participants’ socio-demographic characteristics and CKD awareness showed a significant relationship between both participants’ socio-demographic factors (level of education) and awareness of chronic kidney disease. This implies that the participants' level of education mirrors their awareness of chronic kidney disease. This is similar to the knowledge level of participants from the studies of Asmelash et al. [8] and Kumela et al. [4], where good knowledge was recorded among participants with higher levels of education. However, the study finding was at variance with Ngendahayo et al. [19] and Asmelash et al. [8]. The relationship observed might be due to the participants' different educational backgrounds ranging from primary to tertiary educational levels.
Furthermore, employment status was found to be significant, with the majority of the participants running their businesses or working in private firms; this could be associated with the level of income which is also significantly associated with awareness of CKD. It is opined that employment status and income level influence better access to health information, thus improving health literacy and awareness of CKD. This was supported by the study of Ngendahayo et al. [19], where a significant relationship between occupation and knowledge of CKD was established. This probably implied that occupation might influence more preventive activities. Duration of hypertension was also found to be significantly associated with awareness of CKD; this may be because people who receive follow-up care might have more access to health information, such as the CKD risk factors and preventive measures. However, this finding was at variance with the study by Kumela et al. [4] that showed no significant relationship between hypertension duration and CKD awareness.
The relationship between participants’ clinical characteristics includes body mass index, systolic and diastolic blood pressure, albuminuria, fasting blood sugar, lipid profile, and development of CKD. Diabetes and hypertension have been established in studies as the two principal causes of CKD. Poorly controlled or untreated diabetes mellitus and hypertension progress rapidly to CKD [24]. According to Shiferaw et al. [25], the predictors of CKD among diabetes and hypertensive patients includes clinical factors, which have also been established in the study. Body mass index (BMI) was significantly associated with the onset of CKD among diabetes participants. This is supported by a previous study by Shiferaw et al. [25], where patients with BMI ≥30 kg/m2 were 2.51 times more likely to develop CKD than those with BMI 18.5–24.9 kg/m2. Geletu et al. [26] also posited that high BMI was significantly associated with CKD. The possible reason could be that an increased BMI promotes kidney damage through direct mechanisms like hemodynamic and hormonal effects, which might also lead to glomerular hyperfusion and glomerular hyperfiltration; as a result, glomerular capillary pressure increases, which may subsequently result in increased urinary albumin excretion, followed by overt proteinuria and declining GFR.
Additionally, glycemic control seems to be the most important determinant factor of developing diabetes-related complications and CKD risk, particularly among patients with type 2 diabetes. Fasting blood glucose was significantly associated with the onset of CKD among the diabetes respondents. This is in line with the study of Kumela et al. [4], where fasting blood sugar of >150mg/dl was independently associated with CKD. Shiferaw et al. [25]) likewise posited that poor glycemic control (HbA1c >7%) is the most important risk factor for diabetic nephropathy.
The study revealed a significant level of lipid profile components, including triglycerides, high-density lipoprotein, low-density lipoprotein, and total cholesterol. This is supported by Shiferaw et al. [25] who opined that CKD onset can be predicted by the typical atherogenic lipid profile, including high triglycerides, which was directly found to be associated with an increased probability of developing reduced eGFR.
Furthermore, the study established a significant relationship between CKD with uncontrolled blood pressure, particularly among diabetics and hypertensives. This is similar to the finding of Kumela et al. [4], where the long duration of hypertension and uncontrolled blood pressure (BP>140/90 mmHg) were independent predictors of CKD. Shiferaw et al. [25] also showed that systolic blood pressure of more than 140mmHg was a strong predictor of CKD, predominantly in diabetes and hypertensive patients, establishing that early treatment of hypertension is vital in the prevention of cardiovascular disease and the progression of diabetic renal disease and retinopathy. Likewise, the benefit of tight blood pressure control may be as great or greater than stern glycemic control.
CONCLUSION
The research study determined the prevalence of CKD, assessed the awareness level, and identified the risk factors of chronic kidney disease (CKD) among diabetes and hypertensive patients receiving follow-up treatment in a tertiary hospital. It was revealed in the study that the prevalence of CKD among diabetes and hypertensive participants were 39.8% and 57.1%, respectively. The study established a low level of awareness of CKD among the study population, while the identified risk factors of CKD were; diabetes mellitus, hypertension, herbal concoction, and NSAID. Furthermore, it was observed that there was a significant relationship between participants’ educational background, age, employment status, income level and duration of hypertension, and awareness level of CKD. Body mass index, lipid profile, fasting blood sugar, and blood pressure were the major identified clinical risk factors that significantly predicted CKD development. The study concluded that the prevalence of CKD is high at 39.8% and 57.1% among diabetes and hypertensive participants, respectively, and that body mass index, lipid profile, fasting blood sugar, and blood pressure significantly predicted the development of CKD among the studied population.
Based on the outcomes of this study, prevention is key when it comes to kidney disease; hence, all stakeholders, including nurses and other healthcare providers, should reinforce preventive activities. Community awareness and campaigns on kidney health should be carried out as often as possible, and risk assessment should be done to recognize CKD cases at the initial stages, as this will facilitate early interventions to slow down its progression and thereby reduce the financial burdens and morbidity and mortality associated with CKD.
The most at-risk population, which includes diabetes and hypertensive individuals, should be well counseled on the importance of compliance with the treatment regimen and the need to avoid other risk factors to prevent delayed renal involvement and progression of CKD stages. Finally, nurses and other healthcare professionals should ensure proper monitoring of diabetes and hypertensive patients through adequate health education in compliance with exercise, diet, medications, and regular medical follow-ups. Additionally, blood pressure should be maintained at 130/80mmHg, HbA1c below 7, and BMI 18.5-24.9.
LIST OF ABBREVIATIONS
| CKD | = Chronic Kidney Disease |
| GFR | = Glomerular Filtration Ratebody mass index (BMI) |
| BMI | = Body Mass Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This Study was approved by the Ethics Committee of the Ekiti State University Teaching with protocol number EKSUTH/A67/2022/01/001.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used per the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data used to support the findings of this study are included within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


