All published articles of this journal are available on ScienceDirect.
Evaluation the Effect of Chronic Obestatin Therapy on the Serum Glucose, Insulin And Lipid Levels in Type 2 Diabetic Rats
Abstract
Background:
Diabetes mellitus (DM) is one of the most common health disorders, which has become increasingly common in recent years. Type 2 diabetes affects about 90-95% of all diabetic patients, and is often associated with obesity and insulin resistance in most patients. The medical treatment aims to reduce insulin resistance and increase the production of insulin by pancreatic β-cells.
Obestatin is a new hormone encoded by the Preghrelin gene. Obestatin is an anorexic hormone that reduces food intake. It has also been shown to play an important role in regulating glucose and lipid levels in the blood.
Study Aim:
Our study aims to evaluate the therapeutic benefit of obestatin in rats with experimental type 2 diabetes in reducing blood glucose and improving insulin levels, and its effect on insulin resistance, TG, TC and pancreatic β-cell survival.
Methods:
A total of 30 male Wister rats (150 -200g) were randomly divided into three groups: group I (control group), group II (T2DM group) induced by administration fructose solution 10% for 14 days, and single injection IP of streptozotocin (STZ) (35 mg/Kg), group III (T2DM treated with obestatin) (25 μg/kg) IP twice daily for 30 days. Blood samples were collected at the end of the experiment by terminal intracardiac sampling for bioassays to estimate fasting glucose, insulin, triglyceride (TG), total cholesterol (TC), and assessment of HOMA-IR. Body weight was also measured. Mean ± STD was calculated. The statistical significance of differences across the groups was determined by one-way ANOVA followed by a post Hoc Turkey’s test. The differences were considered significant at 0.05˃P.
Results:
After 30 days of obestatin treatment, the diabetic group showed a significant increase in glucose, TG, TC and HOMA-IR values and a significant decrease in insulin levels compared to the control group. In comparison, the obestatin-treated group of diabetic patients showed a significant decrease in glucose, TG and TC levels, with a slight increase in the insulin level compared to the diabetic group. In addition, the histological study (H&E) of isolated pancreatic tissue from the second group showed deformed, shrunken Langerhans islets with significant loss of their β- cells, and some cells with vacuolated cytoplasm. Moreover, the histological features of the treatment group were somewhat similar to those of the control group.
Conclusion:
The results of our study showed the efficacy of obestatin as a treatment in reducing the levels of all glucose, triglycerides and total cholesterol in the blood to normal limits in induced experimental rats with type 2 diabetes. Moreover, the improvement of insulin levels in the blood, and the results of the histological study showed an improvement in the size of the islet and an increase in the number of β-cells. Thus, obestatin can be used as a promising target in the treatment of metabolic diseases such as diabetes and obesity.
1. INTRODUCTION
Diabetes is among the top ten causes of death in the world [1]. In 2019, the statistic indicates that 463 million people suffer from diabetes and it is expected to rise to 578 million in 2030. There are three main categories of diabetes: type 1, type 2 and gestational diabetes mellitus (GDM) [2]. The most common type is T2DM, which is characterized by chronic hyperglycemia resulting from insulin resistance and lack of insulin secretion [3]. T2DM is often associated with central obesity and fat mass resulting in increased secretion of pro-inflammatory cytokines. This contributes to a disruption of insulin signaling in cells, causing insulin resistance, which is a precursor to T2DM [4].
Controlling blood glucose or raising tissue insulin sensitivity is the only way to treat T2DM [5], this raises the need for developing a new strategy for the prevention and treatment of T2DM.
In 2005, obestatin was discovered by Zhang et al., which is an anorexic hormone released from the gastrointestinal tract and also in the spleen, mammary gland, and adipose tissue. It is a 23-amino acid peptide, it has been reported to bind to G Protein-coupled Receptor-39 (GPR39) [6-8].
Studies have shown that obestatin acts as a physiological antagonist of ghrelin, by decreasing body weight and food intake and by delaying gastric emptying [9, 10]. Obstatin enhances cell survival and proliferation, reduces apoptosis of pancreatic islets, increases insulin secretion from pancreatic β-cells, and decreases insulin resistance [11, 12].
Based on these findings, obestatin has been proposed as a potential therapeutic agent in T2DM therefore studied the long-term therapeutic efficacy of obestatin on glucose homeostasis, serum insulin levels, total cholesterol, and triglycerides, insulin resistance. We evaluated the effects of Langerhans Islands in infected rats with experimental diabetes.
2. MATERIALS AND METHODS
2.1. Animals
This study was conducted according to the controls of animal experimental protocols of the Faculty of Pharmacy, Damascus University, with the approval of the ethical committee. This study was conducted on 30 male Wistar rats of local strain, weighing 155 – 200 g. The rats were housed in clean cages, with a suitable temperature (22 ± 2°C) under a controlled 12 – 12 h light–dark cycle and had free access to food and water throughout the period of the study.
2.2. Study Design
The rats were randomly divided into three groups, each group had 10 rats.
Group I (Control group n=10): The rats were left without intervention.
Group II (T2DM group n=10 rats): A group that had induced T2DM, and did not receive any treatment.
Group III (T2DM+obestatin n=10): A group that had induced T2DM and treated with obestatin at a dose of (25μg/kg) (IP) twice daily for thirty days.
2.3. Induction of T2DM
T2DM was stimulated by providing the mice with a 10% fructose solution, only for the first 14 daysand then with normal drinking water for the remainder of the experiment. On day 14, the mice were injected with (IP) a single dose of streptozotocin STZ 35 mg/kg. After 7 days of STZ injection, animals with a fasting glucose level greater than (mMol/L 11) (mg/dL 200) were considered to have type 2 diabetes. Diabetes was confirmed by measuring the fasting blood glucose level of rats after a fasting period of 9-8 hours, using a blood glucose meter (ACCU CHEK Active). The total experimental period was eight weeks.
2.5. Blood Samples and Tissue Collection
At the end of the experiment, blood samples were obtained by taking a terminal intracardic sampling for bioassays. The pancreas was also excised from the studied groups and fixed in formalin 10% for histological examination.
2.6. Assessment of Biochemical Parameters
The fasting glucose level was measured using a glucose meter (ACCU CHEK Active), whereas fasting serum insulin level was evaluated using an enzyme-linked immunosorbent assay kit (Human Insulin ELISA Sigma Aldrich). TC and TG level were determined colorimetrically according to the method (Roche Diagnostics GmbH, Mannheim).
2.7. Homeostasis Model Assessment-insulin Resistance (HOMA-IR)
It was calculated according to the following formula proposed by Matthews et al.: Fasting glucose (mg/dL) x fasting insulin (µU/ml) /405.
3. RESULTS
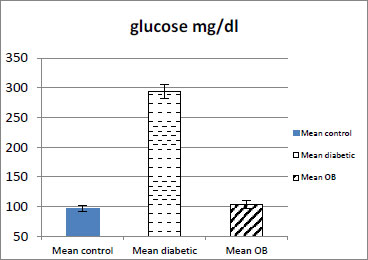
3.1. Effect of Obestatin on Serum Glucose Level
The serum glucose level in the obestatin-treated diabetic group showed a significant decrease as compared with the diabetic group (p < 0.001) (Table 1 and Fig. 1).
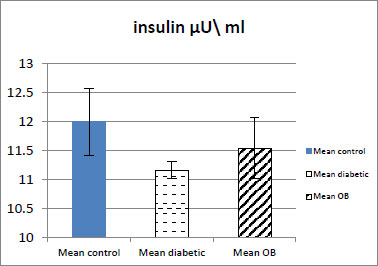
3.2. Effect of Obestatin on Serum Insulin Level
The serum insulin level showed a slight increase in the obestatin-treated diabetic group compared with the diabetic group. There was also no significant difference in the level of insulin in the obestatin-treated diabetic group compared with the control group (p ˃ 0.05) (Table 1 and Fig. 2).
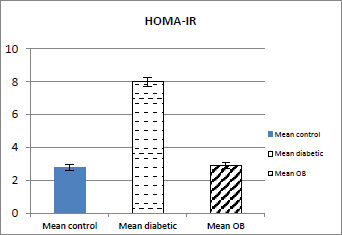
3.3. Effect of Obestatin on HOMA-IR
Treatment with OB caused a significant decrease in HOMA-IR (p < 0.001) in the obestatin-treated diabetic group compared with the diabetic group as shown in (Table 1 and Fig. 3).
| - | Group I (Control Group) Mean ± STD | Group II (T2DM Group) Mean ± STD | Group III (T2DM+Obestatin Mean ± STD |
|---|---|---|---|
| Serum Glucose mg/dl | 96.8±5.29 | 293±11.13### | 103±6.46*** |
| Serum insulin µU/ml | 11.99±0.57 | 11.16±0.14## | 11.54±0.33 |
| HOMA-IR | 2.8±0.17 | 8.1±0.28### | 2.9±0.16*** |
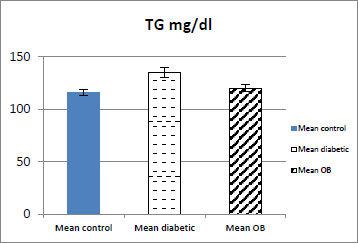
| TG mg/dl | 116±3.14 | 135±4.51### | 120±3.31*** |
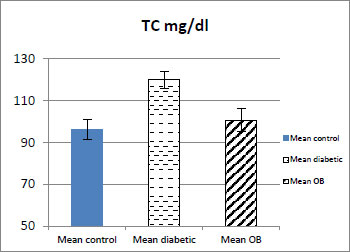
| TC mg/dl | 95±4.58 | 120±4.11### | 100±5.56*** |
| Body weight g |
304.4±5.77^^^ | 253.2±4.94### | 252.8±7.8 |
###. P < 0.001 Compare the group(II) wit the group(I)
## . P < 0.01 Compare the group(II) wit the group(I)
^^^ . P < 0.001 Compare the group(I) wit the group(III)
3.4. Effect of Obestatin on Serum TG and TC Level
As shown in (Table 1, Figs. 4 and 5) the serum TG and TC levels showed a significant decrease in the obestatin-treated diabetic group compared with the diabetic group (p < 0.001).
3.6. The Effect of Obestatin on Body Weight
The results showed a significant decrease (P < 0.001) in the average body weight of the rats of the diabetic group and obestatin-treated diabetic group when compared with the control group (Table 1).
3.7. Histological Results
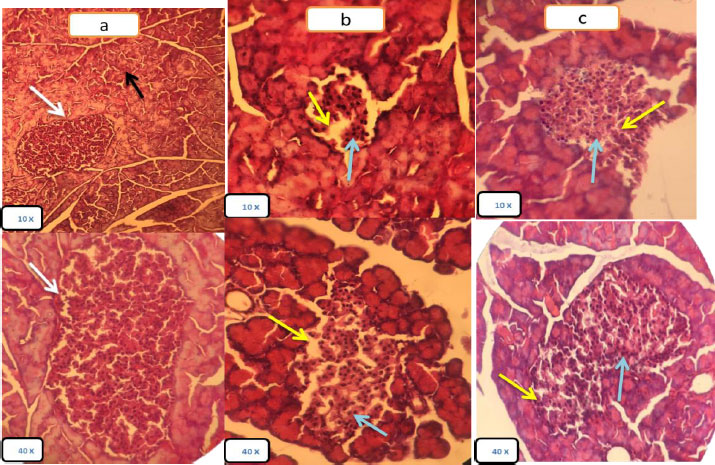
The control group showed a normal architecture and histological structure with the normal Langerhans islet with a compact arrangement of beta and non β-cells, and normal acini composed of aciners cells with basal nuclei (Fig. 6a).
Group II (T2DM): Pancreatic tissues of the diabetic group showed decreased islets size, decreased β- cells number and irregular islets shape, the islets of Langerhans appeared to be deformed and of a smaller size than normal, with wide intercellular spaces resulting from damage to β-cells and dissolution of the cytoplasm, as well as severe dysfunction of the islets, compared to the control group. In contrast, the epithelium of the pancreas (acinar), connective tissues and ducts appeared normal (Fig. 6b).
Group III (T2DM+obestatin): Pancreatic tissue showed an increase in the size of the islets of Langerhans and hyperchromatic nuclei in the stained sections, an increase in the number of islet cells and the restoration of the islets to their nearly regular shape, and narrow intercellular spaces compared to Group II (T2DM) as shown in Fig. (6c).
4. DISCUSSION
In the current study, we evaluated the effect of chronic Obestatin therapy on levels of glucose, insulin, triglycerides, total blood cholesterol, and insulin resistance in type 2 diabetic rats.
In our study, rats were fed a 10% fructose solution for two weeks, and followed by a single STZ injection. It led to a significant increase in the levels of glucose, triglycerides TG and total cholesterol TC in the blood, an increase in insulin resistance expressed by an increase in HOMA-IR, and a decrease in insulin levels in the Group II that had type 2 diabetes events compared to the control group. Furthermore, histopathological examination of pancreatic tissue showed a decreased islet size, decreased β- cells number and irregular islet shape.
These findings are consistent with other studies [13] showing that feeding rats with a 10% fructose solution followed by a single injection of STZ induced progressive β-cell dysfunction in T2DM rats, causes toxic damage to DNA [14]. STZ is transported intracellularly by glucose transporter (glucose transporter protein, GLUT2), and is not recognized by other glucose transport proteins. This explains its selectivity for pancreatic β-cells, because these cells have high levels of GLUT2 [15]. Also, Song et al. showed that STZ leads to degenerative histological changes and shrinkage of the pancreatic islet of Langerhans. They also reported that STZ destroys the islet cells through several mechanisms, including the production of reactive oxygen species ROS, stimulation of inflammatory and immune responses, and activation of pancreatic nuclear factor-κB [16].
In addition, fructose plays an important role in developing insulin resistance and causing metabolic disorders through several mechanisms. The high influx of fructose into the liver (which is a key organ of fructose metabolism) disrupts glucose metabolism and its uptake pathways, leading to a significant increase in the formation of lipids and triglycerides (TG) from fructose catabolism. In addition, fructose may reduce circulating insulin levels resulting from its very high glycation potentials that can lead to the formation of advanced glucose which is accompanied by the generation of oxidative stress, which contributes to significant damage to pancreatic cells in diabetes [13, 17].
On the other hand, our study showed improvement in glucose, insulin, insulin resistance, TG and TC levels after obestatin administration. The positive effect of Obestatin was also confirmed by observing the improvement in the histological structure of the pancreas by H&E stain.
These findings are in agreement with Granata et al. who showed that obestatin enhanced glucose uptake, decreased insulin resistance, increased insulin secretion, and β-cell damage in mice fed with high-fat diet, this could be explained by the increased signaling protein kinase/phosphoinositide 3-kinase (PI3K/Akt) and MPAK, which play a key role in the sensitivity of peripheral tissues to insulin. Obistatin also enhances AMP-activated protein kinase phosphorylation, adiponectin secretion, and reduced leptin secretion. In addition, obestatin improves glucose uptake in the absence or presence of insulin, as it increases the transport of the glucose transporter GLUT4, which plays a key role in glucose uptake in peripheral tissues [18].
Another mechanism of obestatin-decreased glucose level has been documented through the induction of adiponectin level [19]. Adiponectin is a positive regulator in glucose homeostasis by increasing glucose uptake by the cells and enhancing insulin sensitivity and decreasing gluconeogenesis [20].
The results of our study were also in agreement with the study [21] On diabetic rats who were treated with obestatin (100 μg/kg) twice daily for a week, the study showed a significant decrease in glucose levels with a significant increase in the level of insulin. In contrast, it showed a slight change in BMI compared to the healthy control group.
A study on the effect of Obestatin on rats with type 2 diabetes who were treated with a single injection of Obistatin (1nmol / 100ml) for ten days, showed improvement in insulin levels and a decrease in glucose levels in the treated group, without reaching the normal level [22].
Another study [23] also showed a significant decrease in blood glucose levels in mice fed a high-fat diet followed by an STZ injection and treatment with obestatin (75 nmol / kg) for 30 days compared to the group with diabetes, while the insulin levels decreased in the opistatin-treated group compared to the diseased group, this difference in insulin levels could be explained due to the difference in the dose of obestatin used and the different nature of the diet.
In a study [24] on pancreatic tissue isolated from rats Obistatin has shown a dual effect on glucose-stimulated insulin secretion: the results of this study showed that obestatin at a low concentration (1 nmol) strengthened the insulin response according to glucose levels, while a high concentration (10 nmol) of obestatin inhibited the secretion of insulin resulting from this stimulation. This indicates that there is more than one receptor for obestatin with different sensitivities to the ligand that might be implicated.
In addition, our study showed a significant increase in TC and TG levels in Group II (T2DM). In contrast, the administration of obestatin significantly decreased levels of TC and TG. The mechanism by which obestatin reduces TG levels may be via its proposed role by binding to the orphan GPR39 receptor that is expressed in the intestine, adipose tissue, and other tissues that play a role in lipid metabolism [25]. Another explanation for the reduction of TG and TC can be attributed to the effects of obestatin on intestinal TG [1, 8]. Furthermore, it may be due to obestatin binding to GLP-1 that is expressed in adipose tissue where it exerts its role as an autocrine/paracrine in inhibiting lipolysis, stimulating glucose uptake, regulating adipocyte function, and inhibiting inflammation [26]. This result was explained by Agnew et al. who showed that obestatin induces phosphorylation AMP-activated protein kinase in adipocytes, the activation of which correlates with the inhibition of lipolysis. Obestatin also appears to reduce the expression levels of ATP-binding cassette A1, which is a major cholesterol transporter [27].
Moreover, the histopathology study of pancreatic tissue isolated from Group III (T2DM+obestatin): showed a significant improvement in islet size and an increase in the number of β-cells with a decreased intercellular space compared to Group II (T2DM). For islets closer to normal appearance, that evidence for the role of obestatin as an apoptotic inhibitor and stimulant for the growth and proliferation of pancreatic β-cells. The antiapoptotic action of obestatin could be explained as follows: STZ produces diabetes by inducing β-cell destruction, partly through stimulation of apoptosis [10]. Subsequently, obestatin treatment may increase the antiapoptotic protein pancreatic BCL2, suggesting that it may prevent β-cell death by enhancing the activity of the antiapoptotic cell mechanism. Moreover, we could attribute the antiapoptotic action of obestatin to increased expression of the transcription factor PDXI, which is involved in the maintenance of β-cell mass and differentiation of pancreatic duct cells into β-cells [12, 28], Moreover, Hussien et al., revealed an increase in pancreatic expression of GLP-1 receptors in type 2 diabetic rats treated with Obestatin, where GLP-1 plays a major role in the growth and differentiation of β-cells and insulin sensitivity [29], and this corresponds to the results of the isolated pancreatic histological examination in terms of the resulting improvement in islet size and number cells in the Obestatin-treated group when compared with the diabetic group.
CONCLUSION
The results of our study showed the efficacy of obestatin as a treatment in reducing the levels of all glucose, triglycerides and total cholesterol in the blood to normal limits in induced experimental rats with type 2 diabetes, as well as the improvement of insulin levels in the blood, and the results of the histological study showed an improvement in the size of the islet and an increase in the number of β-cells. Thus, obestatin can be used as a promising target in the treatment of metabolic diseases such as diabetes and obesity.
SUGGESTIONS
We recommend conducting a comparative study of different doses of obistatin to assess its effects more clearly on insulin levels, as well as to study its effect on ghrelin levels, given that it is the hormone that physiologically counteracts the effect of obistatin in the blood in type 2 diabetes.
In light of the effective role of obistatin in improving insulin resistance, we recommend conducting further studies focusing on the importance of obistatin as a therapeutic target for diseases related to insulin resistance such as polycystic ovary syndrome.
LIST OF ABBREVIATIONS
| DM | = Diabetes mellitus |
| TG | = Triglycerides |
| STZ | = Streptozotocin |
| TG | = Triglyceride |
| TC | = Total cholesterol |
| H&E | = Hematoxylin and eosin |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted according to the controls of animal experimental protocols of the Faculty of Pharmacy, Damascus University, with the approval of the ethical committee.
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. All the animals were used in accordance with The US National Research Council's “Guide for the Care and Use of Laboratory Animals,” ❍ The US Public Health Service's “Policy on Humane Care and Use of Laboratory Animals,” and “Guide for the Care and Use of Laboratory Animals.”
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
The data and supportive information is available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
I would like to thank the University of Damascus - the College of Pharmacy and the College of Dentistry - for providing the necessary facilities and conditions required to complete the research.


