All published articles of this journal are available on ScienceDirect.
Effects of COVID-19 Vaccines on the Menstrual Cycle: A Cross-Sectional Study
Abstract
Background:
Prior clinical studies that sought to investigate the safety and efficacy of COVID-19 vaccines did not list menstrual cycle changes as a side-effect. However, following reported cases of menstrual cycle disturbances after vaccination, this study sought to examine the link between COVID-19 vaccination and post-vaccine menstrual cycle abnormalities in pre-and post-menopausal women.
Methods:
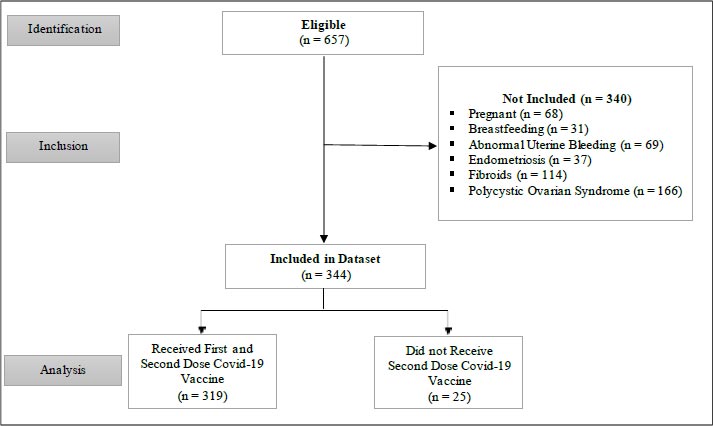
A cross-sectional research design approach using online surveys were employed to investigate the link between vaccination and changes in the menstrual cycle. The participants consisted of a cohort of 657 pre-and post-menopausal women, with the majority drawn from the reproductive age group (25-44 years). The inclusion criteria were that participants must have received any type of COVID-19 vaccine, not be pregnant and those that did not have a negative diagnosis in any gynecologic condition. Of the eligible sample size, only 344 participants met the inclusion criteria.
The sociodemographic and menstrual cycle data were collected from an online survey. Data was analyzed using descriptive, inferential chi-square tests, logistic regression, and correlation.
Results:
The results partially confirmed the findings from prior studies that COVID-19 vaccination is associated with significant changes in the women’s menstrual cycle flow and menstrual period length even after controlling for age, Body Mass Index, and ethnicity. Other menstrual cycle disturbances such as missed periods, cycle regularity, and spotting/vaginal bleeding were noted to be less significant. However, the extent of menstrual cycle changes was less severe and decreased after the second dose of vaccination. It was found that 11.1% and 37.5% of post-menopausal women reported menstrual symptoms after the first and second dose cycles, respectively.
Conclusion:
The study concludes that although COVID-19 vaccines tend to adversely affect women’s menstrual cycle, these changes are short-lived. The findings have important implications in enhancing the success of COVID-19 vaccination programs by reducing cases of vaccine hesitancy among reproductive-age women.
1. INTRODUCTION
COVID-19 vaccination is considered the best option for protection against the potentially adverse effects of SARS-CoV2 infections [1]. Some of the common side effects associated with COVID-19 vaccines, as listed by the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) as well as the U.S. Vaccine Adverse Reporting System (VAERS), include a sore arm, fever, fatigue, myalgia, and headache [2]. However, in prior clinical studies, changes in menstrual cycles, period flow, menses length, and vaginal bleeding were not identified and listed as common side effects following COVID-19 vaccination [3]. By May 2021, fewer than 200 young vaccinated women had self-reported a menstrual-related disturbance following vaccination to VAERS [4].
However, by September 2021, more women (at least 30,000 reports) had complained of the adverse side-effects related to menstrual cycle abnormalities following vaccination to the UK’s MHRA yellow card surveillance scheme [2].
There were concerns that a possible link between COVID-19 vaccination and menstrual cycle disturbances might lead to vaccine hesitancy, especially among young women [5]. Therefore, clinical studies were needed to evaluate the extent of this relationship in order to assure the public and maintain the trust that vaccines interfere substantially with fertility [6]. Menstrual cyclicity is an obvious sign of health and fertility in young women and its variation from month to month across a person’s lifespan is considered normal [7]. Specifically, changes in menstrual cycle length, which can be between 24-38 days is considered normal if it falls within 8 days [3]. The normal variation in the menstrual cycle can be a concern for young women, especially if it is associated with COVID-19 vaccination exposure [4].
The U.S. National Institute of Health (NIH) allocated $1.67 million to fund clinical research on the possible association between COVID-19 vaccination and menstrual cycle abnormalities [2]. The findings based on the most recent clinical studies indicate that there is significant evidence that women tend to experience menstrual cycle disturbances following COVID-19 vaccination [3, 4, 6, 8]. A study by the Norwegian Institute of Public Health [9] reported that 13.6% of young women, 18-30 years had experienced heavier periods after the first dose, while 15.3% of them experienced heavier menstrual periods after the second dose. Research commissioned by the U.S. National Institute of Health (NIH) further reported [4] that COVID-19 vaccination was associated with less than a day (i.e., 0.71 days) increase in menstrual cycle length for both vaccine-dose cycles compared to the pre-vaccine menstrual cycle. Similarly, using a cohort of 79 spontaneously cycling young women, the study by Woon and Male [3] found that COVID-19 doses were associated with a delay in the menstrual cycle (2.3 days after the first dose and 1.3 days after the second dose). However, most of these studies found that menstrual changes tend to reverse in subsequent cycles [4, 6, 8-11]. A study by Muhaidat also found that 66.3% of the participants experienced menstrual symptoms in the period following vaccination [11]. The insight based on clinical studies indicate that the association between COVID-19 vaccination and the menstrual cycle changes are linked to immune activation in response to stimuli [2]. Biologically, it has been noted that the COVID-19 vaccines, similar to the human papillomavirus (HPV) vaccines, tend to create immune stimulation on the hormones that control the menstrual cycle [7]. According to the UK Yellow Card Surveillance Scheme, there were 51, 435 suspected menstrual reactions experienced by persons obtaining three SARS-CoV-2 vaccinations by August 2022, which included heavier periods, delayed periods and unexpected bleeding.Variations in cycle lengths have been linked to the follicular and luteal phases of the menstrual cycle. It was unearthed that individuals who received vaccinations during the follicular phase of their cycle experienced longer cycles than those who were vaccinated during the luteal phase.
Despite reported menstrual cycle changes, it was noted that those who experienced menstrual abnormalities after receiving COVID-19 vaccinations found that their cycle quickly returned to normalcy in the proceeding months after vaccination. This was corroborated by the findings of a study conducted by Alvergne et al. which revealed that the average cycle length between COVID-19 vaccination doses and post-vaccination were not significantly dissimilar to pre-vaccination cycles.
The twin island Republic of Trinidad and Tobago has an estimated population of 1.4 million [12]. The country reported its first case of SARS-CoV-2 on March 12, 2020 [13]. Since then, public health measures such as border closures, social distancing, and mask-wearing have been implemented to limit the spread of the virus [14]. On February 17th, 2021, Trinidad and Tobago joined the global effort to control the pandemic through vaccination when the Ministry of Health embarked upon the Phase 1 rollout of its National COVID-19 Vaccination Program, with healthcare workers are among the first groups to receive the first doses of the vaccine, along with persons aged 60 years and over and persons with non-communicable diseases. By April 2021, subsequent phases (2 and 3) of the campaign offered essential frontline workers and the eligible public the opportunity to be inoculated.
A study investigating the acceptance of the vaccine among healthcare workers in Trinidad and Tobago found that age, profession and trust in international public health organizations and other healthcare professionals predict their vaccine uptake [15]. Researchers in Trinidad and Tobago also reported on the safety of the COVID-19 vaccine by examining the side effects of the ChAdOx1 nCov-19 (Oxford, AstraZeneca COVID-19 vaccine) among healthcare workers16. The study demonstrated that the rate of occurrence of most local and systemic side effects was less than 50%, corroborating the manufacturer’s claim that the vaccine is safe, with implications to reduce vaccine hesitancy through public health efforts [16]. Other studies in Trinidad and Tobago have been limited to investigating COVID-19 patients’ epidemiological characteristics [17] as well as laboratory predictors of COVID-19 admissions to the ICU [18]. The most frequent comorbidities were found to be hypertension and diabetes mellitus, while the most prevalent symptoms were non-productive coughs and fevers [17]. As for laboratory factors, neutrophils, aspartate transaminase (AST), lactate dehydrogenase (LDH) and C-reactive protein (CRP) were suitable predictors of COVID-19 patients in need of ICU care [18]. Both studies allude to the unique characteristics of COVID-19 patients in Trinidad and Tobago and the greater need for research, especially in this region.
The present study aimed to investigate the association between COVID-19 vaccination and changes in menstrual cycle among young women employed at the North Central Regional Health Authority of Trinidad and Tobago who had been vaccinated between 1st June 2020 and 18th March 2022. The study examined whether both the first and second vaccine-dose cycles had a significant effect on variation in the participants’ menstrual cycle. A cross-sectional study design was undertaken using online self-administered surveys, which were employed to collect sociodemographic and menstrual cycle data from the women. The survey was administered from December 2021 to March 2021. The eligible participants consisted of 657 adult healthcare workers who currently menstruate or who have had menstrual cycles in the past and who received at least one dose of the COVID-19 vaccine. However, of those, only 317 women met the inclusion criteriafor this study indicated in Fig. (1). The data were analyzed using both descriptive and inferential statistical analysis in order to examine the link between COVID-19 vaccination and variation in a menstrual cycle. Inferential statistical analysis included logistic regression, correlation analysis, and Chi-square tests of association.
2. METHODS
2.1. Research Design
The study employed a cross-sectional research design approach to investigate the effect of COVID-19 vaccines on the menstrual cycle of healthcare workers (HCWs) employed at the North Central Regional Health Authority (NCRHA) of Trinidad and Tobago. The NCRHA was selected as the setting for HCWs as it was the first RHA to distribute COVID-19 vaccines to HCWs at the outset of the country’s national vaccination program. Data capture was conducted via the electronic distribution of a self-administered questionnaire to NCRHA HCWs.
The survey remained open for responses from December 18th 2021 to March 18th 2022 . Using the stated research design approach, participants who included vaccinated women were required to indicate their sociodemographic information and their corresponding menstrual cycle details before and after vaccination. The anonymous responses were automatically collated via the online platform to which only the principal investigator had access. The collated responses were downloaded as a Microsoft Excel file by the principal investigator and subsequently coded into an SPSS database and analyzed using IBM SPSS V.21 software.
The study protocol was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies.
2.2. Participants
A judgment sampling method was used to obtain the sample of HCWs for this study. The electronic questionnaire was distributed via email to all female NCRHA HCWs. Of the 4,205 NCRHA HCWs to whom the questionnaire was sent, 657 HCWs returned a completed questionnaire during the study period. This cohort of 657 COVID-19 vaccinated women included those who were over 18 years (Mean age = 36.42 years). Their mean body mass index (BMI) before vaccination was 29.24 (29.24 ± 8.38 kg/m2). The participants consisted of both pre-and post-menopausal women who had either experienced or not experienced a menstrual period in the last 12 months and who had received either the first or second dose of the vaccine between 1st June 2020 and 18th March 2022.
2.3. Data Collection Instrument
Sociodemographic and menstrual cycle data before and after vaccination was collected sing a self-administered survey/questionnaire. This data collection instrument was distributed and administered to the email addresses of the female NCRHA HCWs.
The instrument was modeled on relevant questions selected from a digital survey investigating the impact of COVID-19 on women’s reproductive health in Ireland and the United Kingdom [19] and a digital survey investigating the changes in menstruation as a possible side-effect of COVID-19 vaccines [20]. The online questionnaire consisted of 57 detailed self-report questions that covered two main domains. The first section sought to collect participants’ sociodemographic details, including age, ethnicity, BMI, pregnancy status, breastfeeding status, the date of first vaccination, pre-existing medical diagnosis, and method of contraception. The second section contained questions on the participants’ menstrual cycle regularity, period flow, menstrual period length, and other abnormalities, which were experienced pre- and post-vaccination. The electronic self-administered questionnaire was prefaced with an informed consent form that explained that the survey was anonymous, that participation was voluntary and explained the purpose of the study. All ethical standards of voluntary participation and confidentiality were maintained.
Participation in this study was voluntary, and HCWs received no form of financial remuneration in order to reduce the risk of response bias. Due to the anonymous nature of the questionnaire, confirmation of participants’ vaccination could not be verified.
2.4. Outcome Measures
The main outcome measures of this study included the association between the COVID-19 vaccine and participants’ reported menstrual cycle disturbances.
2.5. Ethics
This study was granted ethical approval by The North Central Regional Health Authority Ethics Committee, Trinidad, and The Ministry of Health of Trinidad and Tobago Ethics Committee (3/13/441 Vol. II).
2.6. Data Analysis
The data was analyzed in SPSS v.26 statistical programs. To examine the association between COVID-19 vaccination and menstrual cycle changes, both descriptive and inferential statistical analysis was performed. The participants’ sociodemographic details were analyzed using descriptive statistical analysis and presented as frequencies and percentage frequencies. The association between COVID-19 vaccination and menstrual cycle disturbances were analyzed using correlation analysis. Finally, logistic regression and the non-parametric Chi-square test were performed to examine the effect of COVID-19 vaccine dose on menstrual cycle changes after accounting for participants’ age, ethnicity, and BMI. A parametric paired t-test was used to compare the mean change in menstrual cycle regularity, menstrual cycle length, and period flow between baseline (pre-vaccination) and after vaccination. A statistical significance level of 0.05 was used to conduct the inferential analysis.
The proposed logistic regression model has a unique working structure that includes inputs or predictor variables, weights/coefficients, the intercept, and the binary outcome [21-27]. The structureof the logistic regression model with a target binary outcome variable that was used to estimate the effect of COVID-19 vaccines on menstrual cycle changes controlling for age, BMI, and ethnicity is defined as follows:
P (Y) = αθ + BX
Where Y is a binary outcome variable {Y = 0 if there are no menstrual cycle disturbances
Y = 1 if there are reported menstrual cycle changes}
αθ = Intercept term
B = A vector of odds ratio coefficients
X = A vector of the predictor variable (First/second dose COVID-19 vaccine) and control variables.
The logistic regression model was applied to estimate the values of the parameters, including the intercept term and the odds ratio coefficients for the predictor and control variables.
There were three main motivations for the usage of logistics regression analysis to estimate the effect of COVID-19 vaccines on menstrual cycle disturbances. First, the results of the model can be interpreted easily to capture the likelihood of menstrual cycle changes occurring post-vaccination.
Second, the logistic regression model is appropriate in estimating statistical relationships where there are large datasets, predictors, and control variables. Finally, the application of a logistic regression model was motivated by the fact that it is reliable, accurate, and robust to variations in outliers and missing data [27].
2.7. Bias
It is possible that observed correlations between menstrual cycles and the SARS-CoV-2 vaccine may be influenced by reporting bias of participants who may experience augmented health awareness due to the pandemic, thereby exacerbating the likelihood that menstrual changes are noticed compared to pre-pandemic menstrual changes. Similarly, this study relies on retrospective, self-reported data collated via questionnaires, therein increasing the chances of self-report bias.
Another source of potential bias when examining the impact of COVID-19 vaccinations on menstrual cycles are confounding bias. Given that many women of reproductive age may be using hormonal contraceptives, this type of contraception may affect menstrual symptoms and immunity leading to fluctuations in menstrual cycles pre- and post-vaccination [21]. Therefore, confounding would make it challenging to identify fluctuations due to pandemic-related factors.
3. RESULTS
3.1. Sociodemographic Data
Six hundred and fifty-seven pre- and post-menopausal women participated in the survey.
However, three hundred and forty-four met the inclusion criteria for this study (Fig. 1).
Participants diagnosed with polycystic syndrome (n = 166), uterine fibroids (n = 114), had abnormal uterine bleeding (n = 69), or were pregnant during the period of study (n = 68), endometriosis (n = 37) and those breastfeeding (n = 31) were excluded.
The participants’ sociodemographic data (Table 1) indicated that the mean BMI was 29.24±8.38 kg/m2. Most women who participated in the study were between 25-34 years (n = 135; 42.6%) and 35-44 years (n = 112; 35.3%). The majority of the women were of the reproductive age group. Approximately forty-four percent of the participants were Africans (n = 138; 43.5%).
In terms of the first dose of COVID-19 vaccine, the majority (n = 157; 49.5%) had received the Oxford AstraZeneca vaccine, while for the second dose of the COVID-19 vaccine, the majority (n =153; 48.3%) of the women had been injected with the Oxford AstraZeneca vaccine, and 30.6% of them had received the Sinopharm vaccine. The descriptive statistics also indicated that only 16.1% of the participants had reported a positive COVID-19 diagnosis prior to the study, which was conducted between April and June 2022.
The majority (n = 280; 88.3%) of the women who participated in the study reported a regular menstrual cycle. In terms of the menstrual period length, most (n = 138; 43.5%) of the women reported an average of 3 to 5 days. The majority (n = 230; 72.6%) had a moderate period flow, while only 12.6% of the women reported a heavy period flow.
| - | Mean | Standard Deviation |
| Body Mass Index (BMI) | 28.63 | 8.33 |
| Menstrual cycle length | 1.83 | 0.51 |
| - | Frequency (n) | Percentage Frequency (%) |
| Age (Years) | - | - |
| 18-24 | 15 | 4.7 |
| 25-34 | 135 | 42.6 |
| 35-44 | 112 | 35.3 |
| 45-54 | 46 | 14.5 |
| 55-64 | 8 | 2.5 |
| ≥ 65 | 1 | 0.3 |
| Ethnicity | - | - |
| African | 138 | 43.5 |
| East Indian | 102 | 32.2 |
| Hispanic, Mixed Races and Others | 77 | 24.3 |
| First Dose COVID-19 Vaccine: | - | - |
| Johnson & Johnson | 10 | 3.2 |
| Oxford AstraZeneca | 157 | 49.5 |
| Pfizer-BioNTech | 46 | 14.5 |
| Sinopharm | 104 | 32.8 |
| Second Dose COVID-19 Vaccine: | - | - |
| Johnson & Johnson | 0 | 0.0 |
| Oxford AstraZeneca | 153 | 48.3 |
| Pfizer-BioNTech | 42 | 13.2 |
| Sinopharm | 97 | 30.6 |
| Did not receive dose 2 | 25 | 7.9 |
| COVID-19 Diagnosis: | - | - |
| No | 238 | 75.1 |
| Yes | 51 | 16.1 |
| I think so | 12 | 3.8 |
| Unsure | 13 | 4.1 |
| Other diseases/infections | 3 | 0.9 |
| Menstrual Cycle Regularity: | - | - |
| Rarely menstruate | 18 | 5.7 |
| Irregular | 19 | 6.0 |
| Regular | 280 | 88.3 |
| Menstrual Period Length: | - | - |
| Rarely menstruate | 18 | 5.7 |
| 1-3 days | 33 | 10.4 |
| 3-5 days | 138 | 43.5 |
| 5-7 days | 121 | 38.2 |
| > 7 days | 5 | 1.6 |
| Other | 2 | 0.6 |
| Menstrual Period Flow: | - | - |
| Rarely menstruate | 18 | 5.7 |
| Heavy | 40 | 12.6 |
| Moderate | 230 | 72.6 |
| Light | 28 | 8.8 |
| Other | 1 | 0.3 |
| - | First (1st) Dose | Second (2nd) Dose | x2-value |
| Change in Cycle Regularity | - | - | 165.82 *** |
| No | 244 (71.0) | 224 (64.7) | - |
| Yes | 100 (29.0) | 95 (27.4) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Change in Period Flow | - | - | 387.64*** |
| About the same | 216 (62.8) | 195 (56.2) | - |
| Heavier | 66 (19.2) | 71 (20.5) | - |
| Lighter | 23 (6.6) | 22 (6.3) | - |
| Not applicable | 39 (11.4) | 32 (9.1) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Change in Period Length | - | 402.77 *** | - |
| About the same | 238 (69.1) | 225 (65.0) | - |
| Longer | 40 (11.7) | 39 (11.4) | - |
| Shorter | 26 (7.6) | 21 (6.0) | - |
| Not applicable | 40 (11.7) | 34 (9.8) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Late Period | - | 156.24*** | - |
| No | 271 (78.9) | 261 (75.4) | - |
| Yes | 73 (21.1) | 58 (16.7) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Missed Period | - | 66.13*** | - |
| No | 323 (94.0) | 299 (86.4) | - |
| Yes | 21 (6.0) | 20 (5.7) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Spotting/Vaginal Bleeding | - | 158.02*** | - |
| No | 310 (90.2) | 287 (83.0) | - |
| Yes | 34 (9.8) | 32 (9.1) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Other Menstrual Bleeding | - | 59.65*** | - |
| No | 322 (93.7) | 303 (87.4) | - |
| Yes | 22 (6.3) | 16 (4.7) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Severe Menstrual Symptoms | - | 200.79*** | - |
| No | 284 (82.6) | 272 (78.5) | - |
| Yes | 60 (17.4) | 47 (13.6) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Other Menstrual Symptoms | - | 96.95*** | - |
| No | 308 (89.6) | 289 (83.3) | - |
| Yes | 36 (10.4) | 30 (8.8) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| No Menstrual Symptoms | - | 159.08*** | - |
| No | 168 (48.9) | 152 (43.8) | - |
| Yes | 176 (51.1) | 167 (48.3) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| Number of Days Before Symptoms Started | - | 278.25*** | - |
| 1-3 days | 16 (4.7) | 13 (3.8) | - |
| 4-7 days | 10 (2.8) | 8 (2.2) | - |
| 8-14 days | 14 (4.1) | 19 (5.4) | - |
| > 14 days | 58 (17.0) | 61 (17.7) | - |
| Menstruating when receiving a vaccine | 20 (5.7) | 1 (0.3) | - |
| Not applicable | 226 (65.6) | 218 (62.8) | - |
| Blank | 2 (0.6) | 0 (0.0) | - |
| Did not receive dose 2 | - | 25 (7.9) | - |
| n | Period Length | - | Period Flow | ||
| Change in Length (x2) | ρ-value | Change in Flow (x ) | ρ-value | ||
| First dose v. before vaccination | 344 | 121.15 | < 0.001 | 119.36 | < 0.001 |
| Second dose v. before vaccination | 319 | 402.77 | < 0.001 | 387.64 | < 0.001 |
3.2. Menstrual Cycle Disturbances after COVID-19 Vaccination
The non-parametric chi-square inferential test was used to compare the extent of menstrual cycle disturbances after the first dose and the second dose cycle. Table 2 shows that there were no statistically significant change in menstrual cycle regularity after the first dose of vaccination compared to the second dose vaccination (ρ > 0.05). The chi-square test indicated that exposure to COVID-19 vaccination resulted in a significant change in the women’s menstrual period flow (ρ < 0.001). Exposure to the COVID-19 vaccination also did not have a significant effect on the women’s menstrual cycle length (ρ > 0.05). The participants’ menstrual period length was not significantly longer after the first dose compared to the second dose vaccination (ρ > 0.05).
The inferential analysis findings based on the Chi-square test also indicated that women experienced other forms of menstrual cycle abnormalities after exposure to the first and the second dose COVID-19 vaccines. The women experienced incidences of late menstrual periods (ρ < 0.001), other menstrual bleeding (ρ < 0.001), severe menstrual symptoms (ρ < 0.001), and other menstrual symptoms (ρ < 0.001). In this case, ‘other menstrual bleeding’ refers to other forms of menstrual bleeding or abnormalities besides those specified in the analysis. However, the majority of the cases, the variation was not statistically significant. For instance, missed periods (ρ > 0.05) and spotting/vaginal bleeding (ρ > 0.05) were found to be statistically insignificant. Finally, the findings based on the inferential chi-square test indicated that there was a significant change in the number of days before menstrual symptoms started after the first and the second dose of COVID-19 vaccination (ρ < 0.001). For those who reported menstrual symptoms, the majority stated that they tend to occur 14 days after receiving the first or the second dose of the COVID-19 vaccine (ρ< 0.001).
Table 3 presents a summary of the chi-square test to assess the difference in menstrual cycle length and period flow before and after the first dose of COVID-19 vaccination. The results indicated that there was a significant variation in the women’s menstrual period length before and after the first dose of vaccination (ρ < 0.001) (Fig. 2). Similarly, the chi-square test findings presented in Table 3 indicated that exposure to the first dose of COVID-19 vaccination resulted in a significant variation in menstrual period flow compared to the situation before vaccination (ρ < 0.001) (Fig. 3). The (n = 344) represents the women who received the first dose of vaccination while the (n = 319) captures the women who received the second vaccination dose (Table 4).
Table 4.
| n | First Dose | - | - | Second Dose | ||
| Chi-square (x2) | ρ-value | n | Chi-square (x ) | ρ-value | ||
| Late period | ||||||
| No | 271 (79%) | - | - | 261 (82%) | - | - |
| Yes | 73 (21%) | 317.00 | < 0.001 | 58 (18%) | 292.00 | < 0.001 |
| Missed Period | ||||||
| No | 323 (94%) | - | - | 299 (94%) | - | - |
| Yes | 19 (6%) | 317.00 | < 0.001 | 20 (6%) | 292.00 | < 0.001 |

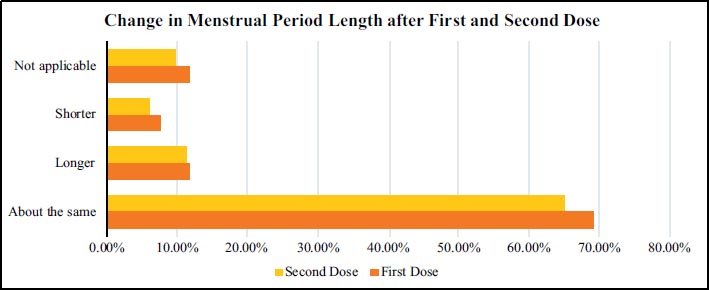
Table 5.
| - | Post Vaccination | Pre-Vaccination | Chi-square (x ) | |
| - | First Dose | Second Dose | ||
| Regular menstruation | 199 (62.8) | 189 (59.6) | 280 (88.3) | 156.14*** |
| Period length | 266 (84.0) | 248 (78.2) | 297 (93.7) | 118.23*** |
| Moderate period flow | 208 (65.5) | 195 (61.4) | 230 (72.6) | 96.55*** |
| Light period flow | 25 (8.0) | 24 (7.5) | 28 (8.8) | 205.13*** |
The results of the non-parametric chi-square test (Table 5) indicate that there was a significant association in menstrual symptoms between the pre- and post-vaccination period. The frequency of menstrual cycle regularity had changed significantly post-vaccination compared to the cycle regularity before COVID-19 vaccination (ρ < 0.001). The frequency of the period length and period flow, as reported by respondents, had significantly decreased after the first and second cycle dose vaccination compared to the menstrual period length and period flow before the COVID-19 vaccination (ρ < 0.001). For instance, moderate period flow decreased after the first dose vaccination (65.5%) and the second dose vaccination (61.4%) compared to 72.6% of respondents who had reported moderate period flow prior to the COVID-19 vaccination.
3.3. Trends in Women who Experienced Menstrual Changes after COVID-19 Vaccination
There are two categories of women who do not menstruate. The first is those that have not yet reached menopause (pre-menopausal) but do not menstruate. The second is those that have reached menopause and were not menstruating prior to receiving COVID-19 vaccines. As shown in Table 6, some of the women in the two categories that were not menstruating previously experienced menstrual cycle after receiving the COVID-19 vaccine.
A summary of the Chi-squared test in pre- and post-menopausal women who experienced menstrual cycle changes following COVID-19 vaccination are presented in Table 6. The findings show that after the first dose of SARS-CoV-2 vaccination, 22 pre- and post-menopausal women (3.3%) reported menstrual cycle abnormalities. However, after the second dose cycle, 28 pre- and post-menopausal women (4.3%) experienced menstrual cycle changes. The increase in the number of women that were not menstruating but reported menstrual cycle abnormalities after the second dose cycle was statistically significant (ρ < 0.001). The results also reveal that among the post-menopausal women (55-64 years and those above 65 years), 37.5% of them reported menstrual cycle abnormalities after the second dose vaccination compared to only 11.1% of the stated group of women that reported menstrual bleeding after the first dose cycle. The change in the proportion of post-menopausal women who reported menstrual cycle changes after the first and the second dose cycle was statistically significant (ρ < 0.001).
3.4. Logistic Regression Analysis
Logistic regression analysis was conducted to examine the effect of COVID-19 vaccination on the women’s menstrual cycle controlling for the participants’ age, BMI, and ethnicity (Table 7). The results indicated that after the first dose cycle, none of the variables had a significant effect on the likelihood of causing missed periods, late periods, spotting, and no menstrual cycle symptoms.
However, the women’s BMI had a greater odds of contributing to cases of missed periods (OR = 1.15; ρ < 0.05) and spotting (OR = 1.55; ρ < 0.05). In addition, the women’s ethnic orientation (OR = 1.87; ρ < 0.05) had a greater likelihood of contributing to ‘no menstrual symptoms’ at 5% significance level.
The logistic regression analysis findings indicated that after controlling for the participants age, BMI and ethnicity, exposure to the second dose had a significant influence on raising the likelihood (odds) of menstrual cycle abnormalities among the women that participated in the study.
Specifically, exposure to the second dose of vaccine increased the likelihood of women reporting late period (OR = 2.16; ρ < 0.001), missed period (OR = 2.03; ρ < 0.001), spotting/vaginal bleeding (OR = 2.27; ρ < 0.001) and no menstrual symptoms (OR = 1.23; ρ < 0.001). The implication is that, on average, women that had received the second dose COVID-19 vaccine were more likely to report incidences of menstrual cycle abnormalities.
The insight based on the logistic regression analysis results indicates that women had a higher risk of experiencing menstrual cycle disturbances after the second dose of vaccine compared to the first dose cycle. The estimated odds ratios for the coefficients of the second dose of vaccines under the ‘late period,’ ‘missed period,’ ‘spotting,’ and ‘no menstrual symptoms’ were significant and greater than 1. A key conclusion based on the logistic regression findings is that controlling for age, BMI, and ethnicity, there is a greater possibility that women who received the second dose of COVID-19 vaccine would report the stated menstrual cycle disturbances. However, the results show that after the first dose, only BMI and ethnicity were found to expose women to a higher risk of reporting missed periods and spotting as menstrual cycle abnormalities.
Table 8 presents the outcome of logistic regression analysis to estimate the effect of the first and second dose COVID-19 vaccine on menstrual cycle disturbances with confounder-adjusted estimates. There were three confounders, which were adjusted to estimate logistic regression model. These include participants’ age, education, and type of COVID-19 vaccines. These variables They were included as confounders because they significantly affect vaccine acceptance [29].
3.5. Correlation Analysis
Pearson correlation analysis was undertaken to examine the relationship between COVID-19 vaccination and changes to women’s menstrual cycle. The Pearson correlation analysis findings for the first dose cycle (Table 9) indicates that the first dose vaccination was not significantly associated with any of the participants’ sociodemographic factors, such as age, BMI, and ethnicity.
| - | First (1st) Dose | Second (2nd) Dose | Chi-square (x2) |
| Do not menstruate but had menstrual changes after vaccination | 22 (3.3) | 28 (4.3) | 299.01*** |
| Post-menopausal women who reported menstrual symptoms | 1 (11.1) | 3 (37.5) | 46.43*** |
| - | Late Period (OR 95% CI) | Missed Period (OR 95% CI) | Spotting (OR 95% CI) | No Menstrual Symptoms (OR 95% CI) |
| First dose | - | - | - | - |
| First dose | 0.78 (0.56 1.11) |
0.75 (0.45 1.14) |
0.66 (0.31 1.08) |
0.96 (0.63 1.46) |
| Age | 0.91 (0.77 1.03) |
0.44 (0.23 0.69) |
2.03** (1.33 2.67) |
0.64 (0.23 1.28) |
| BMI | 0.89 (0.67 1.23) |
1.15*** (0.78 1.49) |
1.55*** (0.93 2.14) |
0.84 (0.54 1.26) |
| Ethnicity | 0.67 (0.81 1.01) |
1.45 (0.97 1.92) |
0.78* (0.42 1.05) | 1.87*** (1.11 2.52) |
| Second dose | - | - | - | - |
| Second dose | 2.16*** (1.43 3.26) |
2.03*** (1.29 3.28) |
2.27*** (1.47 3.28) |
1.23*** (0.67 1.89) |
| Age | 0.56 (0.22 0.93) |
0.69 (0.33 1.32) |
0.75* (0.47 1.22) |
0.77 (0.41 1.13) |
| BMI | 0.97 (0.55 1.38) |
0.58 (0.38 0.84) |
1.68** (0.88 2.61) |
0.91 (0.53 1.42) |
| Ethnicity | 0.92 (0.58 1.46) |
1.55 (0.92 2.01) |
0.67 (0.31 1.02) |
2.08** (1.312.88) |
| Confounder-adjusted Estimates | Late Period (OR 95% CI) | Missed Period (OR 95% CI) | Spotting (OR 95% CI) | No Menstrual Symptoms (OR 95% CI) |
| First Dose | - | - | - | - |
| First dose | 0.57 (0.35 0.90) |
0.65 (0.35 1.04) |
0.61 (0.26 1.03) |
0.92 (0.59 1.44) |
| Age | 0.78 (0.64 0.90) |
0.38 (0.17 0.63) |
1.93** (1.23 2.57) |
0.61 (0.20 1.25) |
| BMI | 0.81 (0.59 1.14) |
1.05*** (0.68 1.39) |
1.35*** (0.73 1.94) |
0.70 (0.40 1.12) |
| Ethnicity | 0.64 (0.78 0.98) |
1.38 (0.90 1.85) |
0.75* (0.39 1.02) | 1.75*** (0.99 2.40) |
| Second Dose | - | - | - | - |
| Second dose | 1.56*** (0.93 2.76) |
1.53*** (0.79 2.78) |
1.77*** (0.97 2.78) |
1.03*** (0.47 1.69) |
| Age | 0.48 (0.14 0.85) |
0.61 (0.25 1.24) |
0.67* (0.39 1.14) |
0.69 (0.33 1.05) |
| BMI | 0.86 (0.44 1.27) |
0.47 (0.27 0.73) |
1.57** (0.77 2.50) |
0.80 (0.42 1.31) |
| Ethnicity | 0.70 (0.36 1.24) |
1.55 (0.70 1.79) |
0.67 (0.11 0.80) |
2.08** (1.08 2.66) |
| - | Type of Vaccine | Age | Ethnicity | BMI |
| Type of Vaccine | 1.000 | -0.011 | 0.004 | 0.048 |
| Age | -0.011 | 1.000 | -0.023 | 0.084 |
| Ethnicity | 0.004 | -0.023 | 1.000 | 0.026 |
| BMI | 0.048 | 0.084 | 0.026 | 1.000 |
| - | Second dose | Age | Ethnicity | BMI |
| Second Dose | 1.000 | -0.051 | -0.133* | -0.086 |
| Age | -0.051 | 1.000 | -0.023 | 0.084 |
| Ethnicity | -0.133* | -0.023 | 1.000 | 0.026 |
| BMI | -0.086 | 0.084 | 0.026 | 1.000 |
The implication is that none of the first dose COVID-19 vaccine types that were given to the women were significantly associated with their sociodemographic profiles.
The Pearson correlation analysis findings for the second dose vaccination (Table 10) indicate that there was a significant negative association between the second dose cycle and the ethnic orientation of women (r = -0.133; ρ < .1). However, the significant relationship between the second dose cycle and ethnicity was only significant at a 10% significance level. The other sociodemographic factors (i.e., age and BMI) were not significantly related to exposure to the second dose vaccination.
4. DISCUSSION
4.1. Limitations
It was recognized that the vaccination status of the participants could not be verified due to the anonymous nature of the questionnaire. However, the anonymity and neutrality of the questionnaire served to mitigate potential self-report bias, which can be prevalent in retrospective and self-reported data.
In addition, our dataset experienced some limitations. Our dataset is limited in its observation of the number of post-vaccine cycles to assess the degree of menstrual cycle length and flow fluctuations among fully-vaccinated persons in the months after vaccination.
Further, the dataset was also limited in its review of variables such as the use of hormonal contraception. Hence, despite rigorous and dynamic methodologies and statistical methods utilized, there is a possibility of confounding bias. Lastly, the data collated on the menstrual cycle length and flow are subjective, and the accuracy of recorded cycle lengths is subject to tracking the behaviour of participants.
4.2. Discussion of Findings
The results based on the descriptive statistics, inferential chi-square test, and logistic regression found that exposure to COVID-19 vaccination had a significant effect in changing the women’s menstrual cycle, although for certain menstrual cycle abnormalities, the effect was not significant. The non-parametric chi-square test findings confirmed that, with the exception of late periods, other menstrual bleeding, severe menstrual symptoms, and other menstrual symptoms, participants did not report significant changes to their menstrual cycle. There was evidence that the majority did not experience menstrual cycle disturbances in the form of changes to their cycle regularity, period flow, and period length. In addition, vaccinated women did not experience other menstrual cycle abnormalities, including missed periods, spotting, and no menstruation in the subsequent period after the first and second dose vaccination. These findings are fairly inconsistent with the outcome of previous studies, which noted that exposure to COVID-19 vaccines was associated with delay in menstrual periods [20], changes in cycle length [21], late periods [22], and substantial changes in the menstrual period flow [23-25]. There are biologically plausible mechanisms that explain the onset of menstrual cycle abnormalities after exposure to the COVID-19 vaccines [26, 27]. According to Male [28], immunological stimulation of the hormones that control menstrual cycle by COVID-19 vaccines plays an important role in influencing changes to the women’s menstrual cycle after the first and second dose cycles. In contrast to the findings from previous studies, this research noted that there was no significant variation in the women’s menstrual period length after the first and second doses cycle vaccination. There was no significant variation in the women who reported having longer menstrual period length after the first and the second dose cycle compared to the menstrual period length before vaccination. The stated outcome contrasts the insight based on the prior research findings by Edelman et al., who noted that [4] exposure to COVID-19 vaccines was associated with less than a 1-day change in the menstrual cycle length. Specifically, Edelman et al. found that pre-menopausal women, 18-45 years, who received COVID-19 vaccination experienced a 0.71 day-increase and 0.91-day increase in period length after the first and the second dose cycle vaccination, respectively. The variation could be explained by differences in the sampling and methodical approach.
This study found that the most common form of menstrual cycle abnormalities following COVID-19 vaccination were late period, heavy period, severe menstrual bleeding, and other forms of menstrual symptoms. The significant evidence on late period abnormalities is consistent with the prior findings by Woon and Male [3], who noted that COVID-19 vaccines were associated with a 2.3-day and a 1.3-day delay after exposure to the first and the second-dose vaccine cycle, respectively. The implication is that receiving COVID-19 vaccination is associated with a significant delay in the menstrual period. Similarly, the evidence that women experienced heavier period flow after vaccination is consistent with the outcome based on the previous study by the Norwegian Institute of Public Health [9] noted that 13.6% and 15.3% of young women, 18-30 years experienced heavier menstrual period flow after the first and the second dose cycle, respectively.
There was no evidence to confirm that the other common menstrual abnormalities, such as cycle irregularity and missed periods were significant following COVID-19 vaccination.
Consistent with the previous research findings, the results of this study also presented evidence that changes to the women’s menstrual cycle after exposure to the COVID-19 vaccination are short-lived [9, 28-30]. The results of the chi-square test analysis indicated that there were fewer incidences of menstrual disturbances after the second dose cycle compared to the menstrual abnormalities after the first dose cycle. The implication is that the extent of immunological stimulation of the menstrual cycle hormones tends to decrease after the second dose cycle.
However, in other studies [9, 31, 32], there was evidence of adverse post-vaccine menstrual cycle flow after the second dose cycle compared to the first dose cycle. For instance, the study published by the Norwegian Institute of Public Health found that [9]15.3% of young women, 18-30 years reported heavier periods after the second dose cycle compared to 13.6% of the participants who reported heavier menstrual periods after the first dose of vaccination. This trend was also noted based on the outcome of logistics regression analysis for this study. The implication is that the extent of menstrual cycle changes depending on the population group being studied and their age profile.
The study findings indicated that among the pre- and post-menopausal women who were not menstruating before vaccination, they reported menstrual cycle changes after receiving the SARS-CoV-2 vaccine. In addition, there was a significant increase in the proportion of post-menopausal women who reported experiencing menstrual symptoms after the second dose vaccination compared to the first dose vaccination. The results indicated that among women who do not menstruate, while 11.1% of post-menopausal women reported menstrual symptoms after the first dose cycle, 37.5% of them experienced menstrual bleeding after the second dose vaccination. These findings are fairly consistent with the outcome of the study by Lee et al. who found that [27] 66% of postmenopausal women had reported breakthrough bleeding after COVID-19 vaccination.
4.3. Implications of the Findings
Although not significant, especially after the first dose cycle, the findings on the link between COVID-19 vaccination and the post-vaccine menstrual cycle, changes have two major implications. First, the research outcome has considerable implications for enhancing the success ofCOVID-19 vaccination program, especially among young reproductive-age women [28]. There are false claims that COVID-19 vaccines could adversely affect women’s fertility and, therefore, their ability to conceive. The stated safety concerns related to COVID-19 vaccines increased substantially after reports that young women who had been vaccinated experienced abnormal menstrual cycles.
These concerns are likely to increase the level of vaccine hesitancy among young women who fear that taking the jab might adversely affect their conception ability [34]. Therefore, the findings from this research is expected to instill trust among young women that the COVID-19 vaccines are safe and do not interfere substantially with their fertility. Specifically, the partial findings of this study that COVID-19 vaccination is not significantly associated with menstrual cycle abnormalities, such as late period and other menstrual bleeding is likely to create trust and confidence among reproductive age women that COVID-19 vaccines are safe.
Second, the research findings on the link between COVID-19 vaccines and post-vaccine menstrual cycle changes are likely to enable young women to effectively plan for potentially altered menstrual cycles in the period after the first and second dose cycles. The knowledge that COVID-19 vaccination alters the period length and cycle regularity can inform young women to effectively plan for their altered menstrual cycle [28]. This will enable women to avoid unplanned pregnancies that might occur due to changes in their menstrual cycles after vaccination [28, 33-38]. This is possible, given that the young women might need to be careful during the few weeks after the first cycle dose and the second cycle dose. Therefore, the findings would be important in minimizing concerns among women that COVID-19 vaccination might place them at risk of experiencing unplanned pregnancies.
CONCLUSION
The findings partially support previous evidence that COVID-19 vaccination is associated with post-vaccine menstrual cycle abnormalities. This study showed that with the exception of late periods, other menstrual bleeding, severe menstrual symptoms, and other menstrual symptoms, the self-reported menstrual cycle disturbances (i.e., missed periods, spotting, change in period length, cycle regularity, and variation in period flow) were not significantly different after the first and the second dose cycle. However, changes in the menstrual cycle were less strong after the second dose compared to the variation after the first dose of vaccination. The findings suggest that the menstrual cycle abnormalities that occurred after the COVID-19 vaccination are temporary (short-lived) given that the menstrual cycle reverts to the normal level after a period of time. The research findings have important implications for reducing vaccine hesitancy among young women and therefore, enhancing the success of the COVID-19 vaccination program. The knowledge of the link between vaccination and post-vaccine menstrual cycle, changes are also expected to help young women to plan for altered menstrual cycles in order to avoid unintended pregnancies.
LIST OF ABBREVIATIONS
| MHRA | = Medicines and Healthcare Products Regulatory Agency |
| VAERS | = Vaccine Adverse Reporting System |
| NIH | = National Institute of Health |
| AST | = Aspartate Yransaminase |
| LDH | = Lactate Dehydrogenase |
| CRP | = C-reactive Protein |
AUTHORS' CONTRIBUTIONS
CDG and BB were responsible for data analysis, with intellectual contributions from DV. CDG and BB drafted the article. All authors contributed to the conception and design of the paper, interpretation of data, and critical revisions contributing to the intellectual content and approval of the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the North Central Regional Health Authority Ethics Committee, Trinidad, and The Ministry of Health of Trinidad and Tobago Ethics Committee (3/13/441 Vol. II) and carried out in accordance with the ethics committees’guidelines.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Participant informed consent to participate in this study was sought via an informed consent form that prefaced the survey. The informed consent form explained that the survey was anonymous, that participation was voluntary, and the purpose of the study. All ethical standards of voluntary participation and confidentiality were maintained. Participation in this study was voluntary, and HCWs received no form of financial remuneration in order to reduce the risk of response bias. Data was collected to ensure patient anonymity, i.e., no identifiers were recorded. Only de-identified data was recorded.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The dataset supporting the findings of the article is available in the Zenodo Repository at https://zenodo.org/record/7957143, reference number DOI 10.5281/zenodo.7957143.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
FUNDING
None.
ACKNOWLEDGEMENTS
We would like to thank Ms. Sacha Singh, for the provision of technical support.


