All published articles of this journal are available on ScienceDirect.
Mortality Rate and Effects of COVID-19 in Cancer Patients versus Non-cancer Patients: A Propensity Score Matching Study
Abstract
Background and Aim:
Coronavirus is still a life-threatening disease around the world. In patients with this disease, having an underlying disease reduces the effectiveness of treatment and increases mortality. This study aimed to examine the effect of cancer history on mortality rates in patients with COVID-19.
Materials and Methods:
This study was a case-control study involving 60 cancer patients with COVID-19 as the case group and 180 non-cancer patients with COVID-19 as the control group. A matching method based on propensity score was used to select patients in the control group. The effect of treatment on the outcome (recovery death) was studied with logistic regression, and the factors affecting patient survival were analyzed with Cox models. R software was used to analyze the data.
Results:
The mean (SD) age of patients in the case and control groups was 61.37 (13.47) and 63.19 (13.95) years, respectively. In the case group, 37 patients (61.7%), and in the control group, 114 patients (63.3%) were male. 23 cancer patients (38.3%) and 26 non-cancer patients (14.4%) died. The results of logistic regression as well as the Cox model showed the variables of age, blood oxygen level (SpO2), admission to the intensive care unit, and cancer history as significant for patient death (P <0.05).
Conclusion:
To study the effect of demographic, clinical, and laboratory results on the risk of death among COVID-19 patients with and without a cancer history, control group in this study was selected by PSM method. The results of this study have indicated cancer history to be one of the factors affecting the mortality of patients with COVID-19 in addition to age variables, blood oxygen levels (SpO2), and admission to the intensive care unit (ICU).
1. INTRODUCTION
Since the report of its first case in Wuhan, China, in December 2019, coronavirus (COVID-19) caused by acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide. According to the Center for Systems Science and Engineering (CSSE) at Johns Hop Keynes University, COVID-19 has infected more than 25 million people in more than 200 countries and has left more than 840,000 dead as of August 31, 2020 [1, 2].
The risk of death among patients with chronic underlying diseases, especially incurable diseases, who are prone to coronary heart disease due to immune suppression, should be evaluated and compared [3]. In patients with COVID-19 who have underlying diseases, such as cancer, the severity of the disease and the risk of death are both high [4-6]. Patients with cancer are typically at high risk for viral pneumonia, with an increased risk of severe infection and also more fatal outcomes than non-cancer patients [7]. The most common symptoms are fever (6.98%), dry cough (4.59%), and shortness of breath, as well as other symptoms, such as fatigue (6.69%), muscle ache, diarrhea, sore throat, loss of olfactory and taste, and abdominal pain. At-risk groups include the elderly, pregnant women, and people with underlying medical conditions, such as chronic bronchitis, emphysema, heart disease, cancer, or diabetes [8]. While no study has yet examined the immune response of patients with malignancy to a co-infection with SARS-CoV-2, it is unclear whether the immunosuppressive status of patients with malignancy predisposes them to a more severe course of COVID-19.
It has been suggested that cancer patients with COVID-19 are at increased risk for a more severe course of the disease and a poorer outcome of treatment than non-cancer patients with COVID-19. However, the studies and patient groups have been mainly composed of patients with solid tumors due to the small number of samples [9-12]. Wang et al. showed the severity of complications to be more severe in patients with malignant diseases [13]. Based on the findings of Dai et al.'s study, cancer patients are significantly more likely to require ICU admission, have severe disease symptoms, or die [9]. In their study, Deng et al. [14] found the risk of mortality in cancer patients to be higher than in patients without cancer, while Brar et al. [15] found patients with COVID-19 and cancer to have similar outcomes to those without cancer.
This study was a case-control study in which the control group using the propensity score matching (PSM) method was matched with the case group since there have only been a limited number of studies comparing mortality and thefactors playing a role in it in patients with a history of cancer. We evaluated the effect of cancer history on patient mortality in the presence of demographic, clinical, and laboratory characteristics, as well as the effect of variables on patient survival.
2. MATERIALS AND METHODS
In this case-control study, 60 hospitalized cancer patients with concomitant SARS-CoV-2 infection confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR) assay were included. The frequency of types of cancer is presented in Flowchart 1. For the control group, 180 patients with similar age, sex, smoking, and other underlying diseases, including hypertension, diabetes, lung and heart disease, selected among 2160 hospitalized patients with confirmed COVID-19 using the PSM method from the same time span without a cancer diagnosis, were recruited. In this study, all demographic and clinical variables were matched to increase the validity of the analysis and results. All demographic characteristics, clinical data, underlying diseases, and disease symptoms were extracted from patients' medical records. Moreover, laboratory tests were performed during hospitalization. In this study, two outcomes were considered, including a binary clinical outcome (death or recovery) and survival time. Survival time was considered as the time from admission to the hospital to death or discharge from the hospital (in days).
2.1. Statistical Analysis
Demographic and clinical characteristics were described as mean and standard deviation (SD) for continuous variables, and categorical variables have been presented as frequency (percentage). To compare the categorical variables, Pearson’s chi-square test was used, and to compare the continuous variables, t-test was used.
A propensity score matching was performed to estimate the effect of cancer by accounting for the covariates statistically significant in the multivariable model. For each cancer patient, three comparable patients were selected in the non-cancer population (1:3 ratio) [16].
The possible factors identified with univariable logistic regression were entered into the multivariable logistic regression to determine independent predictors of patient outcome. Univariable and multivariable logistic regression was used to estimate the odds ratio (OR) of patients' death and to explore the risk factors of death from COVID-19 among patients with cancer. Stepwise regression using the backward selection (Wald) method was also utilized to conduct variable selection. The final model only included the variables that contributed significantly to the model.
The univariable and multivariable Cox models, and their 95% confidence intervals (CI) were also used to compare patient survival time and the hazard ratio (HR). Kaplan–Meier curves were plotted for the time from admission to death (in days) for patients who died.
Statistical analysis was performed using SPSS software version 27 and the R package. A P-value less than 0.05 was considered statistically significant. The “MatchIt” package was used for PSM. The “GLM”, “survival” and “survminer” package was used to fit the logistic and Cox proportional hazard regression model.
3. RESULTS
The steps of selecting control group patients using PSM are shown in Flowchart 1. Flowchart 2 shows the frequency of types of cancer and the patients affected by active cancer or in treatment. A mean (SD) age of 61.37(13.47) in the case group and 63.19 (13.95) in the control group years was found, and 61% of patients in the case group and 63% of patients in the control group were male. Tables 1 and 2 show the laboratory parameters, demographic and clinical characteristics of patients in the case and control groups. On the other hand, the results of Table 1 show no statistically significant differences in the other laboratory characteristics between cancer and non-cancer groups of patients with COVID-19, except for NUT and lymphocyte variables. The table shows 23 deaths among 60 cancer patients (38%) and 26 deaths among 180 non-cancer patients (14%). There was no Statistically significant difference in the variables of age, gender, smoking and use of tobacco, and underlying diseases, such as hypertension, heart disease, diabetes, lung disease, renal failure, in the two groups, and they were minimized by comparison based on the skewed score between the two groups, and the relative risk of death (HR) and mortality (OR) in the two cancerous groups (case group) and non-cancerous (control group) were completely matched. Table 3 shows the results of univariable logistic regression used to compare mortality rates among patients. The multivariable model was applied to all variables with a value of less than 0.1, and then the most appropriate variables were selected by stepwise and regression methods and tested; the results are shown in Table 4. This resulted in a modified logistic regression model. The variables of age, blood oxygen level, admission to the intensive care unit, and cancer history, were found to affect the risk of death in patients (P <0.05). According to the results of Table 4, with one year of age increase or one unit of decreased blood oxygen level, the risk of death of patients increased by 4%. It was estimated that patients admitted to the intensive care unit had a 6.57-fold higher chance of dying than other patients, and that patients with a history of cancer had a 5.03-fold higher chance of dying.
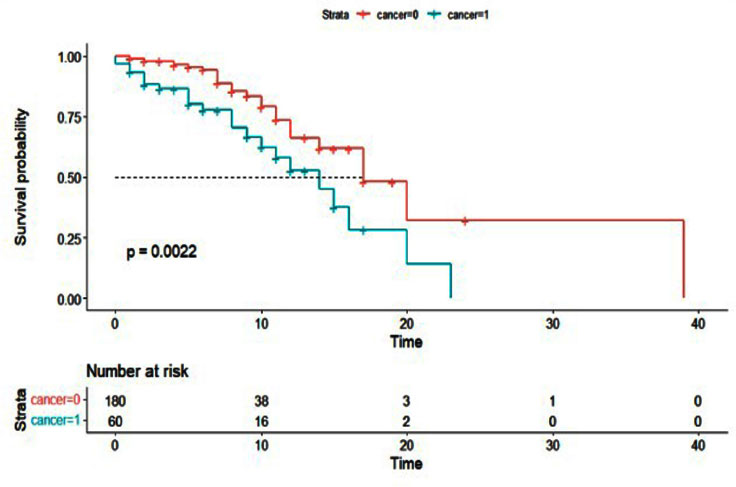
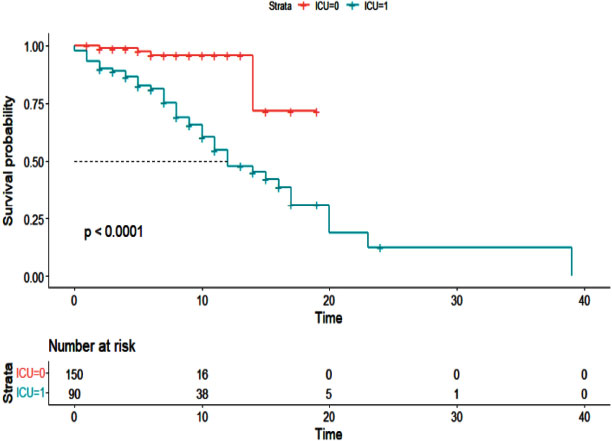
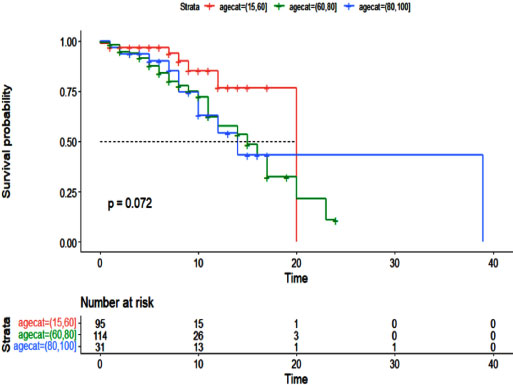
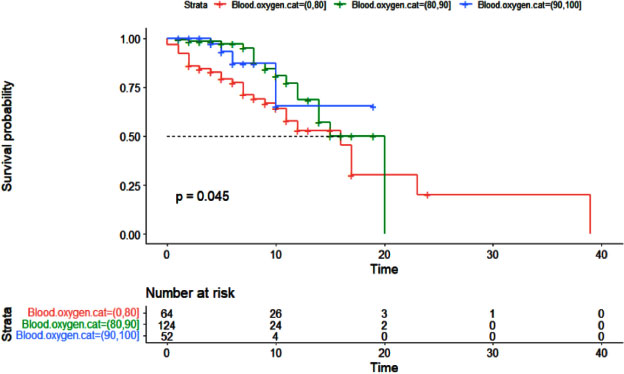
Additionally, the Cox proportional hazards model was used to examine the effect of cancer history on the survival of patients with COVID-19 (Table 5). As with the logistic regression model, all variables were entered into the multivariable model with a value of less than 0.01. The results of the multivariable Cox hazard model are shown in Table 6. The results showed the variables of age, blood oxygen level, admission to the intensive care unit, and cancer history to affect the survival of patients with COVID-19 (P <0.05). An increase of one year in age or a decrease of one unit in blood oxygen level increased the hazard of death for patients by 2%. Intensive care unit patients had a 5.10 times higher hazard of death than other patients, and cancer patients had a 2.43 times higher hazard of death. Figs. (1-5) display the survival rate, as well as the half-life of patients from admission to death or discharge (days) using the Kaplan-Meyer method. The median duration of hospitalization in cancer patients was 14 days, and in non-cancer patients, it was 17 days.
| Variable |
Case group (n=60) Mean(SD) |
Control Group (n=180) Mean (SD) |
p-value |
|---|---|---|---|
| Lymphocyte | 23.8 (11.5) | 20.1 (10.8) | 0.02 |
| Mono | 3.2 (6.6) | 3 (1.8) | 0.82 |
| NUT | 71.9 (12.3) | 75.7 (12) | 0.03 |
| CBC | 6.6 (3.3) | 8.2 (5.3) | 0.01 |
| LDH | 578 (277.4) | 662.5 (616.3) | 0.19 |
| HCT | 41.9 (7) | 42.9 (6.4) | 0.31 |
| Hb | 13.7 (2.4) | 14.2 (3.6) | 0.22 |
| K | 4.2 (0.6) | 4.2 (0.5) | 0.36 |
| Plat | 186.6 (64.9) | 196.8 (72.9) | 0.29 |
| Alp | 202 (105.9) | 206.6 (75.1) | 0.74 |
| PTT | 32.7 (10.2) | 32.9 (6.5) | 0.89 |
| SGPT | 34.1 (44.1) | 52.7 (117.7) | 0.11 |
| SGOT | 38.7 (36.4) | 79 (263.5) | 0.07 |
| CPK | 261.9 (506.4) | 209.3 (336.4) | 0.49 |
| ESR | 39.1 (28.8) | 39.5 (25.8) | 0.93 |
| BUN | 20.7 (15.7) | 21.3 (15.5) | 0.79 |
| BS | 133.4 (58.5) | 145.3 (69.1) | 0.25 |
| CR | 1.3 (1.6) | 1.3 (0.9) | 0.72 |
| PT | 13.5 (2.8) | 13.4 (1.7) | 0.78 |
| Characteristics | |||
|---|---|---|---|
|
Case Group (n=60) |
Control Group (n=180) |
p-value | |
| Age [mean (SD)] | 61.37 (13.47) | 63.19 (13.95) | 0.37 |
| Sex=male (%) | 37 (61.7) | 114 (63.3) | 0.94 |
| Resident (urban) | 53 (88.3) | 145 (80.1) | 0.83 |
| Temperature [mean (SD)] | 37.31 (0.75) | 37.47 (0.88) | 0.20 |
| Systolic BP [mean (SD)] | 123.27 (17.97) | 12.48 (20.8) | 0.34 |
| Diastolic BP [mean (SD)] | 76.03 (12.80) | 76 (11.05) | 0.99 |
| SpO2 [mean (SD)]* | 84.28 (10.34) | 79.85 (15.24) | 0.04 |
| LOH in ICU [mean (SD)] | 6.43 (3.4) | 5.27 (4.48) | 0.26 |
| Total LOH [mean (SD)] * | 9.13 (16.4) | 5.7 (3.9) | 0.01 |
| Oxygen therapy (yes) (%) | 54 (71.2) | 164 (91.1) | 0.42 |
| Admission to ICU (yes) (%) | 29 (48.3) | 61 (33.9) | 0.06 |
| Length of ICU stay [mean (SD)] | 3.02 (4.72) | 2.24 (5.05) | 0.30 |
| Mechanical ventilation (yes)* | 21 (35) | 31 (17.2) | 0.01 |
| Addict | 4 (6.7) | 15 (8.3) | 0.79 |
| Smoking (yes) (%) | 3 (5) | 12 (6.7) | 0.77 |
| Pulmonary disease (yes) (%) | 4 (6.7) | 13 (7.2) | 0.99 |
| Heart (yes) (%) | 7 (11.7) | 17 (9.4) | 0.80 |
| Kidney | 6 (10) | 11 (6.1) | 0.38 |
| Hypertension | 15 (25) | 41 (22.8) | 0.86 |
| Diabetes | 14 (23.3) | 25 (13.9) | 0.13 |
| Other | 19 (31.7) | 62 (34.4) | 0.81 |
| Death* | 23 (38.3) | 26 (14.4) | <0.01 |
| Diarrhea (yes) (%) | 4 (6.7) | 13 (7.2) | 0.98 |
| Dry cough (yes) (%)* | 14 (23.3) | 72 (40) | 0.03 |
| Sputum cough (yes) (%) | 17 (28.3) | 35 (19.4) | 0.20 |
| Muscle pain (yes) (%) | 20 (33.3) | 80 (44.4) | 0.17 |
| Fever (yes) (%) | 29 (48.3) | 82 (45.5) | 0.82 |
| Chills (yes) (%) | 23 (38.3) | 73 (40.5) | 0.88 |
| Sore throat (yes) (%) | 0 | 9 (5) | 0.12 |
| Nausea (yes) (%) | 12 (20) | 36 (20) | 0.97 |
| Headache (yes) (%) | 7 (11.7) | 36 (20) | 0.21 |
| Fatigue (yes) (%) | 0 | 7 (3.9) | 0.20 |
| Vomiting (yes) (%) | 7 (11.7) | 29 (6.1) | 0.53 |
| Asthma (yes) (%) | 33 (55) | 103 (57.2) | 0.88 |
| Runny nose (yes) (%) | 0 | 2 (1) | 0.96 |
| Characteristics ID | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age* | 1.05 | 1.02 | 1.07 | <0.01 |
| Sex (male) | 1.43 | 0.74 | 2.88 | 0.29 |
| Smoking | 0.26 | 0.01 | 1.36 | 0.20 |
| Hypertension (yes) | 1.74 | 0.59 | 2.51 | 0.55 |
| Temperature | 2.53 | 0.65 | 1.46 | 0.93 |
| SpO2* | 0.93 | 0.90 | 0.95 | <0.01 |
| Pulmonary disease (yes) | 1.69 | 0.52 | 4.83 | 0.34 |
| Kidney failure (yes) | 1.68 | 0.51 | 4.82 | 0.33 |
| Diabetes (yes) | 1.43 | 0.62 | 3.11 | 0.38 |
| Heart disease (yes) | 1.99 | 0.25 | 1.09 | 0.09 |
| Cancer (yes)* | 3.68 | 1.89 | 7.19 | <0.01 |
| Other diseases (yes) | 1.18 | 0.60 | 2.52 | 0.62 |
| Admission to ICU (yes)* | 5.74 | 11.31 | 23.83 | <0.01 |
| Oxygen therapy (yes) | - | - | - | 0.98 |
| Length of ICU stay* | 1.19 | 1.11 | 1.28 | <0.01 |
| Mechanical ventilation* | 72.95 | 31.58 | 403.83 | <0.01 |
| Asthma (yes) | 1.14 | 0.60 | 2.18 | 0.69 |
| Diarrhea (yes) | 0.82 | 0.18 | 2.66 | 0.77 |
| Dry cough (yes)* | 0.45 | 0.21 | 0.90 | 0.03 |
| Muscle pain (yes) | 0.55 | 0.27 | 1.06 | 0.08 |
| Fever (yes) | 1.27 | 0.68 | 2.39 | 0.45 |
| Chills (yes) | 0.84 | 0.43 | 1.59 | 0.60 |
| Sore throat (yes) | 1.12 | 0.16 | 4.81 | 0.89 |
| Nausea (yes) | 0.74 | 0.30 | 1.62 | 0.47 |
| Vomit (yes) | 0.93 | 0.35 | 2.17 | 0.87 |
| Fatigue (yes) | 0.64 | 0.03 | 3.88 | 0.68 |
| Headache (yes) | 0.46 | 0.15 | 1.14 | 0.12 |
| Sputum cough (yes) | 1.22 | 0.57 | 2.51 | 0.59 |
| p-value | 95% CI | OR | Characteristics | |
|---|---|---|---|---|
| Upper | Lower | |||
| 0.02 | 1.07 | 1.01 | 1.04 | Age* |
| <0.01 | 13.26 | 2.04 | 5.03 | Cancer (yes)* |
| <0.01 | 0.99 | 0.93 | 0.96 | SpO2* |
| <0.01 | 23.52 | 7.32 | 6.57 | Admission to ICU (yes)* |
| Characteristics ID | HR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 1.02 | 0.99 | 1.04 | 0.09 | |
| Sex (male) | 1.16 | 0.63 | 2.13 | 0.64 | |
| Smoking | 0.26 | 0.36 | 1.96 | 0.19 | |
| Hypertension (yes) | 0.88 | 0.46 | 1.70 | 0.71 | |
| Temperature | 1.02 | 0.68 | 1.54 | 0.90 | |
| SpO2* | 0.98 | 0.96 | 0.99 | <0.01 | |
| Admission to ICU (yes)* | 7.00 | 2.71 | 18.13 | <0.01 | |
| Oxygen therapy (yes) | - | - | - | 0.89 | |
| Length of ICU stay* | 1.01 | 0.90 | 0.99 | 0.04 | |
| Mechanical ventilation* | 3.54 | 10.6 | 111.8 | <0.01 | |
| Pulmonary disease (yes) | 1.38 | 0.54 | 3.52 | 0.49 | |
| Kidney failure (yes) | 1.93 | 0.76 | 4.90 | 0.17 | |
| Diabetes (yes) | 0.85 | 0.41 | 1.72 | 0.65 | |
| Heart disease (yes) | 1.80 | 0.96 | 2.68 | 0.07 | |
| Cancer (yes)* | 2.34 | 1.33 | 4.14 | <0.01 | |
| Other diseases (yes) | 0.82 | 0.45 | 1.50 | 0.53 | |
| Asthma (yes) | 1.25 | 0.70 | 2.24 | 0.45 | |
| Diarrhea (yes) | 1.06 | 0.33 | 3.46 | 0.92 | |
| Dry cough (yes) | 0.67 | 0.34 | 1.32 | 0.24 | |
| Muscle pain (yes) | 1.08 | 0.57 | 2.02 | 0.82 | |
| Fever (yes) | 1.28 | 0.73 | 2.27 | 0.39 | |
| Chills (yes) | 1.25 | 0.69 | 2.28 | 0.46 | |
| Sore throat (yes) | 1.78 | 0.43 | 7.40 | 0.43 | |
| Nausea (yes) | 0.89 | 0.41 | 1.92 | 0.77 | |
| Vomit (yes) | 1.14 | 0.51 | 2.57 | 0.75 | |
| Fatigue (yes) | 0.75 | 0.10 | 5.44 | 0.77 | |
| Headache (yes) | 0.85 | 0.33 | 2.18 | 0.74 | |
| Sputum cough (yes) | 1.32 | 0.69 | 2.55 | 0.40 | |
| Characteristics ID | HR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age* | 1.02 | 0.95 | 0.99 | 0.049 | |
| Admission to ICU (yes)* | 5.10 | 1.93 | 13.44 | <0.01 | |
| SpO2* | 0.98 | 0.96 | 0.99 | 0.049 | |
| Cancer (yes)* | 2.43 | 1.30 | 4.54 | <0.01 | |
4. DISCUSSION
By matching based on propensity scores, 60 cancer patients with COVID-19 were matched with 180 non-cancer patients with COVID-19. Moreover, none of the underlying diseases had a significant relationship with patient mortality in patients with a history of cancer. As reported by Sorouri et al. and Meng et al., there was no significant association observed between underlying diseases and patient mortality [10, 17]. Other studies have shown that high blood pressure or ischemic heart disease can increase the risk of death among patients with COVID-19 and a history of cancer [18, 19]. In the present study, due to the modified logistic regression result and Cox model, increasing age increased the death rate of COVID-19 patients with a history of cancer, despite the fact that in this study, the age variable was matched between the treatment and control groups. Therefore, we managed to reduce the number of significant variables by matching the variables, and due to the small amount of data, this work is of particular importance. However, the only variable that remained significant after adjustment was age. In the case and control groups, the mean age was 61 and 63 years, respectively (P = 0.37). Comparing the data and examining the patients in the two groups of patients with and without a history of cancer with the same age group, we concluded the presence of a significant relationship between a history of cancer and patient mortality. Immune function has been reported to largely reduce with aging [20]. There has been a significant correlation observed between the age and mortality of patients with COVID-19 [18, 20-22]. Also, studies indicate no significant correlation between age and mortality for COVID-19 patients [10, 17].
The results did not show a significant relationship between gender and mortality rate in the present study. Other studies have not found a correlation between gender and mortality in COVID-19 cancer patients [10, 17, 23]. Although, some studies have shown a relationship between gender and patient mortality [18-22]. It may be due to the matching of demographic variables. Our study found 87% of participants to experience shortness of breath, 76% to have fever, and 62% to have muscle pain. Sorouri et al.'s study reported shortness of breath and fever as the two most common symptoms [17]. In this study, shortness of breath was the most common symptom among deceased cancer patients. Sorouri et al., Young et al., and Lee et al. found shortness of breath to be significantly higher in cancer patients who died than other symptoms [17, 19, 22].
The percentage of cancer patients who died in this study was significantly higher than that of non-cancer patients (38% vs. 14%). The results of this study are consistent with those of Meng et al. 's study [10], in which the mortality rate of cancer patients versus non-cancer patients was reported to be 29% and 10%, respectively. Hospitalized patients' poor health may have contributed to the high mortality rate in this study. With univariable and multivariable logistic regression, the odds ratio of death in cancer patients was 3.68 and 5.03, respectively. Meng et al. reported a 2.98 odds ratio of a cancer patient dying [10]; Sorouri et al. reported a 5.4 odds ratio in the univariable and a 3.57 odds ratio in the multivariable model [17].
Additionally, in the present study, the univariable and multivariable Cox regression models were used to compare the survival rate and the relative risk of death of the patients. In the univariable model, the relative hazard of death was 2.34, and in the multivariable model, it was 2.43. In the study conducted by Cai et al., cancer patients were reported with a death risk that was 1.99 times greater in univariable Cox regression and 2.02 times greater in multivariable regression than other patients [24].
Hypoxia (SpO2) was significantly associated with patient mortality in this study. The results of logistic regression and Cox showed the mortality of patients to be increased by 4% and their risk of death by 2% with each unit of reduction in blood oxygen levels. Sorouri et al. and Lee et al. showed oxygen saturation to be lower in deceased patients with a history of cancer [17, 19].
In the current study, patients admitted to the intensive care unit had a 2.43 times higher relative risk of death than other patients. In the study of Elfaituri et al., the relative risk of death for patients admitted to the intensive care unit was 2.32 times higher than for other patients [23]. Safari et al. also found a significant relationship between intensive care unit admissions and mortality in patients with COVID-19 [25]. In other studies, patients with a history of cancer were more likely to be admitted to the intensive care unit [21, 26].
5. LIMITATION
The most important limitation of this study was the small number of cancer patients. Lack of staging of cancers and their therapies was the other limitation in our study.
CONCLUSION
A PSM method was used to select the control group in this case-control study. The effect of demographic, clinical, and laboratory characteristics on the risk of death in COVID-19 patients with and without cancer history was studied. Cancer history was found to be among the factors affecting death, in addition to age variables, blood oxygen level (SpO2), and admission to an intensive care unit. With a history of cancer, patients were at a higher risk of mortality, and they required intensive care to prevent infection with COVID-19 as well as timely treatment. In addition, cancer history was the only independent risk factor for COVID-19 disease among the common diseases, while other comorbidities may be influenced by other factors. A number of laboratory parameters also differed significantly between cancer and non-cancer patients, suggesting lower immunity and inflammatory reactions in COVID-19 cancer patients.
LIST OF ABBREVIATIONS
| SpO2 | = Blood Oxygen Levels |
| ICU | = Intensive Care Unit |
| COVID-19 | = Coronavirus 2019 |
| CSSE | = Center for Systems Science and Engineering |
| RT-PCR | = Reverse-transcriptase Polymerase Chain Reaction |
| OR | = Odds Ratio |
| SD | = Standard Deviation |
| CI | = Confidence Intervals |
| HR | = Hazard Ratio |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Ethic Committee of the Hamadan University of Medical Sciences, Shahid Fahmideh Ave, postal code: 6517838695, Hamadan, Iran.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee, and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


