All published articles of this journal are available on ScienceDirect.
Ranking and Prioritizing Risk Factors for Gastric Cancer
Abstract
Background:
Gastric cancer is one of the gastrointestinal tract cancers with the highest mortality rate; hence, accurate knowledge of the main causes of this disease is of paramount importance to plan, monitor, and evaluate national and regional programs to control this cancer. The present study was to rank and prioritize gastric cancer risk factors.
Methods:
In this study, gastric cancer risk factors were first extracted in a systematic review, and they were then ranked and prioritized using a focused group discussion. MICMAC software was used to rank the factors.
Results:
According to the findings, the following 13 factors had the highest effect on the incidence of gastric cancer: socioeconomic status, age, consumption of pickles and sour vegetables, salt consumption, meat consumption (red, smoked, and processed and salty), consumption of fried foods, consumption of fats and oils, consumption of fish (Salty, smoked, and processed), consumption of bread and leftovers and moldy foods, irregular eating habits, excessive daily calorie intake, smoking (cigarettes, opium, and hookah), and alcohol consumption.
Conclusion:
Prioritizing risk factors would help policymakers identify and present executive strategies and detect action priorities to manage gastric cancer risk factors. According to the findings of the present study, national planning to support vulnerable socio-economic groups, the development of screening programs, and the early detection of diseases in the early stages at an early age, and diet adjustment to increase the consumption of fresh fruits and vegetables and reduce salt, high-fat and fried foods, salted foods, and processed meats are recommended.
1. INTRODUCTION
Cancer is one of the major health problems worldwide [1]. Cancer has emerged since the beginning of human existence, and three factors ‘heredity’, ‘lifestyle,’ and ‘environment’ separately or jointly increase the risk of developing this disease in individuals [2]. The growing trend of cancers has doubled in the last three decades and is predicted to be tripled by 2030 [3]. Among cancers, gastric cancer is one of the most common gastrointestinal tract cancers [4]. Although the incidence of gastric cancer is decreasing in some advanced societies due to appropriate interventions such as health education, proper nutrition, and controlling predisposing behaviors, its incidence is rising in developing countries due to aging, poor nutrition culture, and lack of control over inappropriate behaviors such as smoking and alcohol consumption [5]. According to GlOBOCAN’s (2012) project, this disease, accounting for one million new cases, is the fifth most common malignancy worldwide, revealing a remarkable change in the incidence of this cancer over recent years [6]. From another perspective, for above eight percent of all cancer deaths, gastric cancer is the third leading cause of cancer death in both genders worldwide [6].
Gastric cancer is a disease with vague and unclear initial symptoms, late-onset, which quickly invades nearby and distant organs [7]. Moreover, it is a multi-causal disease resulting from the continuation of injuries caused by constant exposure to carcinogens [8]. Invasiveness and lack of clear clinical symptoms have made gastric cancer incurable; hence, most patients are in the advanced stage of the disease at the time of referral [7]. This cancer develops by creating cancerous tissues in the stomach at several stages over several years [8]. Gastric cancer often begins to develop in the mucosal layer and slowly spreads to other layers. Before initiating cancer, changes emerge in the mucous membranes. These initial changes rarely cause symptoms, which are often not visible [9]. Gastric cancer emerges in different parts of the stomach and is accompanied by different symptoms, leading to different treatment options for each symptom [10]. The prevalence of this disease is higher in the lower socioeconomic classes, individuals with blood type A, or positive family history [11-16]. Some risk factors for gastric cancer are unhealthy, high-salt, high-nitrate diets, a history of Helicobacter pylori infection, genetic factors, precancerous gastric lesions, and smoking [14, 17-24]. On the other hand, given the gentle nature of this disease, its diagnosis is highly challenging, and patients are usually diagnosed in advanced stages, which leads to high mortality of this disease [25]. However, if proper diagnosis and treatment are adopted in the early stages, the disease progression and its distant metastasis can be prevented; hence, the prognosis of the disease can be improved [26].
In addition to the psychological and physical burden posed by this disease, gastric cancer imposes a high economic burden on individuals and society [27]. The global economic burden of cancers in 2009 was estimated to be 280 billion US dollars. In this regard, five major cancers (stomach, lung, colon, breast, and prostate) accounted for 55% of the costs of new cancers in 2009. Of these five cancers, gastric cancer accounts for 26% of total costs [28].
According to what was mentioned and regarding the economic burden posed by gastric cancer, identifying, evaluating, and prioritizing gastric cancer risk factors and then relevant planning for prevention regarding the risk factors can be an effective measure to reduce the burden of this disease. The accurate knowledge of these factors is also essential for planning, monitoring, and evaluating national and regional cancer control programs. Accordingly, the present study was to rank and prioritize gastric cancer risk factors.
2. MATERIALS AND METHODS
This study was conducted in 2020 to rank and prioritize gastric cancer risk factors. To this end, the risk factors were first extracted in a systematic review [29]. These extracted factors were 52 and classified into eight main categories. From the 52 factors, those referred to in two or more studies were selected for ranking (25 factors). Moreover, before importing the selected factors to the ranking phase, experts’ opinions were obtained using the “focus group discussion” approach, and agreement was then reached. During the “focus group discussion” session, individuals were asked to comment on the 25 preliminary factors selected. Regarding the methodology of the focus group discussion and to reach an expert consensus on selected factors, the following steps were adopted:
2.1. Step 1. Form a Focus Group
The members of this group encompassed experts in gastrointestinal diseases and cancers. In this study, 15 experts with at least five years of experience in gastrointestinal diseases and activities in specialized cancer research centers were selected using the purposive snowball sampling method. The participants were the faculty members of medical departments affiliated with medical universities in Iran.
2.2. Step 2. Hold a Meeting
At this step, after selecting the group members, three sessions were held to examine 25 selected factors. These sessions were organized with the coordination and willingness of the members and at the Gastroenterology and Liver Research Center of Namazi Teaching and Medical Hospital located in Shiraz, Iran. Each session lasted about 1-2 hours, and the experts' comments regarding the introduced factors were found and categorized.
2.3. Step 3. Conclude
After having discussions in meetings and interacting with experts to reach an agreement on the selected factors, the findings were summarized. In cases of disagreement, the issues were scored to reach an agreement. Finally, out of the 25 factors, 23 factors were selected.
In the ranking phase, the significant risk factors for gastric cancer were ranked and prioritized using the future study approach and the MICMAC software.
A future study is a set of efforts that visualize potential futures and plan for them by searching for sources, patterns, and factors of change or stability [ 30 ]. The future study reflects how the reality of “tomorrow” is born from the change (or stability) of “today” [ 30 ].
MICMAC software is used to perform structural analysis in future studies [ 31 ]. This software is used to identify key components for extracting future scenarios, as well as prioritizing these factors in terms of effectiveness and influence [ 31 ].
This phase was conducted in four stages.
2.3.1. Step 1: Interactions of Effective Factors in Preventing Gastric Cancers
After determining 23 factors, interaction analysis was used to investigate the interactions of these factors. To this end, the matrix form of paired interaction, which consisted of the gastric cancer risk factors (23 * 23 matrix), was distributed among the experts to determine the interactions between the concerned factors. In this study, 23 gastric cancer risk factors were included in the interaction analysis matrix (a square matrix in which one factor is placed in each row and column). In this matrix, the experts were asked to answer the following question: “Does the factor in this row affect the occurrence of other factors in the columns? If yes, how much is the effect size?” In each cell, the number ‘zero’ was set to represent “no effect” if the factor in the row did not affect the occurrence of those in the columns. Otherwise, regarding the severity of this effect, the numbers ‘1,’ ‘2,’ ‘3,’ also indicated “weak effect,” “consistent effect,” and “high effect,” respectively. After completing the matrix form, the data were imported into the MICMAC software, and the data analysis was performed.
2.3.2. Step 2: System Stability-instability
After importing the interaction matrix into the MICMAC software, the system’s stability - instability status was examined since this issue would affect the analysis of the factors. To this end, a distribution diagram (dispersion map) was used, which was drawn separately regarding the direct and indirect effects of the MICMAC software.
2.3.3. Step 3: Determining the Role of each Factor and Evaluating their Effectiveness and Effect
In this phase, the role of each factor was analyzed. In this regard, each factor can play one of the following roles: “bidirectional,” “effective,” “influenced,” and “independent.” Bidirectional variables act simultaneously in a highly effective and highly influential way. Effective variables are more influential and less affected; hence, the system mainly depends on these variables. Influenced variables have a low impact and are highly affected; hence, they are highly sensitive to the evolution of effective and bidirectional variables. Independent or excluded variables have a low impact and seem to have nothing to do with the system since they do not stop the main variable or do not make it evolve in the system.
3. RESULTS
Table 1 shows 23 gastric cancer risk factors extracted from a systematic review [29] and experts’ opinions.
In the ranking phase of the study, 23 gastric cancer risk factors were examined and analyzed.
3.1. Interaction Analysis
The results of the primary analysis of matrix data and the interaction of factors, the matrix filling degree was 50.07, indicating that the selected factors had a moderate effect on each other and that the system was relatively stable.
| No. | Category | Risk Factors |
|---|---|---|
| 1 | Diet | Salt and salty diets |
| 2 | Pickles and pickled vegetables | |
| 3 | Fried foods | |
| 4 | Meat (red, smoked, processed, and salted) | |
| 5 | Irregular eating habits | |
| 6 | Fish (salted, smoked, and processed with salt) | |
| 7 | Tea and hot foods | |
| 8 | Bread and moldy leftovers | |
| 9 | Not consuming or consuming insufficient and little fresh fruits and vegetables | |
| 10 | Fats and oils | |
| 11 | Lifestyle | Alcohol |
| 12 | Smoking (tobacco, opium, and hookah) | |
| 13 | Excessive daily calories | |
| 14 | Genetic susceptibility | Gastric cancer-related genes and genotypes(role of some polymorphisms) |
| 15 | Family history | Family history of gastric cancer |
| 16 | Treatments and medical conditions | Chronic atrophic gastritis |
| 17 | History of esophageal cancer (the risk of precancerous lesions | |
| 18 | Ulcers | |
| 19 | Infections | Helicobacter pylori |
| 20 | Demographic information | Increasing age |
| 21 | Socio-economic status(income and education level) | |
| 22 | Race | |
| 23 | Occupational exposures | Occupational exposures (cement, chrome, etc.) |
3.2. System Analysis: Stability-instability Status
Fig. (1) shows the distribution map of gastric cancer risk factors in the impact-effectiveness diagram (regarding the direct effects of variables).
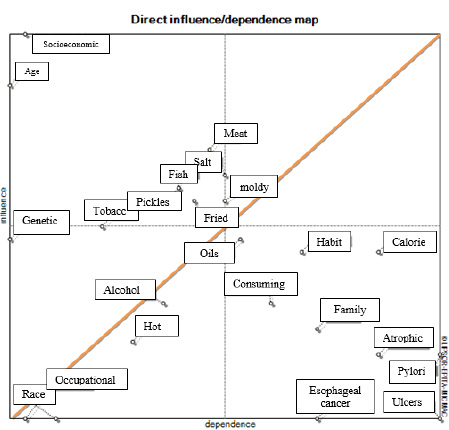
The system is relatively stable since the variables are scattered in the first and third quarters, and eight factors are in the second quarter. These factors have a high impact and are less affected. Moreover, nine factors are in the fourth quarter, which has low impact and is highly affected. Some of these variables are scattered around the diagonal axis, indicating that these variables have similar degrees of being effective and affected.
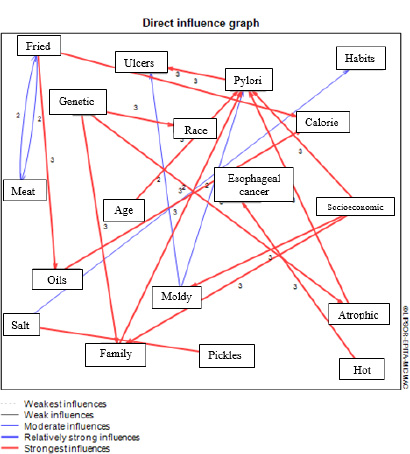
Fig. (2) shows the research matrix form based on the direct effects of the factors on each other, according to which 18 factors were detected. These factors had a direct effect on at least another factor, implying that five factors were only influenced and not presented in the diagram.
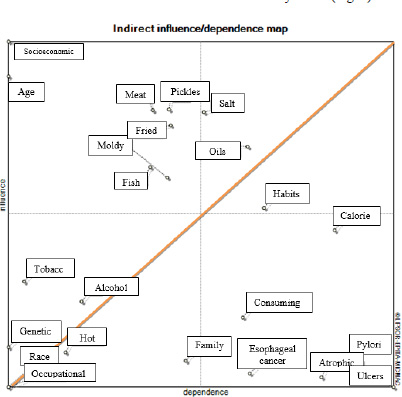
Regarding the indirect effects of the variables, the system is relatively stable (Fig. 3).
| No. | Category | Risk Factors | Sum of the Numbers in Row (Change Range 0 to 66) (Degree of Influence) | Sum of the Numbers in Column (Change Range 0 to 66) (Degree of Affected) |
|---|---|---|---|---|
| 1 | Diet | Salt and salty diets | 20 | 14 |
| 2 | Pickles and pickled vegetables | 19 | 12 | |
| 3 | Fried foods | 18 | 12 | |
| 4 | Meat (red, smoked, processed, and salted) | 22 | 13 | |
| 5 | Irregular eating habits | 14 | 19 | |
| 6 | Fish(salted, smoked, and processed with salt) | 19 | 11 | |
| 7 | Tea and hot foods | 7 | 8 | |
| 8 | Bread and moldy leftovers | 18 | 14 | |
| 9 | Not consuming or consuming insufficient and little fresh fruits and vegetables | 10 | 17 | |
| 10 | Fats and oils | 15 | 15 | |
| 11 | Lifestyle | Alcohol | 10 | 10 |
| 12 | Smoking (Tobacco, opium, and hookah) | 16 | 6 | |
| 13 | Excessive daily calories | 14 | 24 | |
| 14 | Genetic susceptibility | Gastric cancer-related genes and genotypes (role of some polymorphisms) | 15 | 0 |
| 15 | Family history | Family history of gastric cancer | 8 | 20 |
| 16 | Treatments and medical conditions | Chronic atrophic gastritis | 6 | 24 |
| 17 | History of esophageal cancer(the risk of precancerous lesions) | 1 | 20 | |
| 18 | Ulcers | 1 | 28 | |
| 19 | Infections | Helicobacter pylori | 6 | 28 |
| 20 | Demographic information | Increasing age | 27 | 0 |
| 21 | Socio-economic status(income and education level) | 31 | 1 | |
| 22 | Race | 1 | 3 | |
| 23 | Occupational exposures | Occupational exposures(cement, chrome, etc.) | 1 | 1 |
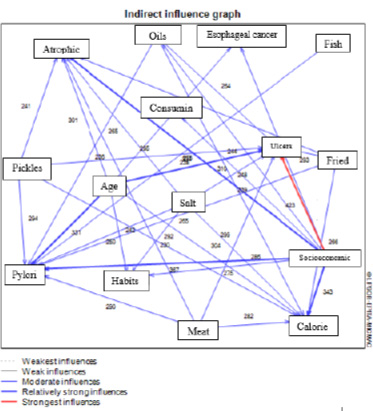
In the research matrix form, 15 factors are presented based on the indirect effects of factors on each other. These factors have affected at least one other factor, and eight factors, which have only been affected by indirect effects and are not included in the diagram (Fig. 4).
4. ASSESSING THE IMPACT AND EFFECTIVENESS OF FACTORS
In the interaction matrix, the sum of the numbers in each row shows the degree of influence of that factor, and the sum of the columns for each factor also presents the degree of its being affected. Table 2 presents the total score of rows and columns for each factor.
The analytical results of this matrix, the most influential factors were socio-economic status (income and level of education), age, meat (red, smoked, and processed), and salt. Moreover, the most affected factors were gastric ulcers, Helicobacter pylori, excessive daily calorie intake, and chronic atrophic gastritis.
Figs. (5 and 6) show the output of the MICMAC software regarding influencing and influenced variables, respectively, indicating the direct impact of the above findings visually.
4.1. System Analysis: Determining the Role of each Factor
Regarding the position of each factor in the distribution map of the impact-effectiveness figure, the role of each factor in the system can be determined. Table 3 presents the classification of variables by their roles.
The first category is influential variables, these factors are located in the second quarter, close to the vertical axis, and include socio-economic status, age, smoking (tobacco, opium, and hookah), meat (red, smoked, processed, and salted), salt, pickles and pickled vegetables, fish (salted, smoked, and processed with salt), and fried foods. The second category refers to the bidirectional variables located in the first quarter and encompasses bread and moldy foods and leftovers. The third category shows the affected variables or outcome variables, which are in the fourth quarter. Nine variables are assigned to this category: stomach ulcers, Helicobacter pylori, excessive daily calorie intake, chronic atrophic gastritis, history of esophageal cancer, family history of gastric cancer, irregular eating habits, and not eating or consuming too little or not enough fruits and vegetables. The fourth category represents independent variables located in the third quarter and consists of alcohol, tea, and hot foods, race, occupational exposure, and genetic factors (Table 3).
5. RANKING AND SELECTING THE MAIN GASTRIC CANCER RISK FACTORS
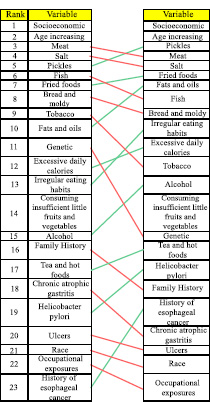
Comparing the ranking results of the variables by the analysis of direct effects and the analysis of their indirect effects showed an insignificant rank shift of the variables. Accordingly, to detect the most influential factors, the factor ranks were not much different regarding the direct effects and indirect effects. Ranking influential variables by the direct effects of factors on each other and their indirect effects are presented in Table 4.
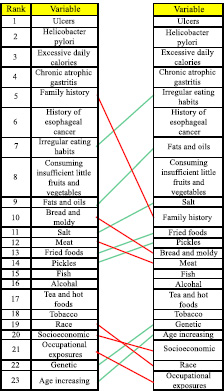
The most influential risk factors for gastric cancer regarding the direct and indirect effects (Table 4) include socio-economic status, age, consumption of pickles and pickled vegetables, consumption of salt, consumption of meat (red, smoked, processed, and salted), consumption of fried foods, consumption of fats and oils, consumption of fish (salted, smoked, and processed), consumption of bread, leftovers, and moldy foods, lack of consumption or insufficient consumption of fresh fruits and vegetables, irregular eating habits, excessive daily caloric intake, smoking (cigarettes, opium, and hookah), and alcohol consumption.
| No. | The Role of Variables based on MICMAC Output | Risk Factors |
|---|---|---|
| 1 | Effective | Socio-economic status(income and education level) |
| Increasing age | ||
| Smoking (Tobacco, opium, and hookah) | ||
| Meat (red, smoked, processed, and salted) | ||
| Salt and salty diets | ||
| Pickles and pickled vegetables | ||
| Fish (salted, smoked, and processed with salt) | ||
| Fried foods | ||
| 2 | Bidirectional | Bread and moldy leftovers |
| 3 | Influenced | Ulcers |
| Helicobacter pylori | ||
| Excessive daily calories | ||
| Chronic atrophic gastritis | ||
| History of esophageal cancer(the risk of precancerous lesions) | ||
| Family history of gastric cancer | ||
| Irregular eating habits | ||
| Not consuming or consuming insufficient and little fresh fruits and vegetables | ||
| Fats and oils | ||
| 4 | Independent | Alcohol |
| Tea and hot foods | ||
| Race | ||
| Occupational exposures(cement, chrome, etc.) | ||
| Gastric cancer-related genes and genotypes (role of some polymorphisms) |
| The Variable Ranking based on Direct Effect | The Variable Ranking based on Indirect Effect | ||||||||||
| Rank of Effectiveness | Variables | Rate of Effectiveness | Rank of Affected | Variable | Rate of Affected | Rank of Effectiveness | Variables | Rate of Effectiveness | Rank of Affected | Variable | Rate of Affected |
| 1 | Socio-economic status | 3811 | 1 | Ulcers | 3749 | 1 | Socio-economic status | 3837 | 1 | Ulcers | 3721 |
| 2 | Increasing age | 3430 | 2 | Helicobacter pylori | 3310 | 2 | Increasing age | 3405 | 2 | Helicobacter pylori | 3326 |
| 3 | Pickles | 3066 | 3 | Excessive daily calories | 3173 | 3 | Pickles | 3071 | 3 | Excessive daily calories | 3162 |
| 4 | Salt | 3058 | 4 | Chronic atrophic gastritis | 3046 | 4 | Salt | 3043 | 4 | Chronic atrophic gastritis | 3061 |
| 5 | Meat | 3037 | 5 | Irregular eating habits | 2487 | 5 | Fried foods | 3031 | 5 | History of esophageal cancer | 2351 |
| (red, smoked, processed, and salted) | |||||||||||
| 6 | Fried foods | 2892 | 6 | History of esophageal cancer | 2348 | 6 | Meat | 3029 | 6 | Irregular eating habits | 2291 |
| (red, smoked, processed, and salted) | |||||||||||
| 7 | Fats and oils | 2660 | 7 | Fats and oils | 2328 | 7 | Fish (salted, smoked, and processed with salt) | 2464 | 7 | Fats and oils | 2287 |
| 8 | Fish | 2434 | 8 | Not consuming or consuming insufficient and little fresh fruits and vegetables | 2281 | 8 | Fats and oils | 2461 | 8 | Not consuming or consuming insufficient and little fresh fruits and vegetables | 2273 |
| (salted, smoked, and processed with salt) | |||||||||||
| 9 | Bread and moldy leftovers | 2321 | 9 | Salt | 1902 | 9 | Bread and moldy leftovers | 2345 | 9 | Family history | 1881 |
| 10 | Irregular eating habits | 1987 | 10 | Family history | 1725 | 10 | Irregular eating habits | 2008 | 10 | Salt | 1801 |
| 11 | Excessive daily calories | 1742 | 11 | Pickles | 1562 | 11 | Excessive daily calories | 1749 | 11 | Pickles | 1541 |
| 12 | Smoking (tobacco, opium, and hookah) | 1179 | 12 | Fried foods | 1579 | 12 | Smoking (Tobacco, opium, and hookah) | 1191 | 12 | Meat | 1505 |
| (red, smoked, processed, and salted) | |||||||||||
| 13 | Alcohol | 860 | 13 | Bread and moldy leftovers | 1546 | 13 | Not consuming or consuming insufficient and little fresh fruits and vegetables | 817 | 13 | Fried foods | 1498 |
| 14 | Not consuming or consuming insufficient and little fresh fruits and vegetables | 786 | 14 | Meat | 1405 | 14 | Alcohol | 806 | 14 | Bread and moldy leftovers | 1481 |
| (red, smoked, processed, and salted) | |||||||||||
| 15 | Genetic | 458 | 15 | Fish | 1380 | 15 | Genetic | 472 | 15 | Fish | 1361 |
| (salted, smoked, and processed with salt) | (salted, smoked, and processed with salt) | ||||||||||
| 16 | Tea and hot foods | 401 | 16 | Alcohol | 712 | 16 | Tea and hot foods | 393 | 16 | Alcohol | 691 |
| 17 | Helicobacter pylori | 346 | 17 | Tea and hot foods | 555 | 17 | Helicobacter pylori | 331 | 17 | Tea and hot foods | 531 |
| 18 | Family history | 309 | 18 | Smoking (Tobacco, opium, and hookah) | 152 | 18 | Family history | 298 | 18 | Smoking (Tobacco, opium, and hookah) | 148 |
| 19 | History of esophageal cancer | 172 | 19 | Race | 0 | 19 | History of esophageal cancer | 189 | 19 | Race | 0 |
| 20 | Chronic atrophic gastritis | 128 | 19 | Occupational exposures | 0 | 20 | Chronic atrophic gastritis | 149 | 19 | Occupational exposures | 0 |
| 21 | Ulcers | 31 | 19 | Genetic | 0 | 21 | Ulcers | 38 | 19 | Genetic | 0 |
| 21 | Race | 31 | 19 | Increasing age | 0 | 22 | Race | 36 | 19 | Increasing age | 0 |
| 21 | Occupational exposures | 31 | 19 | Socio-economic status | 0 | 23 | Occupational exposures | 18 | 19 | Socio-economic status | 0 |
6. DISCUSSION
The present study was to rank and prioritize the main gastric cancer risk factors. Many studies have reported gastric cancer risk factors; however, to the best knowledge of the researchers, this is the first study in which gastric cancer risk factors were prioritized. According to the findings, socio-economic status was the most influential cause of gastric cancer. Similarly, Heidariarjloo et al. (2015), in their study, concluded that socioeconomic factors such as unemployment rate, household size, low level of education, urbanization ratio, and household expenditure were associated with the prevalence of gastric cancer [32]. Moreover, Ueda et al. (2009) conducted a study to analyze socio-economic differences in the prevalence, mortality, and survival rates of cancer in Japan. They reported a strong inverse relationship between homeownership with age-adjusted cancer mortality [33]. Given that socioeconomic inequalities have a great impact on the incidence of gastric cancer, and since the management and control of social and economic factors are beyond the scope of the health system, each country’s general policies should consider the fair distribution of resources, support a healthy lifestyle among deprived groups, and address social factors affecting health, including recreation and employment rates, to prevent the occurrence of disease by promoting socio-economic indicators in the long term.
In the present study, aging as the second gastric cancer risk factor has a high significance and priority among other risk factors. In this regard, Rajaeefard et al. (2011), in their study in Fars province, Iran, showed that most patients with this disease are usually affected during the seventh and eighth decades of their lives [34]. Nourozinia et al. (2013) stated that individuals above 70 years of age are more prone to cancer [35]. Considering gastric cancer screening for individuals aged above 70 years in the health centers’ programs can be effective in the prevention and timely detection of this disease.
Findings of the present study revealed that high salt intake also is of high priority (the third most influential factor) for gastric cancer, among other risk factors. Park et al. (2016) stated that in the age group >40 years, the consumption of salty foods increases the risk of gastric cancer [36]. The results of a meta-analysis showed that the consumption of salt and salted foods is associated with an increased risk of gastric cancer [37]. Pourfarzi (2009) also mentioned that high salt consumption in northern Iran has a double effect on gastric cancer [38]. Accordingly, interventions should be developed to reduce the amount of salt consumed in families. For example, they recommended the reduction of salt for baking bread in bakeries and preparing food in restaurants, as well as general training to consume less salt.
Meat consumption (red, smoked, and processed) also is of high priority as one of the risk factors for gastric cancer. In line with this finding, a study in Ardabil found that the predominant use of red meat in the northern part of Iran increases the risk of gastric cancer [39]. The findings of this study also indicated that some other dietary factors such as consumption of fried foods, consumption of fats and oils, consumption of fish (salty, smoked, and processed), consumption of bread, leftovers and moldy foods, irregular eating habits, and excessive daily calorie intake are the most influential factors in the development of gastric cancer. Iravani (2013) stated that environmental factors such as nutrition and health, Helicobacter pylori infection, and genetic background are the risk gastric cancer factors; however, each of these factors affects other factors. For example, poor nutrition increases the inflammatory effect of bacteria [40]. In their study, Daniyal et al. (2015) concluded that diet is effective in the development of gastric cancer [19]. Hoshyar (2015) reported that proper diets are one of the effective ways to prevent gastric cancer [41]. Gao et al. (2011), in their case-control study, revealed that individuals with gastric cancer regularly consume pickles in their meals [42]. Accordingly, these findings, in line with those of the present study, highlight the role of diets as an effective factor in gastric cancer. In contrast, Niknam and Azadbakht (2012) claimed that there was no clear evidence on the relationship between meat, fish, black tea, and coffee with gastric cancer [43].
As the findings of this study suggested, the lack of consumption or insufficient consumption of fresh fruits and vegetables is another underlying cause of gastric cancer with high priority and ranking. In this regard, several studies have reported the effect of obesity, non-consumption of fruit and vegetables, and smoking among individuals and regions with lower social status on the incidence of gastric cancer [32, 44, 45]. The relationship between the prevalence of gastric cancer and the low costs of fruit and household food has also been proven in several studies [46, 39]. This finding is consistent with the findings of this study. Lai et al. (2016) concluded that the consumption of fruits and citrus reduces the risk of gastric cancer [47]. The findings of a high-indexed study (2012) revealed that gastric cancer risk was reduced by the high consumption of fresh fruits and vegetables [41]. Further, a study in Iran suggested that a health education program based on a health belief model can be effective and influential in observing the nutritional points associated with gastric cancer [48]. Accordingly, holding training classes at health centers and using specialists in health education and nutrition sciences is recommended to promote individuals’ awareness, attitude, and practice.
According to the present study, the use of tobacco, including cigarettes, hookah, and alcohol, as the causes of a higher incidence of gastric cancer in individuals, is of high priority. Icli (2011) noted that smoking had a significant effect on cancer. In this regard, Lai et al. (2016) mentioned that hookah use increased the risk of cancer by one and a half times compared to cases, not hookah and that individuals who use hookah continuously and frequently are 2.7 times more likely to develop cancer. However, they concluded that the disease was not associated with alcohol or smoking [47]. One of the reasons could be the low number of smokers among their participants. Many studies, including Rajaeefard et al. (2011), Heidariarjloo et al. (2015), Hiscock et al. (2012), and Di Cesare et al. (2013) [32, 34, 44, 45] have documented the relationship between smoking and diseases.
CONCLUSION
The findings of this study indicate that socio-economic status, aging, diet-related factors, smoking, and alcohol are of high priority among other risk factors for gastric cancer. Accordingly, national planning to support vulnerable socio-economic groups, screening programs, and the early detection of disease in the early stages at an early age, diet adjustment to increase the consumption of fresh fruits and vegetables and reduce salt intake, high-fat and fried foods, salty foods, and processed meats are recommended to prevent this disease. It is also suggested to hold training classes in health centers and use specialists in health education and nutrition sciences to promote health awareness and literacy in the community.
AUTHORS’ CONTRIBUTIONS
Study conception (ARY, GM, KBL, ZK, SHB), Data acquisition, analysis (ARY, ZK, SHB), Data interpretation (KBL, ZK, ARY, SHB, PB), Manuscript drafting (ARY, ZK, MK), Manuscript substantial revisions (ARY, GM, KBL, ZK), Approved submitted version (ARY,SHB, GM, KBL, ZK, PB). All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the Shiraz University of Medical Sciences (Code: 13918).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All participants provided written informed consent to participate in this study.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author [Z.K] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise
ACKNOWLEDGMENTS
The researchers express their gratitude to the esteemed Vice Chancellor for Research and Technology of the Shiraz University of Medical Sciences, who made the research possible with their material and spiritual support.


