All published articles of this journal are available on ScienceDirect.
Environmental Exposures, Characteristics, and Treatment Outcomes of Hypersensitivity Pneumonitis at a Rural Appalachian Academic Medical Center: A Cross-sectional Study
Abstract
Purpose:
Hypersensitivity pneumonitis (HP) is a form of interstitial lung disease (ILD) caused by inhalational exposure to an antigen. Little is known about the exposures, outcomes, and management of HP in rural Appalachian patients.
Methods:
A retrospective cross-sectional study was conducted from January 1, 2017, to June 30, 2022, at a tertiary academic medical center. Sixty-two patients were initially screened, and seven patients fulfilled the inclusion criteria. The primary outcome was the exposure leading to HP. Secondary outcomes included the disease stage at diagnosis, smoking rate, treatment modalities, ILD exacerbation rate, and mortality.
Results:
Birds and mold are the more common exposures attributed to HP (both n=2, 28.57%). Novel exposures to continuous positive airway pressure devices, vapor and/or fumes, and fiberglass were noted (each n=1, 14.28%). Three patients (42.85%) had fibrotic HP at presentation. Most patients were ever-smokers (n=4, 57.14%). All patients (n=7, 100%) received corticosteroids with a mean duration of use of 2.50 ± 0.65 months, and a mean dose of 37.14 ± 12.54 mg. One (14%) patient was compliant with antigen elimination. ILD exacerbation and mortality rate was high (both n=2, 28.57%).
Conclusion:
The exposures identified in rural Appalachian HP patients were similar to other rural and urban populations in the United States. Risk factors associated with poor outcomes, such as smoking, fibrotic HP subtype, and non-avoidance of antigen were higher in this cohort. The rate of ILD exacerbation and mortality were similarly higher. Larger studies are needed to investigate longitudinal trends of exposure, characteristics, and management of HP to improve outcomes in rural populations.
1. INTRODUCTION
Hypersensitivity pneumonitis (HP) is a subtype of diffuse interstitial lung disease (ILD). Formerly termed allergic alveolitis, the pathophysiology of HP is characterized by dysregulated immune-mediated inflammatory injury to the alveoli and terminal bronchioles following exposure to inhaled antigens in susceptible individuals [1]. HP may present as a self-limiting disease, follow a relapsing-remitting pattern, or slowly progress into a fibrotic form of the disease [2].
HP is the most common subtype of ILD after idiopathic pulmonary fibrosis, with an annual incidence of 30 per 100,000 persons in the United States (US) [3, 4]. The prevalence of fibrotic HP and exposure associated ILD, like pneumoconiosis, is higher in rural than urban areas [5]. According to the US 2010 Census, 51% of the population in West Virginia (WV) is considered rural [6]. It is unsurprising that WV has the highest prevalence of HP, with 3-3.99 cases per 100,000 persons [3].
Epidemiologic studies of Farmer’s Lung have shown a higher rate of HP among men compared to women [7,8], but more recently rates have been higher in women [3]. The recent gender shift may be related to a higher representation of women in the workforce with consequent exposure to antigens causing HP, and the increased access to healthcare among women [3]. It is unknown if sex differences affect genetic susceptibility to HP [3].
Historically, HP has been subdivided into acute, subacute, and chronic subtypes to characterize the temporal relationship of disease development with exposure. This categorization has fallen out of favor due to the lack of correlation with treatment strategies, prognosis, and patient outcomes [2]. The 2020 American Thoracic Society/Japanese Respiratory Society/Asociación Latinoamericana de Tórax (ATS/ JRS/ALAT) clinical practice guidelines for the diagnosis of HP dichotomized HP into fibrotic and non-fibrotic subtypes based on radiological features [9]. This distinction is important due to the differing management and prognosis of patients with fibrotic and non-fibrotic HP [9].
Since its first mention in the early 20th century among farm workers, the spectrum of causative agents leading to the development of HP has grown exponentially [10]. Chemical agents, plant and/or animal proteins, and microbial particulate matter are among the major categories of HP triggers [10]. The duration of exposure, in addition to the host's aberrant inflammatory response and genetic susceptibilities plays a key role in the progression of disease [11]. Identification of the causative agent increases the diagnostic confidence of HP. Elimination and avoidance of exposures is a crucial step in the management of HP to halt disease progression, improve symptoms, and potentially cure HP if undertaken expeditiously [1].
In practice, despite extensive exploration through meticulous history-taking and the utilization of comprehensive exposure questionnaires, the identification of HP antigens in an individual patient remains elusive [12]. Much of the complexity lies in the variability of occupational, geographic, socioeconomic, cultural, and climate factors influencing the prevalence of HP triggers [13]. The Appalachian region carries a disproportionate burden of pulmonary diseases due to socioeconomic and environmental factors [14]; hence, it is foreseeable that the exposures, clinical characteristics, and outcomes of HP in this population may differ in comparison to other rural and urban regions in the US. Recognizing the dearth of studies specifically examining the exposures, outcomes, and management strategies of HP in Appalachia, we sought to examine the frequency and types of exposures; characterize the features; describe management strategies; and finally, to determine the outcomes of HP patients in this region.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
This was a retrospective cross-sectional study conducted at our institution in WV and its affiliated hospitals. We extracted data and compared the outcomes of patients with the diagnosis of HP. The study was reported in line with the “STrengthening the Reporting of Observational Studies in the Epidemiology (STROBE) guidelines” [15].
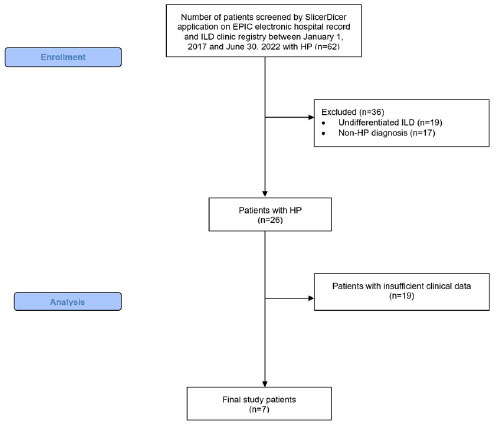
Abbreviations: HP, hypersensitivity pneumonitis; ILD, interstitial lung disease.
2.2. Participants and Study Size
The SlicerDicer application within EPIC electronic health record and our ILD clinic registry were utilized to identify patients with the diagnosis of HP between January 1, 2017 and June 30, 2022 (n=62). The “Consort Flow Diagram” [16] illustrates the patient selection process (Fig. 1). The study was conducted in line with “Sex and Gender Equity in Research” (SAGER) guidelines [17]. Patient genders were self-reported. No participants were excluded based on their gender. We excluded patients who did not meet the ATS/JRS/ALAT 2020 Clinical Practice Guidelines for the diagnosis of HP [9]. Patients (n=36) were excluded due to the lack of the following criteria: 1) clinical presentation, radiological and pathological findings consistent with diagnosis of HP, and 2) received specific treatment for HP. After individual review of all cases by BB, VD, and BSB, patients with insufficient clinical data were excluded (n=19). A total of 7 patients met all the inclusion criteria of the study.
2.3. Study Group Interventions
Patient characteristics were recorded and analyzed (Table 1). A detailed chart review was performed to determine HP exposures as documented by the clinician during clinical encounters (Fig. 2). The exposures were grouped according to the HP Exposure Assessment Tool [10]. The radiological patterns, distributions, and abnormalities were reviewed (Fig. 3) as outlined in the HP Diagnostic Clinical Practice Guidelines by Raghu et al. [9]. The fibrotic pattern on high-resolution computed tomography (HRCT) of the chest was defined by the presence of all the following: 1) septal thickening, 2) honeycombing, 3) reticulation, and 4) traction bronchiectasis. The absence of one or more of these findings was defined as the non-fibrotic pattern. Histopathology consistent with HP was defined as the presence of loosely formed granulomas and interstitial lymphocytic infiltrates.
| Variables | n = 7 |
|---|---|
| Age: mean (SD) | 66.14 (11.19) |
| Female: n (%) | 5 (71.43) |
| Never-smoker: n (%) | 3 (42.86.0) |
| Ever-smoker: n (%) | 4 (57.14) |
| • Amount in pack-years: mean (SD) | 14.00 (7.12) |
| Occupation | - |
| • Bar Manager: n (%) | 2 (28.57) |
| • Fiberglass factory worker: n (%) | 1 (14.29) |
| • Electronic engineer: n (%) | 1 (14.29) |
| • Elderly care provider: n (%) | 1 (14.29) |
| • Office manager: n (%) | 1 (14.29) |
| • Not reported: n (%) | 1 (14.29) |
| Caucasian: n (%) | 7 (100.0) |
| Duration of follow-up in months: mean (SD) | 59.67 (35.71) |
| BMI: mean (SD) | 30.05 (6.48) |
| Dyspnea with exertion: n (%) | 7 (100.0) |
| Cough: n (%) | 5 (71.43) |
| Malaise and/or fatigue: n (%) | 2 (28.57) |
| Weight loss: n (%) | 2 (28.57) |
| Dyspnea at rest: n (%) | 1 (14.29) |
| Erythema nodosum: n (%) | 1 (14.29) |
| Night sweats and/or fever: n (%) | 0 |
| Joint pain: n (%) | 2 (28.57) |
| Raynaud’s phenomenon: n (%) | 2 (28.57) |
| Dry eyes and mouth: n (%) | 1 (14.29) |
| Inspiratory squeaks on lung auscultation: n (%) | 1 (14.29) |
| Wheezing: n (%) | 1 (14.29) |
| mMRC dyspnea scale on presentation: mean (SD) | 1.85 (0.90) |
| Duration of symptoms at first visit in months: mean (SD) | 72.29 (89.06) |
| Oxygen dependence at rest/exercise: n (%) | 3 (42.86) |
| Oxygen requirement, L/min: mean (SD) | 2.66 (0.57) |
| Gastroesophageal reflux disease: n (%) | 4 (57.14) |
| Asthma: n (%) | 3 (42.86) |
| Chronic obstructive pulmonary disease: n (%) | 2 (28.57) |
| Pulmonary hypertension: n (%) | 2 (28.57) |
| Congestive heart failure: n (%) | 1(14.29) |
| Obstructive sleep apnea: n (%) | 1 (14.29) |
| Bronchiectasis, venous thromboembolism, connective tissue disease, chronic kidney disease, chronic kidney disease, cerebrovascular disease: n (%) | 0 |
| Forced vital capacity, Liters: mean (SD) | 2.22 (0.71) |
| Forced vital capacity, percent predicted: mean (SD) | 71.29 (21.55) |
| Forced expiratory volume in 1 second, Liters: mean (SD) | 1.83 (0.49) |
| Forced expiratory volume in 1 second, percent predicted: mean (SD) | 80.86 (23.33) |
| FEV1/FVC: mean (SD) | 83.90 (8.37) |
| DLCO, mL/min/mmHg: mean (SD) | 12.13 (4.28) |
| DLCO, percent predicted: mean (SD) | 51.29 (23.31) |
| Restrictive pattern on pulmonary function testing: n (%) | 5 (71.43) |
| Obstructive pattern on pulmonary function testing: n (%) | 0 |
| Normal pattern on pulmonary function testing: n (%) | 2 (28.57) |
| Positive serum HP panel: n (%) | n = 4, 1 (25.0) |
| Pathology consistent with HP: n (%) | n = 5, 4 (80.0) |
| Medication | n (%) | Mean (SD) in Months | Average Daily Dose (SD) in mg |
|---|---|---|---|
| Oral corticosteroids | 7 (100) | 2.50 (0.65) | 37.14 (12.54) |
| Azathioprine | 3 (43) | 6.50 (9.96) | 83.33 (28.87) |
| Mycophenolate mofetil | 1 (14) | 18.0ᵻ | 1400.0 ᵻ |
| Nintedanib | 3 (43) | 4.33 (2.52) | 300.0 (0) |
| Antigen Elimination | 1 (14) | unclear | n/a |
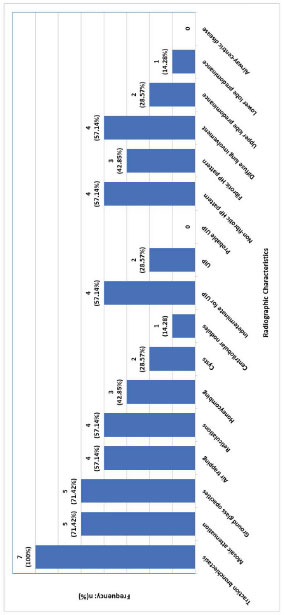
Abbreviations: HP; hypersensitivity pneumonitis; UIP, usual interstitial pneumonia.
| Clinical Parameter | n = 7 |
|---|---|
| Symptoms progression: n (%) | - |
| • Improved | 4 (57.14) |
| • Worsened | 2 (28.57) |
| • Stable | 1 (14.29) |
| Oxygen supplementation requirement: n (%) | - |
| • Improved | 0 |
| • Worsened | 5 (71.43) |
| • Stable | 2 (28.57) |
| Compliance to exposure elimination: n (%) | 1 (14.29) |
| Mortality: n (%) | 2 (28.57) |
| • Time to death from diagnosis, months: mean (SD) | 34.0 (36.77) |
| Imaging: n (%) | - |
| • Improved | 0 |
| • Worsened | 1 (14.29) |
| • Stable | 6 (85.71) |
| PFT: n (%) | - |
| • Improved | 0 |
| • Worsened | 4 (57.14) |
| • Stable | 1 (14.29) |
| • Post-treatment PFT not available | 2 (28.57) |
| mMRC dyspnea scale | - |
| • Improved | 3 (42.86) |
| • Worsened | 1 (14.29) |
| • Stable | 3 (42.86) |
| ILD exacerbation | 2 (28.57) |
The treatment utilized by the study group is shown in Table 2. The clinical course of all 7 patients was recorded and analyzed (Table 3). ILD exacerbation was defined as visits to the emergency department or admissions to the hospital due to acute respiratory failure. Treatment response was measured by similar criteria as established for idiopathic pulmonary fibrosis [18]. An improved response was defined by a decrease in the modified Medical Research Council (mMRC) dyspnea scale, a reduction in the radiographic parenchymal abnormalities, and physiologic improvement by ≥ 10% increase in the forced vital capacity (FVC) or ≥ 15% increase in diffusion capacity of lungs for carbon monoxide (DLCO). Conversely, failure to respond to treatment was defined as an increase in the mMRC dyspnea scale, an increase in the radiographic parenchymal abnormalities, including progression to fibrotic pattern, and physiological deterioration by ≥ 10% decrease in the FVC or ≥ 15% decrease in DLCO.
2.4. Outcomes
The primary outcome of this study was the exposures associated with HP in the study population. The secondary outcome was the disease stage at the time of HP diagnosis. Other endpoints included the rate of smoking, treatment modalities, rate of ILD exacerbation, and mortality.
2.5. Data Collection
All study records were securely stored in our institution’s network of computers in the pulmonary office.
3. RESULTS
3.1. Study Participants Characteristics
Baseline patient characteristics of the 7 patients included in the study are shown in Table 1. All patients were White. Most of the patients were female (71.43%, n=5), with a mean age of 66.14 ± 11.19 years. The mean BMI was 30.05 ± 6.48 kg/m2. The most common occupation in the cohort was bar management (28.57%, n=2). The most common comorbidities were gastroesophageal reflux disease (57.14%, n=4), asthma (42.86%, n=3), chronic obstructive pulmonary disease (28.57%, n=2), and pulmonary hypertension (28.57%, n=2). All patients had exertional dyspnea at presentation (100.0%, n=7). Other symptoms include cough (71.43%, n=5), weight loss (28.57%, n=2), and fatigue (28.57%, n=2). The duration of symptoms at the time of initial evaluation was 72.29 ± 89.06 months. The mean mMRC dyspnea scale was 1.85 ± 0.90, with 42.86% (n=3) of the patients requiring oxygen supplementation at the time of presentation. Pulmonary function tests (PFT) for all patients were available prior to treatment initiation. Five patients (71.43%) had a restrictive pattern on their PFT. The mean FVC and FEV1 were 2.22+0.71 L and 1.83 ± SD0.49 L, respectively. The percent predicted DLCO was 51.29 ± 23.31%.
All patients (100.0%, n=7) had traction bronchiectasis (Fig. 3). Other common radiographic findings were mosaic attenuation (71.42%, n=5), ground glass opacities (71.42%, n=5), and air-trapping (57.14%, n=4). Non-fibrotic and diffuse changes were noted in 57.14% (n=4). Only 2 patients (28.57%) had upper lobe predominant abnormalities. Of the 3 (42.85%) patients with fibrotic HP subtype, only 2 (28.57%) conformed with the usual interstitial pneumonia (UIP) pattern. Lung histopathological data was available in 71.42% (n=5) of the study participants. Two patients (40.0%) underwent surgical lung biopsies, while 3 patients (60.0%) underwent transbronchial biopsies. Four patients (80.0%) had histopathology consistent with HP (Table 1).
The clinical course of our study patients is illustrated in Table 3. More than half of these patients noted symptomatic improvement (57.14%, n=4). The reported mMRC improved over the course of their treatment in 42.86% (n=3) of patients. However, oxygen requirements worsened in 71.43% (n=5) of them. Radiographic changes remained stable in 85.71% (n=6) and worsened in 14.29% (n=1) of the patients. PFT decline was noted in 57.14% (n=4) patients, while 1 patient (14.29%) remained stable following initiation of treatment for HP.
3.2. Main Results
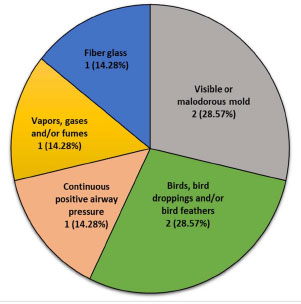
In our study population, the most common exposure leading to HP was mold and birds (both 29.57%, n=2). Use of continuous positive airway pressure (CPAP), and exposure to gases or fumes, and fiber glass were noted in 14.28% (n=1 each) of patients. Fibrotic HP was noted in 42.85% (n=3) of the patients (Fig. 3). At least half (57.14%, n=4) of the patients were ever-smokers (Table 1). Cigarette exposure among ever-smokers was 14.0 ± 7.12 pack-years (Table 1).
All patients received oral corticosteroids at some point in their disease course for a mean duration of 2.50 ± 0.65 months with an average dose of 37.14 ± 12.54 mg (Table 2). All fibrotic HP patients (43.0%, n=3) received antifibrotic therapy (Nintedanib). Azathioprine (AZA) (43.0%, n=3) and Mycophenolate mofetil (MMF) (14.0%, n=1) were also utilized. Only 1 (14.0%) patient was compliant with exposure elimination. Two (28.57%) patients had an ILD exacerbation during the disease (Table 3). The mortality rate was 28.57% (n=2) with a mean of 34.0 ± 36.77 months from time of diagnosis to death.
4. DISCUSSION
This study characterizes the exposures, management strategies, and outcomes of HP patients in the rural Appalachian region of WV. Avian and fungi exposure are the predominant antigenic source of HP in this cohort. This finding is in keeping with the most common exposure causing HP worldwide [19-21] and in urban areas in the US [13, 22]. Historically, agriculture-related moldy-hay exposure was the most common rural HP antigen [23, 24]. Agricultural pesticide use has been suggested to trigger Farmer’s Lung [25]. Outside the US, there is a different pattern to HP triggers. For example, in urban Indian communities, air-conditioners have been reported as the most common HP trigger [26]. Another study highlighted a strong association of environmental air pollution with HP in India [27]. In China, low molecular weight chemicals and animal proteins were the most common exposures leading to HP [28]. This underscores the importance of tailoring the clinical evaluation of patients suspected of having HP depending on geographical and socioeconomic determinants. Antigen detection and its subsequent elimination, not only improves diagnostic yield and treatment expediency, but also improves survival and halts the progression of disease [29].
There is a growing body of evidence of novel antigens causing HP. This cohort revealed an association of HP with CPAP utilization. Chang et al. reported the first and only case of HP associated with CPAP due to improper cleaning of the device [30]. Contaminated water harboring molds and fungi has been thought to cause HP [1]. With the rising prevalence of OSA [31] and the consequent use of CPAP, it is expected that the incidence of HP will rise due to the obesity epidemic in this region [32]. Additionally, exposure to fiber glass was noted in this cohort. Fiber glass is routinely used as insulation in industrial and domestic products. Exposure to fiber glass by inhalation has been associated with a variety of pulmonary abnormalities [33-35].
Elimination of HP antigens has been shown to improve mortality and symptom burden in both fibrotic and non-fibrotic HP [12, 29, 36]. Nevertheless, the elimination of HP triggers continues to be a challenge. The emotional attachment to pets among owners who are allergic or sensitized to their pets is a common obstacle [37]. The feasibility of eliminating exposures when it is encountered at the workplace is low in some instances as it may lead to loss of income and financial instability [37]. Compliance to exposure elimination was profoundly low in this cohort compared to other centers [38]. The observed difference may be rooted in the economic disparity of rural populations whereby housing and employment mobility may not be realistic [39]. Furthermore, inequity of education level of rural populations may lead to poor health literacy and distrust in physicians, which ultimately results in poor compliance to treatment [39].
A significant portion of the patients (n=3, 42.85%) in this cohort had fibrotic HP at the time of diagnosis. This finding is unique in that, at most, only one-fourth of patients are diagnosed with fibrotic HP at initial presentation in the US [3]. It is foreseeable that health disparities of rural populations may have contributed to this finding [39]. Unfortunately, patients with fibrotic HP have a significantly higher mortality [40]. To reduce diagnostic delays, and consequently improve health outcomes of patients with HP, effort must be made to improve the expediency of referral to ILD centers.
More than half (n=4, 57.14%) of HP patients in this cohort had tobacco use disorder. The rural Appalachian region has one of the highest smoking rates in the US [41]. It is unsurprising that the number of ever-smokers in this cohort was higher than urban populations [13, 36]. Early in the disease, tobacco exposure has been thought to be protective due to the dampening of the immune system’s reaction to antigenic material [42]. This is reflected in observation of the low prevalence of smokers in non-fibrotic HP [43]. Overtime, this trend is reversed in that cigarette smoking is associated with progressive fibrosis, worsening mortality outcomes, symptoms, and quality of life [12, 44, 45]. Thus, ongoing cigarette smoking should be strongly discouraged in patients with HP.
Corticosteroids are the most frequently used therapy for HP patients in this cohort. Compared to Morisset et al., the average daily doses were higher in this cohort (37.14 ± 12.54 mg vs 10-30 mg) [46]. The duration of corticosteroid use was also longer in this cohort. While corticosteroids were effective in slowing the rate of PFT decline in fibrotic HP, and may even improve symptoms in non-fibrotic HP, survival benefits have not been robustly demonstrated [38]. Immunomodulators like AZA and MMF, are attractive options that can be used to reduce the adverse events profile associated with corticosteroid [22]. The addition of AZA and MMF allows for the reduction of the duration and cumulative dose of steroid therapy. Both agents were infrequently used in this cohort in contrast other ILD centers in the country where at least 50% of chronic HP patients were managed with either AZA or MMF [22]. It is unclear why there is a difference in the prescribing habits of pulmonologists in this region.
Antifibrotic therapy was used in all patients with fibrotic HP in our cohort. Benefits of antifibrotics include significantly lowering the rate of PFT decline in patients with progressive fibrosing ILD as demonstrated in the INBUILD trial which enrolled 26 patients with chronic HP [47]. Symptomatic improvement was observed in 57.14% (n=4), stabilization of PFT in 14.29% (n=1) and radiographs in 85.71% (n=6) of patients in this study. However, 5 patients (71.43%) had worsening oxygen requirement despite treatment.
The mortality and ILD exacerbation rates (28.57%, n=2) were higher in this cohort compared to a larger study of chronic HP patients (12%) [48]. The median survival time from diagnosis was similarly lower in our study compared to a cross-sectional European study [49]. In an observational study, of the 14% of patients who had exacerbation of chronic HP, they were more likely to be smokers [50]. While causality cannot be determined, the observed higher mortality and ILD exacerbation rates may be related to the higher prevalence of cigarette smoking, longer lag-time between symptom onset to diagnosis, and non-avoidance of antigenic source and advanced fibrotic HP at the time of presentation. These factors may be associated with health disparities commonly encountered in rural populations, not exclusive to the WV Appalachian region [6, 39, 51].
Other notable trends in this study include a higher proportion of patients undergoing lung biopsy, either surgical lung biopsy or transbronchial biopsies, for the evaluation of diffuse lung disease compared to other retrospective studies of HP patients [13, 22]. We speculate that this practice may be related to the complexity of patients in this area requiring invasive testing for diagnostic conundrums. A vast majority of patients seen in this center present with wide-ranging exposures, including those related to the predominant occupation of coal mining [52]. Thus, pneumoconiosis due to coal dust and silica may confound the diagnosis. Furthermore, consistent implementation of the ILD multidisciplinary collaboration between chest radiologists, thoracic pathologists, rheumatologists, thoracic surgeons, and pulmonologists is challenging due to the lack of specialist availability [39].
4.1. Limitations
Our study has several limitations. The results of this study are not generalizable outside of the WV Appalachian region. This study was designed to measure the outcomes of the population regardless of gender. Therefore, a gender-based analysis of outcomes was not performed. The retrospective nature of the study may potentially lead to selection bias. The sample size of our study was small in comparison to other studies with similar design. The rarity of the disease and the shifting diagnostic criteria of HP are reasons why a larger sample size for the study was not achievable. The magnitude of effect analyses could not be performed. The smaller sample size allowed for extensive examination to identify HP exposures and their temporal relationship to symptom onset. Smaller studies like this allow for the investigation of nuanced features specific to a certain region and/or population.
4.2. Future Direction
Collaboration with medical centers in the Appalachian region will enable the accumulation of a larger cohort of patients with HP to investigate trends longitudinally and prospectively in exposures, characteristics, outcomes, and management for HP. The identification of factors leading to poor compliance to antigen identification and elimination in this population is essential to improve patient outcomes.
CONCLUSION
The most common HP-inducing exposures in rural Appalachia are avian and fungi. Novel exposures, including those related to CPAP use, gases and/or fumes, and fiberglass have been reported in a minority of patients. Most patients had fibrotic HP at the time of initial evaluation. The ILD exacerbation and mortality rates are higher due to the presence of a greater magnitude of risk factors (smoking, non-avoidance of exposures, fibrotic HP at presentation) associated with poor outcomes. This study underscores the diagnostic and therapeutic challenges associated with management of HP in the rural WV Appalachian region.
AUTHORS’ CONTRIBUTIONS
BB had full access to all study data and took responsibility for the integrity of the data and the accuracy of the data analysis. BB, VD, BSB, and TL were involved in the study conception, design, and data acquisition. BB, VD, BSB and RS interpreted the data. VD, BSB, and RS provided the statistical analysis reported in the study. BB, VD, RS, and TL were involved in drafting and writing the manuscript. All authors contributed to the critical revision of important intellectual content and approved the final version for publishing.
LIST OF ABBREVIATIONS
| ATS/JRS/ALAT | = American Thoracic Society/Japanese Respiratory Society/Asociación Latinoamericana de Tórax |
| AZA | = Azathioprine |
| CPAP | = Continuous Positive Airway Pressure |
| DLCO | = Diffusion Capacity of Lungs for Carbon Monoxide |
| FEV1 | = Forced Expiratory Volume in One Second |
| FVC | = Forced Vital Capacity |
| HRCT | = High-resolution Computed Tomography |
| HP | = Hypersensitivity Pneumonitis |
| ILD | = Interstitial Lung Disease |
| mMRC | = Modified Medical Research Council Dyspnea Scale |
| MMF | = Mycophenolate Mofetil |
| PFT | = Pulmonary Function Testing |
| US | = United States |
| WV | = West Virginia |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was reviewed and approved on November 17, 2022, by the West Virginia University Institutional Board Review (#2209654138) under the category of flexible exemption as it poses minimal to no risk to subjects.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
The informed consent requirement was waived. Only deidentified data obtained for clinical evaluation purposes was used.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data sets generated during the current study are available from the corresponding author [B.D] upon reasonable request.
FUNDING
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
We thank the West Virginia Clinical and Translational Science Institute for their assistance and support.


