All published articles of this journal are available on ScienceDirect.
Prevalence of Sexually Transmitted Infections and Related Sexual Behaviour among Pregnant Women 18-49 years Old Attending Antenatal Clinic at a Primary Health Care in Maseru, Lesotho
Abstract
Background:
The prevalence of STIs among pregnant women attending Antenatal care (ANCs) clinics in Maseru remains unknown. Therefore, there is a need to determine the prevalence of STIs among pregnant women attending ANC in Maseru and to assess their sexual behaviour.
Methodology:
A quantitative cross-sectional survey methodology using medical records and patient surveys were conducted. Firstly, the records of pregnant women attending ANC at three clinics-Queen Elizabeth, Seventh Day Adventist, and Thamae clinic from March to May 2021 were used to determine the prevalence of STIs, including HIV. A count of all records with an STI diagnosis and syndromic management regimen over three months was performed. Prevalence was determined by calculating the quotient of STI-positive records over all the records for three months. Secondly, a cross-sectional descriptive study of the same pregnant women was used to assess their sexual behaviors. Both the patient record for STI data and the questionnaires for sexual behaviors were included. Overall, 405 pregnant women were investigated at the three clinics in Maseru, Lesotho.
Results:
The prevalence of STIs including HIV in the three clinics over the three month period was found to be high. The prevalence of STIs in the Seventh Day Adventist clinic was the highest of the three clinics. The prevalence varied across three months with cumulative prevalence of 17.0%, 8.7%, and 9.7% respectively. The overall cumulative and comparative prevalence in the three months and in the three clinics was 12.2% for STIs against 29.0% for HIV. There was low uptake of STI treatment, inconsistent use of condoms, and the practice of having multiple sexual partners among pregnant women.
Conclusion:
The prevalence of STIs including HIV in the three clinics was found to be high. The inconsistent use of condoms and the practice of having multiple sexual partners were found to be the main predictors of STI and HIV acquisition among pregnant women in these three clinics.
1. INTRODUCTION
STIs constitute a serious public health problem all over the world, particularly in developing countries including Lesotho. STIs are infections that are spread by having unprotected sex with someone who has an STI, including HIV. Corno et al. (2010) reported that STIs are one of the strongest risk factors for HIV infection, that facilitate the transmission of the virus. To date, very few studies have reported the prevalence of STIs in the general population in Lesotho. One of the very few studies done in 1995 on the general population, reported a prevalence of 28.4% for chlamydia, 5.9% for gonorrhea, and 11.3% for syphilis in a rural community in the Lesotho highlands [1]. The Lesotho Demographic Health Survey [2] cited that the self-reported prevalence of sexually transmitted infections and STI symptoms in the country for people aged 15-49 years was 17% for women and 15.1% for men. In Maseru, the prevalence was 14.8% in women and 14.9% in men [3]. The prevalence of STIs among pregnant women attending ANC in Maseru remains unknown. Although the syndromic management of STIs is provided as a standard treatment, health facilities still see high number of STI patients, including pregnant women. Therefore, there is a need to determine the prevalence of STIs among pregnant women attending ANC in Maseru and to assess their sexual behaviour, hence the aim of conducting the study.
According to Simon et al. [4], STIs are a major public health concern globally among women in resource-limited countries. Risky sexual behaviour can contribute to the transmission of STIs by pregnant women and include unprotected sex, having multiple sexual partners, early sexual intercourse, and extra-marital sex. Motsima and Malela [5] indicate that the age at first sexual intercourse may be a predictor of future sexual behaviour and sexual risk. Early sexual intercourse at an average age of 16 years of age and below is associated with subsequent high-risk behaviour [6], including having multiple sexual partners, lower levels of condom use, and an increased likelihood of extra-marital sex and unintended pregnancy, which may result in long-term health problems and social disadvantages. Having multiple sexual partners increases the likelihood of an individual’s transmitting or acquiring an STI. Furthermore, a person who has only one partner is at risk, depending on his/her partner’s actions [7].
The Lesotho Demographic Health Survey [3] states that 54% of women and 65% of men reported using a condom at last sexual intercourse. The symptomatic or syndromic management approach includes symptomatic screening and management of STIs during pregnancy and continues to be the standard care in Lesotho. Due to the vast impact of the antenatal period on the child and subsequent adult health, visiting an antenatal care clinic is important in preventing perinatal mortality. However, inequality exists, and young, rural, poor, and less-educated women may have less access to antenatal clinic services. The high burden of HIV and STI infections was suggestive of the need to strengthen the national prevention programmes, including the education of the people on the risk factors pertaining to the transmission of HIV and STIs [8]. In screening and treating pregnant women in Haiti, Bristow et al. [9] observed that routine screening for chlamydia trachomatis, Neisseria gonorrhoea, and trichomonas vaginalis among pregnant women is not done although these STIs are associated with adverse birth and newborn health outcomes.
In South Africa, Marikina et al. [10] concluded that integration of point-of-care diagnostic testing for STIs in a South African antenatal care programme, for HIV-infected pregnant women would be acceptable and feasible. The literature shows that in Lesotho consistent and correct condom use is being promoted to diverse population groups through the health promotion media. Lesotho’s National AIDS Commission (NAC) distributed 31 condoms per adult man in 2015, which exceeds the United Nations Population Fund’s regional benchmark of 30. Condom use among adults aged 15-49 years with more than one sexual partner in the past 12 months was reported as 76% in 2016. A key indicator in the Lesotho Demographic Health Survey [3] showed that 92% of women and 88% of men know that consistent use of condoms is a means of preventing HIV and STIs. About 99% of women and 87% of men know that limiting sexual intercourse to one faithful and uninfected partner can reduce their chances of contracting HIV and STIs.
In Lesotho, there are 265 healthcare centers where people access healthcare services, including the treatment and management of STIs using the syndromic management approach, according to the WHO STI guidelines [11]. Primary health care is meant to be the first level of care and first-contact personal healthcare services that people have with the healthcare system when they have a health problem [11]. As most of the population in Lesotho live in rural and semi-urban areas, services related to health including sexually transmitted infections (STIs) must be available at the grass-root level. People are highly likely to be exposed to STIs, including pregnant women, whether in rural or urban areas. Pregnant women can also infect their unborn babies. Thus, all pregnant women attending antenatal clinics in the Mother-and-Child-Health (MCH) and Primary Health Care (PHC) Programme are screened for syphilis, HIV, and other STIs [12].
This study was undertaken to assess the prevalence of STIs and HIV among pregnant women attending ANC in Maseru and to assess their sexual behaviour. It is envisaged that the findings and recommendations of this study would provide vital data and inform the government, policymakers, and health professionals in terms of the prevalence of STIs among pregnant women, on the association between their sexual behaviour and their socio-demographics. The outcome of this study can further be used to develop appropriate health education and health promotion interventions for patients and communities. The control of STIs in Lesotho would help to reduce the incidence of HIV.
2. MATERIALS AND METHODS
2.1. Study Design
A quantitative approach using cross-sectional survey of medical records and patient surveys was employed for this study. The methodology consisted of two phases, the first phase determined the prevalence of STIs in the three clinics in Maseru, namely Queen Elizabeth, Thamae, and Seventh Day Adventist clinics, while the second phase assessed sexual behaviours of the pregnant women attending the above clinics, using descriptive study design. Phase 1, which had to do with establishing the prevalence of STIs, involved a retrospective review of three months’ medical records, from March to May 2021, of participants who were syndromically diagnosed and treated for STIs. Phase 2 had to do with establishing the nature of their sexual behaviour and involved the administration of a questionnaire to participants.
2.2. Study Setting
The three selected facilities provide services to about 50,000 people in their catchment areas, with an expected daily headcount estimated at 15 pregnant women visiting the maternal and child health (MCH) department. The health services are accessed at no cost to the general population, including pregnant women. The clinics have a reliable system of recording data in the ANC registers, are cost-effective, and provide services, including the systematic screening of STIs and the prompt provision of syndromic management to pregnant women diagnosed with STIs. They also have a committed, well-trained, and experienced staff support.
During the COVID-19 pandemic, the daily headcount of pregnant women attending ANC at such primary healthcare facilities noticeably declined, according to different records in the MCH and the clinics. Many pregnant women prefer to stay at home, avoiding the risk of COVID-19 infection during their ANC activities. In February 2021, record shows that only 153 pregnant women attended ANC in the past 3 months at the Seventh Day Adventist clinic, making it difficult to reach the calculated sample size of 321 participants in the study from that clinic alone. A survey was therefore organized and administered to four more clinics in Maseru to establish among other things the daily headcount of pregnant women attending ANC activities during the pandemic period in June 2021. From the information gathered, two more clinics were added to the Seventh Day Adventist clinic, these being the Thamae clinic with 149 participants and the Queen Elizabeth clinic with 138. This made a total of 440 participants, a number was considered sufficient to meet the requirements of the first phase of the study.
2.3. Study Population
Pregnant women aged 18-49 years old who were attending antenatal care at the primary health care clinics were selected to participate in this study.
2.4. Inclusion and Exclusion Criteria
Pregnant women aged 18 to 49 years old attending antenatal care at the primary health care facility who consented to participate in the study were included as a matter of convenience, but pregnant women younger than 18 and older than 49, those who were ill, and those who did not consent were excluded from participating in the study.
2.5. Sample Size
Three clinics were selected for the collection of data, and the sample size of each clinic was calculated, considering the daily headcount of pregnant women attending antenatal care at the MCH department, and the number of days per week that each MCH department was engaged in antenatal care. The MCH department at each primary health care clinic had an average of 15 pregnant women attending ANC as a daily headcount. All three clinics selected scheduled first-visit antenatal care activities for one day per week. To determine the population of each clinic, we multiplied the daily headcount of pregnant women by the number of days of antenatal care per week, then, by the four weeks per month, and finally by the three months which was the period under review. This generated a population of 180 participants attending the antenatal clinic in each facility. The RAOSOFT sample size calculator was used to determine the minimum size of the sample. The determination of the size of the population at each MCH department mentioned above, with a margin of error of 5%, a confidence level of 95%, and a response rate of 50% was used. It was calculated that 123 participants were needed from each clinic. A 10% buffer was included in each sample size calculation, thus increasing the final sample size to 135 for the clinics. This was done to accommodate for any non-responses. A total sample of n=405 pregnant women attending antenatal care in these three clinics was used as the sample for data collection in the second phase of the study, the purpose of which was to describe the sexual behaviour of pregnant women in Maseru, Lesotho.
2.6. Sample Recruitment
Pregnant women presented themselves at the clinic during the collection of data, and were recruited using convenient sampling and taken one by one from the queue to a private consulting room to avoid disrupting the flow of the queue. The purpose of the study was clearly explained to the participants. They were asked if they wanted to participate in the study or not and those who agreed were recruited and given a consent form to sign.
2.7. Data Collection
The collection of data used a methodology comprising two phases to determine the prevalence of STIs among pregnant women and to assess their sexual behaviour. An appointment was scheduled to meet the District Health Management Team (DHMT) to obtain permission to access the study setting. Permission was also secured from the managers of the three clinics. The questionnaire was distributed to each participant and the estimated time to complete the questionnaire was 20 minutes. The completed questionnaires were collected back from the participants and ensured that all questions were answered, for data quality control.
2.8. Data Collection Tools
In phase one, a count of all records with an STI diagnosis and syndromic management regimen over three months was done. Prevalence was determined by calculating the quotient of STI-positive records over all the records over three months. In phase 2, both the patient records for the STI data and the questionnaire about sexual behaviour were included. A closed-ended validated questionnaire about the socio-demographic and sexual behaviour of the participants, developed by Madiba and Mokgatle [13], was used in this study. The questionnaire, which had been developed in English, was translated into Sesotho (local language) by an expert before the data collection stage. The questionnaire comprised two sections. Section A was used to obtain sociodemographic data like the age, nationality, race, occupation, marital status, and educational qualifications of the participants. Section B included questions on sexual behaviours of the participants. The questions were simple and clear, understandable, concise, and not ambiguous.
2.9. Data Analysis
All questionnaires were carefully checked to make sure that they had been completed before starting the data analysis. An Excel spreadsheet was used to enter the cleaned, coded data, which were then imported into STATA 13 (Stata Corp., Texas, USA) for statistical processing and analysis after a double-check to ensure that the data had been correctly entered. Descriptive statistics were to analyze the data. Data was presented as the mean, median, standard deviation, and frequencies. Bivariate analysis was conducted, with treatment uptake as the dependent variable. Independent variables were socio-demographics and sexual behaviors.
2.10. Reliability
The questionnaire was pretested on 10 women in one of the clinics to identify any unclear or ambiguous items in the questionnaire and to verify its accuracy. The data collected for pretesting was excluded from the main study. No problems were identified in the tool and no changes were made. The Cronbach alpha analysis was calculated to verify the reliability and internal consistency of the data collection instrument. The score of internal consistency on risky sexual behaviour was at Cronbach´s alpha = 0.79.
2.11. Validity
The validity of an instrument is the degree to which it is able to measure the variable it is intended to measure. For this study, the data collection tool was previously used and validated to respond to the set objectives of measuring the sexual behaviors of the participants. The same questionnaire was administered to all the participants. Standardized questions with standardized pre-categorized responses will be used to ensure the reliability and validity of the tool and the tool will be translated into the local language of Setswana to ensure that it is well understood by participants. The participants were encouraged to respond honestly and due to the sensitive nature of the sexual behaviour questions, privacy was provided to encourage honest responses.
2.12. Measures to Minimize Bias
At the stage of sampling, all pregnant women 18-49 years old available at the clinic were included in the study. A 10% buffer was added to the to reduce non-response bias. Social desirability bias was reduced using self-administered questionnaires for data collection.
2.13. Ethical Considerations
Ethical approval was obtained by the School Research Ethics Committee (SREC) and Sefako Makgatho University Research Ethics Committee (SMUREC). Permission to conduct the study was given by the Lesotho Ethics Committee. The District Health Management Team (DHMT) was informed of the research and facility managers gave permission for the study to be conducted. The participants were informed about their right to withdraw from the study freely and at any time and that participating in the study was voluntary. The participants were assured that the information derived from this study would remain confidential and would never be shared with anyone who was not part of the study. The participant’s identity was not required in this study. Each participant was identified by a code given as a unique number, from number one to number four hundred and five. A private room was arranged for the sake of the privacy of the participants, and they were assured that the information they gave would remain private.
3. RESULTS
From a sample size of n=405, the mean age of the pregnant women was 27.5 years old, the median was 27 years old, a standard deviation of 6.1 and the age range was 18-45 years. Table 1 shows that 77.53% were married, 16.54% were single, 5.93% were cohabiting. 26.2% were employed, and 97.4% had some form of education. The table further shows that more than 50% of the sample were in the age group of 25 to 45 years of age.
| Socio-demographic Characteristics | Frequency (N) | (%) |
|---|---|---|
| Marital status | ||
| Cohabiting | 24 | 5.93 |
| Married | 314 | 77.53 |
| Single | 67 | 16.54 |
| Total | 405 | 100 |
| Type of employment | ||
| Full time | 106 | 26.17 |
| Part-time | 21 | 5.19 |
| Self-employed | 64 | 15.80 |
| Not employed | 214 | 52.84 |
| Total | 405 | 100 |
| Educational level | ||
| Did not attend school | 12 | 2.96 |
| Primary school | 73 | 18.02 |
| Secondary | 193 | 47.65 |
| Tertiary | 127 | 31.36 |
| Total | 405 | 100 |
| Age group | ||
| 18-24 | 148 | 36.54 |
| 25-35 | 192 | 47.41 |
| 36-45 | 65 | 16.05 |
| Total | 405 | 100 |
3.1. Description of Sexual Behaviour of the Participants
Table 2 shows that 99.8% (n=404) of the participants were currently in a sexual relationship, 67,7% (n=274) had been with the same partner for the past 12 months, and 32.3% (n=131) had two or more partners in the last 12 months. As observed, multiple sexual partners among pregnant women are among the causes of STIs in the three clinics in Maseru.
Table 3 shows that 84.2% (n=341) of the participants agreed that they could access condoms without being embarrassed, 89.1% (n= 361) of the participants reported being able to ask their partners to use condoms when having sex, 20% (n= 81) of the participants said that they always use condoms. About 52% (n= 210) of the participants reported having a good chance of successfully refusing sex when the partner did not use a condom. The reasons for not using condom and inconsistent condom use were, having trust for the partner 38% (n=154), 32.1% (n= 130) partners dislike for condoms.
| Variable | Frequency (N) | Percentage (%) |
|---|---|---|
| Currently in a sexual relationship | ||
| No | 1 | 0.20 |
| Yes | 404 | 99.80 |
| Total | 405 | 100 |
| Have been with the same partner for the past 12 months | ||
| No | 131 | 32.00 |
| Yes | 274 | 67.70 |
| Total | 405 | 100 |
| Number of partners had in the past 12 months | ||
| One partner | 274 | 67.70 |
| Two or more | 131 | 32.30 |
| Total | 405 | 100 |
| Variable | Frequency (N) | Percentage (%) |
|---|---|---|
| I can access condoms without being embarrassed | ||
| Agree | 341 | 84.20 |
| Disagree | 28 | 6.90 |
| Not sure | 36 | 8.90 |
| Total | 405 | 100 |
| I can ask my partner to use a condom when having sex | ||
| Yes | 361 | 89.10 |
| No | 28 | 6.90 |
| Not sure | 16 | 4.00 |
| Total | 405 | 100 |
| Rate of condom usage | ||
| I always use condoms | 81 | 20.00 |
| I never use condoms | 62 | 15.30 |
| I sometimes use condoms | 262 | 64.70 |
| Total | 405 | 100 |
| Chances of refusing sex when a partner doesn’t want to use a condom | ||
| High chances | 210 | 51.90 |
| Low chances | 195 | 48.10 |
| Total | 405 | 100 |
| Reasons for not using condoms | ||
| I don’t like it | 58 | 14.30 |
| I trust my partner | 154 | 38,00 |
| My partner doesn’t like it | 130 | 32.10 |
| Always use a condom | 63 | 15.60 |
| Total | 405 | 100 |
3.2. Prevalence of STIs among Pregnant Women who Attended ANC at the Three Clinics in Maseru
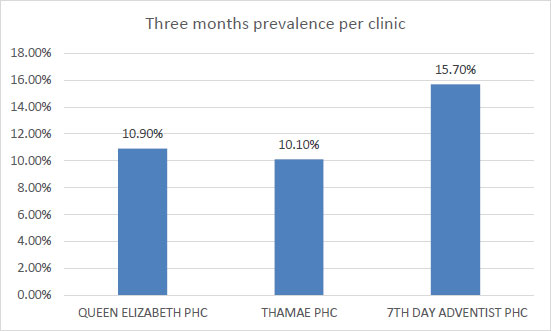
Fig. (1) shows three months’ prevalence of STIs per clinic in Maseru. The Seven Day Adventist has the highest prevalence of 15.7% among the three clinics, the lowest is Thamae at 10.1%.
Fig. (1) shows three months’ prevalence of STIs per clinic in Maseru. The Seven Day Adventist has the highest prevalence of 15.7% among the three clinics, the lowest is Thamae at 10.1%.
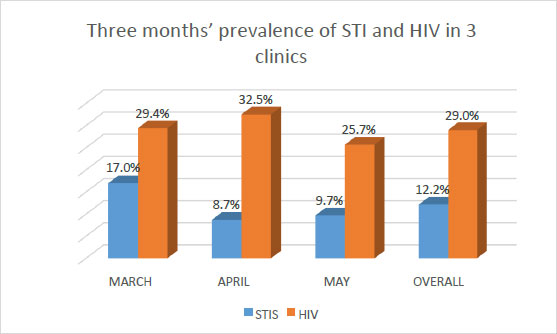
Fig. (2) shows a cumulative and comparative prevalence of STIs and HIV among pregnant women who attended ANC at the same clinics and in the same period. HIV prevalence is much higher than STIs prevalence. Overall, the prevalence of STIs was 12.2% as against 29.0% for HIV.
3.3. Participants’ Medical Records for the three Clinics: Overall Syphilis Screening among Pregnant Women
All the pregnant women who attended ANC at the three clinics during this period were systematically screened for syphilis.
We reported that only 4.4% of the pregnant women received STI treatment however, none of the pregnant women in the three clinics received treatment for syphilis.
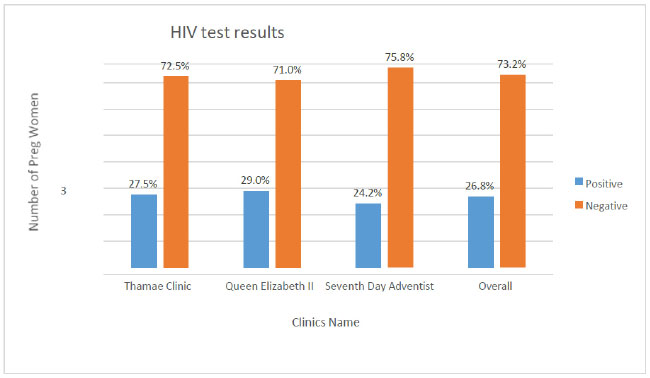
Fig. (3) shows the highest prevalence (29%) of HIV in Queen Elizabeth clinic, and the lowest prevalence of 24.2% was at the 7th Day Adventist clinic. The overall prevalence was 26.8% across the three clinics.
3.4. Alcohol Use during Pregnancy Per Clinic
Our result shows that 100% of the pregnant women who attended ANC at the Thamae and Seventh Day clinics did not use alcohol, as against 1.4% who used alcohol at Queen Elizabeth clinic over the three months period. Overall alcohol use was 0.5% among the women who attended antenatal care services.
3.5. Smoking during Pregnancy Per Clinic
The result shows that 2.0% of the pregnant women who attended ANC at the Seventh Day Adventist clinic were smokers, and the overall rate of smoking was 0.7% of the women who attended antenatal care across the clinics.
| Variable | On STI Treatment | Not on STI Treatment | p-value |
|---|---|---|---|
| Marital Status | |||
| Cohabiting | 0.7% (3) | 5.2% (21) | 0.149 |
| Married | 9.1% (37) | 68.4% (277) | |
| Single | 3.4% (14) | 13.1% (53) | |
| Age group | |||
| Younger women <=35 | 10.6% (48) | 75.8% (307) | 1.000 |
| Older women >=36 | 1.5% (6) | 10.9% (44) | |
| Educational level | |||
| Did not attend school | 0.7% (3) | 2.2% (9) | 0.450 |
| Primary school | 1.5% (7) | 16.3% (66) | |
| Secondary school | 6.6% (27) | 41.0% (166) | |
| Tertiary | 0.6% (17) | 27.2% (110) | |
| STI treatment uptake by sexual partners | |||
| Currently in a sexual relationship | |||
| No | 0,00% (0) | 0.20% (1) | 1.000 |
| Yes | 13.30% (54) | 86.40% (350) | |
| Have been with the same partner for the past 12 months | |||
| No | 13.10% (53) | 19.30% (78) | 0.000 |
| Yes | 0.20% (1) | 67.40% (273) | |
| Number of partners had in the past 12 months | |||
| One partner | 0.20% (1) | 67.40% (273) | 0.000 |
| Two or more | 13.10% (53) | 19.30% (78) | |
The data in Table 4 show of the n=54 who were on STI treatment, there is an association between uptake of STI treatment and having one partner for the past 12 months. The association was statistically significant at a p-value =0.000.
4. DISCUSSION
4.1. Prevalence of STIs among Pregnant Women
This study was conducted to determine the prevalence of STIs among pregnant women attending ANC at three clinics in Maseru and to assess their sexual behaviour. A total of 405 women were selected to participate in the study from a population of 440 pregnant women in the three selected clinics. The mean age of the participants was 27.5 years with a standard deviation of 6.1 and an age range of 18 years to 45 years.
The overall prevalence of STIs was 12.2% across the clinics, which is quite low compared to other countries in the African region such as Gambia, with prevalence of 53.6%, and South Africa reported 37% diagnosis of at least one STI in pregnant women, of which 76% were asymptomatic [14, 15]. Christine et al. [16] reported that in Lesotho, 30.4% of teenagers had at least one diagnosed STI, 25.3% had one, 3.8% had two, and 1.3% had three STIs across gestation. Dvora et al. [17] indicate that overall, the region with the highest adjusted mean prevalence of curable STIs among pregnant women is Southern which includes South Africa, included. Malawi, Madagascar, Zambia, Mozambique, and Zimbabwe. It is important to note that Lesotho is not included in the list, maybe because of the dearth of research in this area.
Gadoth et al. [18], indicated that 18.1% of the women were found to be positive for at least one STI at the time of interview in the Democratic Republic of the Congo. Of the 18.1%, 3.1% had chlamydia, 1.4% had gonorrhoea, and 14.6% had trichomonasis. Most of these studies focused on isolating the agents linked to the STIs and measured the prevalence. However, in our study, we did not concentrate on detecting the types of agents linked to STIs. Our aim was to determine the prevalence of STIs and to assess sexual behaviour of the participants.
In our study, the overall prevalence of HIV is lower than the 39% HIV prevalence in an urban area of South Africa [19]. The findings are in accord with the reality in Lesotho in terms of STI and HIV in the general population and in the three clinics in Maseru. The prevalence of both STI and HIV is high, as was also found in a study conducted in Tanzania where the prevalence of STIs and HIV infection were high [20]. A study in Sudan found high prevalence of STIs [21]. In their study, Davey et al (2018) highlighted a higher STI prevalence among HIV-positive women compared to their HIV-negative counterparts, but our study focused mainly on STIs and STI treatment uptake [19].
In a Lesotho Ministry of Health Survey [2], self-reported prevalence of STIs and STI symptoms in the country for people aged 15-49 years was 17% for women and 15.1% for men, and in Maseru, the prevalence was 14.8% in women and 14.9% in men. Furthermore, the 2014 data from the Lesotho Ministry of Health Survey [3] showed that the self-reported prevalence of STIs and STI symptoms in the country for people aged 15-49 years was 15.2% for women and 11.8% for men. In Maseru alone, the prevalence was 17.7% for women and 14.4% for men, which probably means that women are more susceptible to STIs in Maseru. According to the HIV Sentinel Survey Report in 2005, the overall prevalence of syphilis among pregnant women in Lesotho was 2.2%. This was reported to be 2% in 2009, 3% in 2011, and 3% in 2013 [22]. Efforts should be made by health providers to systematically continue screening for STIs, including chlamydia trachomatis, Neisseria gonorrhoeae, and trichomoniasis vaginalis to treat them.
This study found that most of the participants who were diagnosed and treated for STIs were married, representing 9.1%. Of the married pregnant women treated for STIs, 8.1% were treated for 7 days and 1% of them for 14 days. Some of the pregnant women have consecutive two periods of the 7 days treatment. This suggests either that these participants were re-infected or that the syndromic management treatment possibly failed to treat the infection in the first 7-day period. This shows that the syndromic management of STIs instituted by the WHO and applied in Lesotho and elsewhere all over the world but mainly in low and middle-income countries has a limit. There is also a possibility of antimicrobial resistance which is a current trend for patients who are placed on broad-spectrum antibiotics. The shortcomings of syndromic management may be augmented with routine screening of STIs and the point of care treatment for STIs.
Over or undertreating pregnant women with the same STI syndromic management for longer than specified treatment days without screening of STI types before providing appropriate treatment can induce resistance and therefore cause treatment failure. Many studies have proven the syndromic management of STIs to be a failure or a weak strategy of STI control mostly in developing countries due to the lack of resources to apply an aetiologic strategy as is done in developed countries [23]. Likewise, a study of HIV-infected pregnant women in South Africa found that only 24% of women who tested positive for a chlamydial, gonococcal, or trichomonas infection had vaginal symptoms, whereas 47% of those with symptoms were negative for all three infections. The poor specificity and sensitivity of syndromic management could lead to both over-treatment and under-treatment and poor antimicrobial stewardship may increase the risk of microbial resistance [24]. The high STI prevalence coupled with the low symptom prevalence among infected individuals justifies the use of diagnostic screening approaches rather than the syndromic management of STIs in this setting [25].
4.2. Sexual Behaviour among Pregnant Women: Multiple Sexual Partners and Condom Use among Pregnant Women
Multiple sexual partners are defined as having more than one sexual partner, which can predispose an individual to various sexual infections such as STIs and HIV. It is one of the practices that can promote the transmission of infections such as STIs and HIV, principally due to inconsistent or no use of condoms during sexual intercourse. The consistent use of condoms during sexual intercourse has been reported to be the most effective way of preventing the acquisition and spread of STIs and HIV. In this study, having multiple sexual partners and unprotected sex were identified as sexual behaviour most often leading to the acquisition of STIs including HIV among the participants. Other authors cited that there is a high number of condomless sex and inconsistent use of condoms among pregnant women, together with having more than one sexual partner in the past 12 months [19, 20].
According to Dvora et al. [20], sexual behaviour in HIV-infected pregnant women in South Africa is not well understood. While there are numerous studies on the physiological changes during pregnancy and postpartum periods which increase the risk of HIV infection, behaviour that increases the likelihood of risky sex during pregnancy, thus increasing the risk of HIV and STI acquisition and transmission, are not well described. A recent study showed that alcohol use in sero-discordant couples was associated with reporting one or more outside sex partners, an increased risk of HIV acquisition, and lower odds of initiating ART in HIV-infected female heavy drinkers [22].
4.3. Limitations of the Study
The study was conducted in three clinics in Maseru, Lesotho over a period of three months. The findings have some limitations which include the following: The study was conducted in the difficult times of Covid-19 whereby the expected daily headcount of pregnant women attending ANC and participating in the study was reduced drastically due to infection control protocols. This affected the number of participants that took part in the study. For sexual behaviour, self-reported measures were used, so, the participants may have exaggerated or underreported or may have reported socially desirable responses, even though privacy and confidentiality were emphasized in the informed consent process. This study was also limited in our inability to isolate the types of STIs involved. The use of a convenient sampling technique makes it difficult to generalize the findings to other pregnant women in Maseru and across Lesotho. One of the major limitations of this study arose from recruiting only pregnant women attending ANC. This might influence the sero-prevalence of syphilis and HIV because women with risky behaviour might not wish to attend ANC because of the routine screening for HIV and syphilis. Furthermore, our study did not compare the STI prevalence according to the HIV serostatus because it would have made comparisons that are confirmatory with other studies.
CONCLUSION
The study was conducted among pregnant women accessing antenatal care services in the three clinics in Maseru, Lesotho. A large proportion of the women were married and only a fraction were not in a sexual relationship at the time of data collection. Inconsistent use of condoms and the practice of having multiple sexual partners were the main predictors of STI and HIV acquisition among the participants in the study. Most of the participants did not use condoms consistently, some never used condoms during their sexual activities, and many of them were also living with two or more sexual partners. STI syndromic treatment was provided to those who were diagnosed with STIs. Some of the participants were co-infected with STIs and HIV, placing the newborns in danger of infection.
RECOMMENDATIONS
Arising from the findings, the following recommendations are suggested:
• Routine and aetiologic screening of all pregnant women for STIs including HIV combined with a targeted or specific treatment, might be an effective intervention to reduce the prevalence of STIs in the country.
• Encourage health providers to systematically apply routine aetiologic screening for STIs for all pregnant women presenting at health facilities, whether symptomatic or asymptomatic, and provide specific and appropriate treatment.
• Update Lesotho’s STI policies and adapt them to the current situation in accordance with the HIV and syphilis guidelines recently published by the Ministry of Health, Lesotho.
• The government of Lesotho should assist and support the National Laboratory by providing the budget to enable them to acquire the reagents to detect and isolate other common STIs including chlamydia trachomatis, trichomonas vaginalis, and gonorrhoea.
• Government and health facilities should continue to develop programmes to educate the general population of the country, including pregnant women, about the consistent use of condoms and the need to confine one’s sexual activities to a single partner and maintain one partner to reduce STIs in the Country.
• Given the increased risk of the horizontal and vertical transmission of HIV associated with STIs, an understanding of the sexual and behavioral risk factors associated with STIs during pregnancy is urgently needed.
• The existing public health intervention programmes should be strengthened to promote the sexual and reproductive health of pregnant women in Lesotho.
• Provide regular health education to the community, including pregnant women and their partners, and encourage them to conduct ANC at their nearest health facilities, consistently use condoms during pregnancy, and stick to one sexual partner to minimize the risk of contracting STIs, including HIV.
• .Emphasize that the treatment of STIs in both infected persons and their partners is pivotal for STI management, thus ensuring a reduction in cases of reinfection or non-resolving infections and preventing adverse outcomes.
• There is a need to empower women, including adolescent girls, with the skills and rights to negotiate sexual matters that must be more successfully addressed.
LIST OF ABBREVIATIONS
| STI | = Sexually Transmitted Infections |
| (ANCs) | = Antenatal Care |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained by the School Research Ethics Committee (SREC) and the Sefako Makgatho University Research Ethics Committee (SMUREC).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


