All published articles of this journal are available on ScienceDirect.
Investigation of Hepatitis B Virus in the Body of the Bed Bug Cimex hemipterus (Hemiptera: Cimicidae) Fed on Infected Human Blood by RT-PCR Method
Abstract
Background
Bed bugs (Hemiptera: Cimicidae) are insects found in abundance among urban and rural communities. Both male and female bed bugs thrive on human blood. The main aim of this study was to detect Hepatitis B Virus (HBV) in the body of the bed bug, Cimex hemipterus (Hemiptera: Cimicidae), fed on naturally infected patient blood by Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Methods
In this experiment, C. hemipterus bugs nourished once with HBV-positive blood were examined by RT-PCR at 1, 2, 3, 7- and 10 days post-engorgement. Bloodsucking was performed using an artificial membrane feeding system. RNA was extracted from infected bed bugs, and cDNA was then synthesized to monitor the HBV mRNA.
Results
The RT-PCR test results for infected specimens were positive on the first to third days post-bloodsucking. Moreover, the sample on days 7-10 was negative for HBV. This study also revealed that the breeding of bed bugs under laboratory conditions lasted for one week from the first to fifth nymph stages.
Conclusion
Since HBV mRNA was detected in infected bed bugs until the third-day post-engorgement, it is likely that they potentially transmitted HBV. It is thus indispensable to conduct more thorough research in the future.
1. INTRODUCTION
Insects are one of the most important pests of human communities, and bed bugs, as annoying urban pests, are usually adapted to live in an environment with close contact and proximity to humans. Bed bugs are cosmopolitan nocturnal insects apparently resurging in High-Income Countries (HIC) [1]. In fact, the behavior of these pests is attributable to the urban lifestyle [2, 3]. They occur disproportionately in city district housing, and the built environment is now the dominant health influence. According to the World Health Organization (WHO), inadequate housing is a determinant of health. Houses significantly affect public health, and bed bugs are of devastating environmental justice concern [4].
The global population of bed bugs (Cimex lectularius and Cimex hemipterus, Family: Cimicidae) has recently earned considerable attention. The universal inundation of bed bugs is a main public health menace [5]. This is most possibly due to a surge in international travel, trade, use of scrap equipment, presence of nesting sites, adaptability to new niches, levitation of the preponderance of bed bugs resistant to insecticides, insufficient knowledge, and lack of effective control measures. The estimated global population of bed bugs is rising by 100-500% annually [6, 7].
Both male and female bed bugs are obligatory ectoparasites. They occur in the phylum Arthropoda, subphylum Hexapoda, class Insecta, order Hemiptera, and suborder Heteroptera [5]. Bed bugs are the sole insects that both nourish human blood and copulate by traumatic insemination. Two species associated with humans include the common bed bug, C. lectularius (L.), and the tropical bed bug, C. hemipterus. They need regular blood meals to produce eggs as adult females and probably sperm production in males. They are intermittent suckers and do not reside in a place for long periods of time [5, 8]. They also feed on bats, birds, and rarely other domestic animals.
The level of hygiene is not, however, a good indicator of the presence of bed bugs [9]. They are a “pest of significant health importance” and appear to harbor microorganisms found in the stomach, feces, exoskeleton, or saliva [10]. The detection of the hepatitis virus in mosquitoes (Diptera) has been conducted by Zheng et. al., whose results provided evidence for the possibility of transmission of Hepatitis B Virus (HBV) through mosquitoes. This implies its epidemiological significance in mosquito-infested areas [11]. In addition, Anopheles are widely distributed in Iran [12-16].
WHO reports about 240 M people have chronic HBV infection. A high prevalence of infection with HBV in Low- to Middle-Income Countries (LMIC) compared to HIC is common. HBV, as the causative agent, is a hepatotropic virus from the Hepadnaviridae family with a small double-stranded circular DNA genome that replicates its nucleotides via a reverse transcription step through an RNA mediator. This reverse transcriptase activity of HBV polymerase was expressed in insect cells using the baculovirus system [17, 18]. In 2001, HBV DNA was detected in C. lectularius L., which demonstrated that HBV DNA persisted in bed bugs and their excrement up to 6 weeks post-feeding on an infected meal [19, 20]. The persistence of HBV antigen (HBsAg) in C. hemipterus has previously been shown for the same period of time following infected blood uptake [20, 21].
HBV contains one of the smallest genomes among human viruses. Its genome contains 3200 bp with a molecular weight of about 2×106 D. The virus contains a reverse transcriptase enzyme. The size of its genomic core (virion) is almost 42 nm. It has a very high replication rate on a scale of 108-1013 ml/day [22, 23]. In addition, a relatively small number of viruses are sufficient to cause infection in humans.
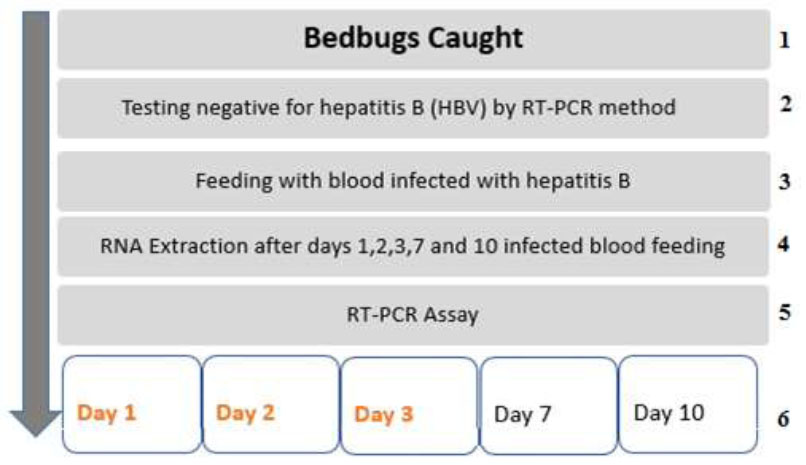
Display different stages of investigation of hepatitis B virus in the body of the bed bug cimex hemipterus (Hemiptera: Cimicidae) fed on infected human blood by RT-PCR method.
HBV infection is an important cause of acute and chronic hepatitis, cirrhosis, and Hepatocellular Carcinoma (HCC) worldwide. A high incidence of HBV infection has been noted in LIC compared to MIC. Although the main modes of transmission appear to be perinatal, conta- minated blood, and sexual exposure, up to 40% of people infected with HBV are not associated with these risk factors. The aim of this study was to detect the hepatitis B virus in the body of the bed bug Cimex hemipterus (Hemiptera: Cimicidae) fed on infected human blood by RT-PCR method.
2. MATERIALS AND METHODS
The research steps were carried out according to the flowchart (Fig. 1 ).
2.1. Collection and Identification of Samples
The current research was an experimental study. Experiments were conducted on Cimex hemipterus bed bugs caught in the city of Abadan, SW Iran. About 110 bed bugs (adults and nymphs) were manually collected from a residential building located in Abadan, using forceps, tweezers, and brushes, in the spring of 2022. The specimens were placed in glass containers with specifications. They were then transferred to the Laboratory of Biology and Control of Disease Vectors at Shiraz School of Health. Samples were identified using valid diagnostic keys to the species level. In addition, for testing the non-infection of caught bed bugs with the HBV virus, randomly, the RNA (Cedbio Co.) from 10 live bed bugs was separately extracted on ice, and then their cDNA (AccuPower®) was synthesized. RT-PCR was carried out to detect the HBV virus.
2.2. Artificial Membrane Feeding System
This system had a glass container with two channels for inlet and outlet water. The water pouring into the system was adjusted to remain at 37°C, so it kept the blood temperature at 37°C and made blood feeding more favorable for bed bugs. This was achieved with a water circulation pump through a glass feeder. At the bottom of it, a parafilm membrane was stretched to maintain and facilitate feeding to strengthen bed bug attachment; 10 ml of HBV-infected blood was poured into the feeder and kept at 37°C in a warm (Bain-Marine) heating bath. The paper strips were placed in the plate in a way to ensure direct contact of bed bugs to reach the food source through the hole of the net and the parafilm membrane, all parts of the membrane needed to be in contact with the blood pool so that the bed bugs could suck blood by mouthparts. The bed bugs were fed with blood for 6 hours until their stomachs were filled with blood. They were then detached from the parafilm and kept at -20°C.
2.3. Blood Samples
Blood samples from patients infected with HBV referred to the Gastroenterology and Liver Research Department of SUMS with high viral titers (5042700 IU/ml) were prepared and defibrinated. The whole blood was transferred to the molecular laboratory under cold chain conditions and kept at -70°C.
2.4. Experiments
From the total of 110 caught bed bugs, 10-bed bugs were used for testing to ensure that they were not infected with hepatitis virus, and the remaining 100 were divided into two groups of 50 each. About 50 C. hemipterus bedbugs (adults and nymphs) were fed with HBV-infected blood by an artificial feeding device, and another 50 bedbugs were fed with uninfected healthy blood (control group). This was conducted to determine how long HBV remains infectious and dangerous in bed bugs. Therefore, on days 1, 2, 3, 7, and 10, two infected blood bed bugs were removed and immediately stored at the -20° C until the experiments (Fig. 2 ). Since the aim of this study was to determine the survival rate of the virus in the body of bed bugs, RNA expression was evaluated.
| Primer Name | Sequences |
|---|---|
| HBV forward primer | 5ʹ- GCTTTGGGGCATGGACATTGAC -3ˊ |
| HBV reverse primer | 5ʹ- TTGATAAGATAGGGGCATTTGGTGGTC -3ˊ |
2.5. Primer Design for Hepatitis B Virus (HBV) Gene Detection
The first step in identifying the virus was to design the primer. For this purpose, the DNA sequence of the HBV virus was extracted from the National Center for Biological Information (NCBI) site with an emphasis on the sequences reported from Iran and neighbouring countries. Using MEGA 6.0 software, alignment was then conducted based on the conserved points. The hepatitis gene with the accession number OP611181.1 was used as a reference genome, and the coding region of the protein pre-core/core protein was amplified by PCR. The primer design was performed using Oligo7.0 and GeneRunner4.0 software on the complete genome of the virus to identify the hepatitis virus (Table 1).
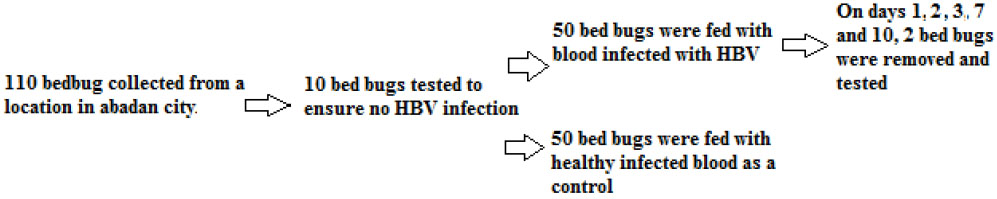
Flowchart of sample selection for Investigation of hepatitis B virus in the body of the bed bug Cimex hemipterus (Hemiptera: Cimicidae) fed on infected human blood.
The forward and reverse primers corresponded to the positions of 1888 and 2319, respectively, and the expected band was 431 bp.
2.6. RNA Extraction
In this study, RNA was extracted using a high-pure RNA purification kit from Roche Company. The steps of this method were carried out according to the manufacturer's instructions, in addition, in order to maintain the quality of the study, DNase was used for RNA isolation. The bed bug specimens on days 1, 2, 3, 7, and 10 were extracted. The concentration of RNAs prepared by spectrophotometer (Analytikjena) was measured, and the required amount of them was calculated for cDNA synthesis. In each reaction of this study, 200 ng of RNA was used to perform each reverse transcription reaction.
2.7. Synthesis of cDNA
A reverse transcription reaction was used to prepare cDNA from the extracted RNA molecules. To this end, the AccuPower Cycle Script RT Pre Mix (d N6) kit of BIONEER Company was used. One μl of extracted RNA and 19 μl of DDW were added to the kit, and then PCR was performed according to the kit program.
2.8. RT-PCR- Reverse Transcription Method
To perform the RT-PCR reaction, a mixture containing 8 microliters of Master Mix, Red amplicon, 500 ng cDNA, 1 microliter (400 micromoles) of forward and reverse primers, and finally 20 microliters of DDW water was prepared. The material was gently pipetted and then spinning was performed. The PCR program was assigned to the thermal cycler, and microtubes were placed in the machine. The PCR program included a 5-minute step at a temperature of 94°C to open the DNA strands. Then at a temperature of 94°C for 30 seconds, then an annealing step at a temperature of 59°C for 30 seconds, an extension step at a temperature of 72°C for 30 seconds (in 35 cycles), and finally, a 10-minute step, the final advance temperature was set at 72°C. The PCR products were then run on 1% agarose gel in Tris-acetate-EDTA (TAE) buffer and stained with Safe Stain (SinaClone Co.). Electrophoresis involved a voltage of 100 mA. A volume of 1μl DNA ladder 100 bp was poured into the first well, and 7 μl of PCR sample was poured into the subsequent wells. After 1 h, the results of the work were observed by the GEL DOC device. The amplicon length was 431 bp. All PCR reactions were also performed using the Analytikjena machine made in Germany.
3. RESULTS
In this research, 110 caught bed bugs were identified in the spring of 2022 from Abadan city. The bed bugs were 5-6 mm long and belonged to the Cimex hemipterus species based on specific morphological criteria. The results showed that the field-caught bed bugs were all free from HBV. The steps of breeding and continuing the study were then carried out. This study showed that 5 nymph stages were completed in one week under laboratory conditions. The blood sample received from the Gastroenterology and Liver Research Department of SUMS was also tested to ensure it was infected with blood. An expected band of 431 bp was thus observed (Fig 3).
In the present study, RNA was extracted from bed bugs fed with infected blood on days 1, 2, 3, 7, and 10. The RT-PCR reaction was performed with specifically designed primers at an annealing temperature of 57°C, on days 1, 2, 3,7, and 10 and run on 1% agarose gel. The size of the expected band for the identification and infectivity of the HBV virus, the 431 bp band, was observed on days 1, 2, and 3. Therefore, our results showed that the HBV was viable and infectious in the body of the bed bugs until the third day (Fig. 1). The positive HBV (mRNA) in bed bugs may be from the HBV mRNA in the blood that was used for feeding. However, bed bugs can still be a big threat because mRNA has been seen in their bodies until the third day. In addition, the infected bed bugs in the samples on days 7 and 10 were negative for the mRNA virus. (Table 2).
The 100 bp ladder was in well designated as L. The wells of 1-3 were positive HBV-RNA samples from Cimex hemipterus bed bug body homogenate detected on days 1, 2, and 3, showing the 431 bp band. In contrast, wells 7 and 10 revealed negative HBV-RNA samples in the bed bug body homogenates on days 7 and 10. Well, Co is negative control. The well Pos was the positive HBV control, showing the size band 431 bp.
| Days |
Number Bedbugs
Test |
RNA Extraction | RT-PCR | cDNA Synthesis | Result |
|---|---|---|---|---|---|
| 1 | 2 | √ | √ | √ | Posetive |
| 2 | 2 | √ | √ | √ | Posetive |
| 3 | 2 | √ | √ | √ | Posetive |
| 7 | 2 | √ | √ | √ | Negative |
| 10 | 2 | √ | √ | √ | Negative |
| Control | 2 | √ | √ | √ | Negative |
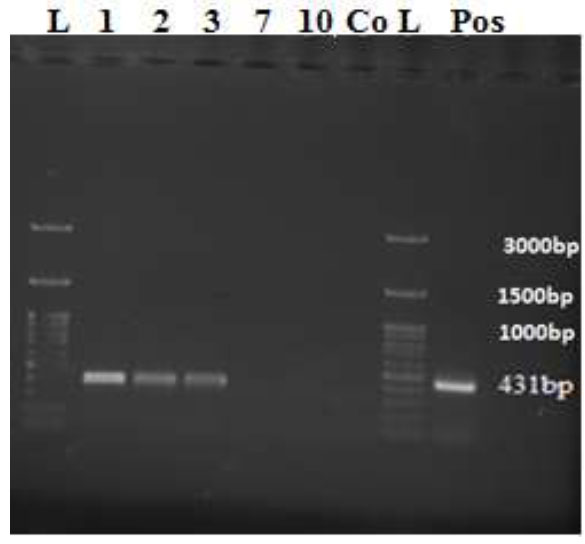
RT-PCR product gel electrophoresis of the 431 bp fragment of the HBV virus genome.
4. DISCUSSION
This study was the first attempt to infect C. hemipterus with HBV via an artificial blood-feeding device in Iran. This finding shows that bed bugs are able to acquire HBV from an infected blood meal via an artificial membrane and retain it in their body for up to 3 days. The presence of HBV-RNA in the homogenate of the whole body of bed bugs is proof of this infection, and to ascertain the biological transmission of HBV, it is necessary to check whether the virus has the ability to infect bed bug somatic cells. Otherwise, there is a likelihood that a number of human White Blood Cells (WBC) remained viable in the body of the bed bug for three days, from which RNA was extracted in this study. The possible role of bed bugs in the transmission of human diseases has been the subject of many studies for more than five decades. Currently, they have become endemic in most big cities, but the biology and main behavior of this pest are still not well understood [23]. In the present study, RNA was detected in the homogenate of bed bugs, and the bed bugs acquired HBV infection through an infected blood meal with a high viral titer via an artificial membrane, maintaining their viability. Similarly, using the PCR molecular technique, Silverman and colleagues detected HBV-DNA in bed bugs (Rhodnius prolixus, Cimex lectularius) and showed that HBV-DNA remains for a long time in the bed bugs and excreted through the feces up to six weeks after an infectious feeding. On the other hand, using the reverse transcription method (in hepatitis C), they found that HCV-RNA cannot be detected in bed bugs after feeding directly on the blood of infected patients with a high viral titer [19, 24]. This is in sharp contrast to a recent identification of HCV in a pool of C. lectularius specimens collected from domestic residences in Europe [25, 26]. For the micro- organisms identified in most of the studies reported on pathogen presence, histological investigations were not performed to investigate whether the microbe could be in a site that suggests the possibility of biological trans- mission (such as salivary glands or ovarian ampulla) [26, 27], and in many studies, whether the pathogen was alive or not. Likewise, most researchers have not performed vector competence tests to determine whether a bed bug is capable of transmitting a pathogen [27, 28]. Other researchers identified HBV-DNA nucleic acids by PCR test in the body and feces of C. lectularius up to 35 days postfeeding with infected blood. The observations exhibited that bed bugs keep the blood in a semi-digested state for several weeks postfeeding, and during this time, they excrete drops of feces consisting of an undigested blood meal. In bed bugs that had inoculation inside the thorax, HBV-DNA was detected 21 days after inoculation [19]. Although bed bugs could potentially act as vectors of viruses and other pathogenic agents, as is the case with the human body lice transmitting agent of trench fever, Bartonella quintana, among homeless people [29, 30], bed bugs have never been confirmed to naturally transmit any disease agents in vivo [31, 32].
This is experimentally examined in a recent attempt to evaluate the competence of C. lectularius to trans mit B. quintana [32, 33]. In an earlier study, researchers detected Human Immunodeficiency Virus (HIV) up to 8 days after feeding blood with a high concentration of the virus in bed bugs. However, no viral multiplication occurred in the insect, and no virus was detected in the bed bug feces [19, 34]. The same group of researchers in South Africa experimentally demonstrated that biological replication of HBV does not occur in C. lectularius, but mechanical transmission occurs (contamination from bed bug crushing, contact with infected feces, or through interrupted feeding or vomiting). They detected HBsAg in feces for 5-6 weeks after feeding contaminated food [34], yet no transmission of HBV was detectable in a chimpanzee model [35, 36]. The first experiment testing the ability of C. lectularius to transmit HBV was undertaken via artificial blood-feeding devices (membrane feeders). However, transmission occurred at very low rates, and they concluded that biological replication of HBV does not occur in bed bugs because virus titer decreases over time in bed bugs fed on HBV-infected blood [37, 38]. The persistence of viral antigen in C. lectularius using artificial feeding through the membrane for 122 days with signs of increasing positive ratio in 2-3 months showed that the virus is multiplying but transmission of HBV through the ovary to the offspring of bed bugs did not occur [39]. In an intervention (using indoor insecticide spraying) study in Gambia, there was no reduction in HBV infection despite a remarkable reduction in bed bug exposure. Studies indicate that inanimate objects infected with HBV may contribute to disease transmission for up to one week and possibly longer. The virus has been reported to be infectious after storage at room temperature for 6 months and exposure to UV irradiation. Therefore, it is possible that HBV shed onto surfaces with bed but fecal material could remain viable and infectious for yet an undetermined length of time. In recent years, machine learning has been used to identify diseases. Based on the study of Gangani Dharmarathne et al., in 2024, machine learning was used to identify hepatitis and and have reported that given the high-risk nature of the medical field, this developed interface can be enhanced to include more extensive clinical data and ultimately assist medical professionals in their decision-making processes [40]. One Health is an approach that optimizes the health of humans, plants, animals and the ecosystem as one of its main goals. Today, due to the expansion of communication and urban life, the abundance of bed bugs in public places and homes has increased, which has caused health concerns. Therefore, studying and planning to control vector-borne diseases is one of the priorities of the World Health Organization in the One Health program.
CONCLUSION
A recent study showed that mRNA-HBV is present in the body of bed bugs until the third day. In addition, the results showed that the bed bugs fed with infected blood were negative for hepatitis B in the samples on days 7 and 10. Since it was found in this study that the virus was active in the body of infected bed bugs until the third day, this issue still needs more basic studies to determine the answers to the following questions. Is the virus able to infect bed bug cells, or can it persist in human WBC cells for three days? Therefore, it can be claimed that bed bugs can probably be a potential vector of HBV. The health authorities should thus pay special attention to controlling bed bugs in centres whose inhabitants are in a cluster, including the most important centres where the transfer capacity potential is extremely high, which are prisons. Therefore, health executives should compile a proper guideline and dispose of it to relevant centres, so that they can control it with the utmost care. On the other hand, the bed bug control program should be a priority for health care centre experts and the control of disease vectors department.
AUTHORS’ CONTRIBUTIONS
HA, MDM, and NKR contributed to the conceptua- lization, NKA, JS, MK and MS, ST contributed to the study’s methodology,; HA and MDM contributed to the study’s validation, NKR curated the data, HA, NKR and MDM wrote original draft preparation, HA and MDM supervised the study. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| WBC | = White Blood Cells |
| RT-PCR | = Reverse Transcription Polymerase Chain Reaction |
| HBV | = Hepatitis B Virus |
ETHICAL STATEMENT
This work was supported in part by an MSc thesis by Mrs. N. Kiani-Ravesh in Biology and Control of Disease Vectors (Grant no.: 21626, Ethics code no.: IR.SUMS.SCHEANUT.REC.1400.003) at School of Health, SUMS, Iran.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This work was supported in part by an MSc thesis by Mrs. N. Kiani-Ravesh in Biology and Control of Disease Vectors (Grant no.: 21626), at the School of Health, SUMS, Iran.
ACKNOWLEDGEMENTS
The authors wish to convey their gratitude to the vice-chancellor for research and technology for SUMS, to all anonymous HBV-positive patients who contributed their blood samples to those staff at the Gastroenterology and Liver Research Department of SUMS whose valuable assistance is appreciable, and to our anonymous reviewers of this paper for their meticulous comments.


