All published articles of this journal are available on ScienceDirect.
Prevalence of Thyroid Dysfunction Disorders among Adult Populations in the Middle–East: A Systematic Review and Meta-analysis
Abstract
Background
Thyroid dysfunction is a systemic disorder that causes severe morbidity and is a public health problem worldwide. This study aimed to evaluate the prevalence of thyroid dysfunction among adults in the Middle East.
Methods and Materials
We searched PubMed, Google Scholar, and Medline databases from 2000–2021 to identify studies that presented the prevalence of thyroid dysfunction, hypothyroidism, subclinical hypothyroidism, hyperthyroidism, and subclinical hyperthyroidism in the Middle East. A random-effects model was used to calculate the pooled prevalence and confidence intervals of thyroid dysfunction. The data were analyzed using STATA-V14.
Results
Generally, 345 studies had eligible criteria to be included in this meta-analysis. The pooled prevalence of thyroid disorders, overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, and subclinical hyperthyroidism in the Middle East were 19.2% (95% CI: 11.0 – 33.2), 7.2% (95% CI: 3.6 – 14.3), 8.3% (95% CI: 5.3 – 13.0), 2.4% (95% CI: 1.4– 3.9), and 3.2% (95% CI: 2.1 – 4.7), respectively. Moreover, the prevalence of thyroid disorders increased from 15.2% (95% CI: 9.8-23.6) to 31.5% (95% CI: 22.5- 44.2) between 2000 and 2022.
Conclusions
Current meta-analysis suggests that thyroid disorders are more prevalent among adults in the Middle East. Moreover, with an increasing trend in the prevalence of thyroid disorders during the last two decades, early screening and prevention of the disease should be practiced.
1. INTRODUCTION
Thyroid dysfunction is one of the most prevalent endocrine disorders [1]. The thyroid gland generates the thyroid hormones thyroxine (T4) and triiodo-thyronine (T3) in response to pituitary production of thyrotrophin (TSH). Overt hyperthyroidism is characterized by subnormal serum TSH levels and elevated free serum triiodo-thyronine (T3) or free T4 levels. In contrast, subclinical hyperthyroidism is defined as subnormal serum TSH levels and normal free triiodothyronine (FT3) and free thyroid hormone (FT4) or thyroxine levels. On the other hand, Overt hypothyroidism is defined by a decrease in free thyroxine levels (T4) in the presence of elevated TSH, while Subclinical hypothyroidism occurs when TSH levels are slightly elevated but T3 and T4 or thyroxine levels are normal [2, 3].
Some possible symptoms of hyperthyroidism include exhaustion, weight loss, increased perspiration, heat intolerance, tachycardia, tremor, and hyperactive reflexes that can develop gradually or abruptly [4]. Exhaustion, dry skin, susceptibility to colds, hair loss, weight gain, constipation, changes in voice, and slower movement and thinking are common symptoms of hypothyroidism that, at onset, are typically slow [5].
Thyroid dysfunction is prevalent at different ages, sexes, race/ethnicity, and geographical locations due to differences in dietary iodine intake [6]. Women, older people (> 60 years old), those with a prior personal history of or a significant family history of thyroid illness, and postpartum women are at a higher risk of developing thyroid dysfunction [2].
Overt thyroid dysfunction has been related to different types of cognitive decline, peripheral neuropathy, and psychological health. Moreover, they have effects on the nervous system, cardiovascular system, bone health, and erythropoiesis [6, 7]. Studies have shown that the prevalence of different types of thyroid disease in the Arab world varies from 6.18 to 47.34%, and thyroid cancer is very prevalent in Arab countries. The diet and lifestyle of the Arab countries have changed over the past years, and thus, thyroid diseases are becoming more prevalent, making the economic crisis greater [8].
Limited studies have been conducted on the prevalence of various types of thyroid disease in the Middle East. Therefore, this study aimed to investigate the prevalence of thyroid dysfunction, namely hypothyroidism, and hyperthyroidism, among the adult population in the Middle East.
2. METHODS
2.1. Search Strategy and Selection Criteria
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [9]. A literature search was conducted in PubMed, Google Scholar, and Medline databases to search for studies that presented the prevalence of thyroid dysfunction, hypothyroidism, sub- clinical hypothyroidism, hyperthyroidism, and subclinical hyperthyroidism in the Middle East. The search was carried out using the search terms “prevalence”, “hyperthyroidism”, “hypothyroidism”, “subclinical hyper- thyroidism”, “subclinical hypothyroidism”, “Thyroid”, “Thyroid Dysfunction Disorders”, “The Middle East”, and “countries in the Middle East area”. The search was limited to studies that were published between 2000 and July 2021.
2.2. Inclusion and Exclusion Criteria
Studies were included if they provided sufficient information to estimate the prevalence with confidence intervals of thyroid dysfunction, which were cross-sectional studies published in the English language. The exclusion criteria were review, not providing sufficient information, not cross-sectional studies, involving adolescents and children, focusing on specific groups like patients with diabetes, hypertension, postmenopausal women, pregnant women, etc. Moreover, the studies measuring the prevalence of Congenital hypothyroidism and Postpartum Thyroiditis were excluded as well.
2.3. Study Selection and Data Extraction
At first, we screened all of the identified studies through databases based on titles and abstracts. Then, the full text of relevant articles was read, and data extraction was performed from eligible studies. The data extracted from the studies included names of authors, year of publication, setting (country’s name, city), sampling method, diagnostic criteria, gender, number of participants, age, prevalence of Thyroid Dysfunction, and its 95% confidence interval. Table 1 illustrates the main characteristics of the included studies in this meta-analysis.
2.4. Statistical Analysis
The data were analyzed using STATA-14 (Stata Corp, Texas, USA) statistical software. Pooled estimates of thyroid dysfunction prevalence and confidence intervals were calculated using a random-effects model. The I2 test was used to measure study heterogeneity. Subgroup analysis was then performed to investigate the sources of heterogeneity. Egger's test was also performed to check for publication bias.
|
Authors/ Year of Publication/Refs |
Setting | Sampling | Dyslipidaemias | Overt Hypothyroidism * | Subclinical Hypothyroidism ** | Overt Hyperthyroidism # | Subclinical Hyperthyroidism $ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | City | Age | Method | Sex | N | Criteria | Prevalence (95% CI) | *Criteria | Prevalence (95% CI) | Criteria | Prevalence (95% CI) | Criteria | Prevalence (95% CI) | Criteria | Prevalence (95% CI) | |
| Elham Faghih Imani,2010 [10] | Iran | isfahan | > 20 | multistage clustering | FM | 263 | - | _ | 5 | 6.5 (3.8 - 10.1) | 1 | 6.9 (4.1- 10.6) | 1 | 0.3 (0.01 - 2.1) | 1 | 0.7 (0.09- 2.7) |
| - | - | - | - | - | M | 142 | - | _ | - | 3.5 (1.1 - 8) | - | 5.6(2.4 - 10.8) | - | _ | - | 0.7 (0.02 - 3.8) |
| - | - | - | - | - | F | 121 | - | _ | - | 10.7 (5.8 - 17.6) | - | 7.4(3.4 - 13.6) | - | 0.8 (0.02 - 4.5) | - | 0.8 (0.02- 4.5) |
| Ebrahim Barzegari,2021 [11] | Iran | Ravansar | 35-65 | _ | FM | 10069 | - | _ | _ | 3.21 (2.8 - 3.5) | _ | _ | _ | 0.44 (0.3 - 0.5) | _ | _ |
| - | - | - | - | - | M | 4752 | - | _ | - | 0.88 (0.6 - 1.1) | - | _ | - | 0.35(0.21- 0.57) | - | _ |
| - | - | - | - | - | F | 5269 | - | _ | - | 5.36 (4.7 - 5.9) | - | _ | - | 0.51(0.34- 0.75) | - | _ |
| Bahram Eshraghi,2022 [12] | Iran | Rasht | 39.5±13.45 | _ | FM | 383 | - | _ | _ | 8.9(6 - 11.8) | _ | _ | _ | 89 (85.4- 91.9) | _ | _ |
| - | - | - | - | - | M | 155 | - | _ | - | 3.8 (1.4- 8.2) | - | _ | - | 94.8 (90 - 97.7) | - | _ |
| - | - | - | - | - | F | 228 | - | _ | - | 11.8 (7.9 - 16.7) | - | _ | - | 85(79.7 - 89.4) | - | _ |
| NIAFAR M,2009 [13] | Iran | East Azerbaijan | 60-89 | random | FM | 1000 | - | 12.7(10.7 - 14.9) | _ | 1.5(0.8 - 2.4) | _ | 5.8 (4.4 - 7.4) | _ | 1.2 (0.6 - 2.09) | _ | 4.1 (2.9 – 5.5) |
| Maryam Rezaei,2019 [14] | Iran | Birjand | _ | voluntarily | FM | 110 | - | _ | 11 | 30 (21.6 - 39.4) | _ | _ | 1 | 30 (21.6 - 39.4) | _ | _ |
| - | - | - | - | - | M | 45 | - | _ | - | 22.2 (11.2 -37) | - | _ | - | 22.2 (11.2 -37) | - | _ |
| - | - | - | - | - | F | 65 | - | _ | - | 35.3(23.9- 48.2) | - | _ | - | 35.3(23.9- 48.2) | - | _ |
| Ladan Mehran,2017 [15] | Iran | Tehran | 40.3 ± 14.4 | multistage cluster random | FM | 5422 | - | _ | 1 | 1.8(1.5 - 2.2) | 1 | 5.4(4.8 - 6.06) | 1 | 1.5(1.2 - 1.8) | 1 | 3.2 (2.8- 3.7) |
| - | - | - | - | - | M | 2318 | - | _ | - | 16.8(15.3 - 18.4) | - | 23.7 (22 - 25.5) | - | 42.7 (40.6 -44.7) | - | 40.4(38.3- 42.4) |
| - | - | - | - | - | F | 3104 | - | _ | - | 83.2 (81.8 -84.4) | - | 76.2(74.6 - 77.6) | - | 57.2(55.5 - 59) | - | 59.6 (57.8 -61.3) |
| Ashraf Aminorroaya,2008 [16] | Iran | Isfahan | > 20 | multistage cluster | FM | 2523 | - | _ | _ | _ | _ | _ | 1 | 0.8(0.5 - 1.2) | 1 | 0.9(0.6 - 1.4) |
| - | - | - | - | - | M | 1275 | - | _ | - | _ | - | _ | - | 0.3 (0.1 - 0.9) | - | 0.7 (0.3 - 1.3) |
| - | - | - | - | - | F | 1248 | - | _ | - | _ | - | _ | - | 1.2 (0.7 - 2.07) | - | 1.2 (0.7- 2.07) |
| Farnaz Rahmani,2018 [17] | Iran | Tabriz | 18-58 | convenient sampling | FM | 261 | - | _ | _ | 1.1(0.2 - 3.3) | _ | 16.8(12.5- 21.9) | _ | 0.3 (0.01 - 2.1) | _ | _ |
| Yusuf Aydin,2014 [18] | Turkey | _ | 18-92 | _ | FM | 2233 | - | _ | 1 | 6.5 (5.5 - 7.6) | 1 | 12 (10.6 - 13.4) | _ | 0.5 (0.2 - 0.9) | _ | 11 (9.7 - 12.4) |
| - | - | - | - | - | M | 803 | - | _ | - | 1.7 (0.9 - 2.9) | - | 2.8(1.8 - 4.2) | - | 0.6 (0.2 - 1.4) | - | 10.8 (8.7- 13.1) |
| - | - | - | - | - | F | 1430 | - | _ | - | 1.3(0.8 - 2) | - | 4.4 (3.4 - 5.6) | - | 0.4 (0.2 – 1) | - | 11.1 (9.6- 12.9 |
| Mustafa Behçet Demirbaş,2019 [19] | Turkey | Istanbul | > 65 | _ | FM | 500 | - | - | _ | 0.6 (0.1 - 1.7) | _ | 2.8 (1.5- 4.6) | _ | 0.6 (0.1- 1.7) | _ | 16 (12.9- 19.5) |
| Faruk Kutluturk,2013 [20] | Turkey | province of Tokat | >18 | random sampling | FM | 1095 | - | _ | 2 | 1.6 (1 - 2.6) | 2 | 2.7 (1.9 - 4) | 1 | 0.5 (0.2 - 1.2) | 1 | 4.9 (3.8- 6.6) |
| - | - | - | - | - | M | 541 | - | _ | - | 0.7 (0.2 - 1.8) | - | 1.8 (0.8 - 3.3) | - | 0.4 (0.05 - 1.3) | - | 3.5(2.1 - 5.4) |
| - | - | - | - | - | F | 554 | - | _ | - | 2.5(1.4 - 4.2) | - | 3.6 (2.2 - 5.6) | - | 0.7 (0.2 - 1.8) | - | 6.4 (4.5 - 8.8) |
| Glay HERGEN,2005 [21] | Turkey | _ | >34 | _ | FM | 512 | - | _ | 3 | 3.1 (1.8 – 5) | _ | _ | 1 | 5.8 (3.9 - 8.2) | _ | _ |
| - | - | - | - | - | M | 239 | - | _ | - | 1.7(0.4 - 4.2) | - | _ | - | 7 (4.2 - 11.1) | - | _ |
| - | - | - | - | - | F | 273 | - | _ | - | 4.4(2.2 - 7.5) | - | _ | - | 5 (2.8 - 8.4) | - | _ |
| Selahittin Çayan,2017 [22] | Turkey | 19 provinces of Turkey | ≥40 | randomly | FM | 2760 | - | _ | _ | 0.5 (0.2 - 0.8) | _ | _ | _ | 1.6 (1.1 - 2.1) | _ | _ |
| Aykut Sarıtaş,2015 [23] | Turkey | Giresun | ≥18 | _ | FM | 10600 | - | _ | 4 | 1.9(1.6 - 2.1) | 1 | 6.7 (6.2- 7.1) | 3 | 0.7 (0.5 - 0.8) | 2 | 1.8 (1.5 - 2) |
| - | - | - | - | - | M | 5697 | - | _ | - | 1.4(1.1 - 1.7) | - | 5.2 (4.6 - 5.8) | - | 0.6 (0.4 - 0.8) | - | 1.7 (1.3 - 2) |
| - | - | - | - | - | F | 4903 | - | _ | - | 2.4 (2 - 2.9) | - | 8.3 (7.5- 9.1) | - | 0.6 (0.4 - 0.9) | - | 1.9 (1.6 - 2.4) |
| Rana Hasanato,2017 [24] | Saudi Arabia | _ | 20-65 | _ | F | 199 | - | _ | 2 | 10(6.2 – 15) | _ | _ | _ | 3 (1.1 - 6.4) | _ | _ |
| Ahmed Ali Gaffer Ali,2016 [25] | Saudi Arabia | AlBAHAH city | ≥18 | _ | FM | 71 | - | 43.6(31.9 - 55.9) | _ | 40.8(29.3 - 53.1) | _ | _ | _ | 2.8 (0.03 - 9.8) | _ | _ |
| - | - | - | - | - | M | 8 | - | 25 (3.1 – 65) | - | 25 (3.1 – 65) | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 63 | - | 46.03(33.3 -59) | - | 42.8 (30.4 -55.9) | - | _ | - | 3.17(0.3 - 11) | - | _ |
| 18Malak A. Al-Shammari,2022 [26] | Saudi Arabia | Dammam City | median: 37 | _ | FM | 240 | - | _ | 5 | 29.2 (23.5- 35.3) | _ | _ | 4 | 15 (10.7 - 20.1) | _ | _ |
| - | - | - | - | - | M | 35 | - | _ | - | _ | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 205 | - | _ | - | _ | - | _ | - | _ | - | _ |
| 19 Saif Aboud M. Alqahtani,2021 [27] | Saudi Arabia | Asir region | 43.4 ±15.8 | _ | FM | 9992 | - | 49.7(48.7- 50.7) | 6 | 5.3(4.8 - 5.7) | 1 | 39.2 (38.2 -40.2) | 1 | 2.49 (2.2 - 2.8) | 1 | 2.72(2.4 - 3) |
| - | - | - | - | - | M | - | - | 18.3(17.6- 19.1) | - | 2.4(2.1 - 2.7) | - | 15.3 (14.3- 15.7) | - | 1.1 (0.9 - 1.3) | - | 0.8(0.7 - 1) |
| - | - | - | - | - | F | - | - | 31.9(30.9- 32.8) | - | 2.9(2.5 - 3.2) | - | 24.2(23.3- 25) | - | 1.4 (1.1 - 1.6) | - | 1.8(1.5 - 2.1) |
| 20 Shahad Lafi Alanazi,2018 [28] | Saudi Arabia | Arar | >20 | multistage stratified random | FM | 160 | - | 22.5(16.2- 29.7) | 7 | 15.6 (10.3 -22.2) | _ | _ | _ | 6.8 (3.4 -11.9) | _ | _ |
| - | - | - | - | - | M | _ | - | _ | - | 17.3(4.9- 38.7) | - | _ | - | 39.1(19.7 - 61.4) | - | _ |
| - | - | - | - | - | F | _ | - | _ | - | 15.3(9.7- 22.4) | - | _ | - | 1.4 (0.1 - 5.1) | - | _ |
| 21 Atheer Mohammed D Alotaibi,2018 [29] | Saudi Arabia | Riyadh city | >18 | Systematic random | FM | 870 | - | _ | 8 | 16.7(14.3 - 19.4) | _ | _ | 5 | 2 (1.1 - 3.1) | _ | _ |
| - | - | - | - | - | M | 297 | - | _ | - | _ | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 573 | - | _ | - | _ | - | _ | - | _ | - | _ |
| 24 Eidan Al Eidan,2018 [30] | Saudi Arabia | Riyadh | >18 | _ | FM | 394 | - | _ | _ | _ | 1 | 10.3(7.2- 14) | _ | _ | 1 | 2.1(0.8 - 4.2) |
| 25 Bassem Refaat,2015 [31] | Saudi Arabia | Makkah | 18-45 | _ | F | 600 | - | 19.6(16.5- 23) | 1 | 6.3(4.5 - 8.5) | 1 | _ | 6 | 1.6(0.8 - 3) | _ | _ |
| 26 Abdelhameed A Fureeh,2019 [32] | Saudi Arabia | l-Baha | >18 | _ | FM | 567 | - | _ | 2 | _ | 1 | 15.9 (12.9 -19.1) | 1 | _ | 3 | 4.4(2.8 - 6.4) |
| - | - | - | - | - | M | 101 | - | _ | - | _ | - | 2.6 (1.4- 4.3) | - | _ | - | 0.7(0.1 - 1.7) |
| - | - | - | - | - | F | 466 | - | _ | - | _ | - | 13.7(10.9- 16.9) | - | _ | - | 3.7(2.4 - 5.8) |
| 27 Mohammed Qashqary,2020 [33] | Saudi Arabia | Jeddah | 16-56 | _ | FM | 346 | - | _ | _ | _ | _ | _ | _ | 1.1(0.3 -2.9) | _ | _ |
| - | - | - | - | - | M | 152 | - | _ | - | _ | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 194 | - | _ | - | _ | - | _ | - | _ | - | _ |
| 34 Abdulaziz A Alghaithy,2013 [34] | Saudi Arabia | Almadinah Almounawarah | >14 | _ | FM | 177 | - | _ | _ | 34.4(27.4 - 41.9) | _ | 19.2(13.6 -25.7) | _ | 12.4 (7.9 - 18.2) | _ | 16.3(11.2 - 22.6) |
| - | - | - | - | - | M | 34 | - | _ | - | 23.5(10.7 41.1) | - | 14.7 (4.9- 31) | - | 14.7 (4.9- 31) | - | 11.7(3.3 - 27.4) |
| - | - | - | - | - | F | 143 | - | _ | - | 37(29.1 - 45.5) | - | 20.2(14 - 27.8) | - | 11.8(7 - 18.3) | - | 17.4(11.6 - 24.7) |
| 35 Abdulwahab Alyahya,2021 [35] | Saudi Arabia | Al Ahsa | 18-60 | randomly | FM | 882 | - | _ | _ | 11.7(9.7- 14.1) | _ | _ | _ | 1.7(0.2 - 2.7) | _ | _ |
| - | - | - | - | - | M | 391 | - | _ | - | _ | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 491 | - | _ | - | _ | - | _ | - | _ | - | _ |
| 36 Khalid SJ Aljabri1,2019 [36] | Saudi Arabia | Jeddah | 12-105 | _ | FM | 3872 | - | _ | 5 | 29.1(27.6 -30.5) | _ | _ | _ | _ | _ | _ |
| - | - | - | - | - | M | 884 | - | _ | - | 18.2(15.7- 20.9) | - | _ | - | _ | - | _ |
| - | - | - | - | - | F | 2988 | - | _ | - | 32.2(30.5- 33.9) | - | _ | - | _ | - | _ |
| 38 Nida Suhail,2020 [37] | Saudi Arabia | Arar | >18 | _ | FM | 150 | - | _ | _ | 48.6(40.4 - 56.9) | _ | _ | _ | 12.6(7.8 - 19) | _ | _ |
| - | - | - | - | - | M | 64 | - | _ | - | 45.3(32.8 - 58.2) | - | _ | - | 29.6(18.9 - 42.4) | - | _ |
| - | - | - | - | - | F | 86 | - | _ | - | 51.1(40.1 - 62.1) | - | _ | - | 13.9(7.4 - 23.1) | - | _ |
| 28 Munir Abu‐Helalah,2020 [38] | Jordan | Karak | 18-79 | _ | FM | 7085 | - | _ | 2 | 14.8(14 - 15.7) | 1 | 5.5 (5 – 6) | 1 | 1.9(1.6 - 2.2) | 4 | _ |
| - | - | - | - | - | M | 2007 | - | _ | - | 9.1(7.8- 10.4) | - | 4.3 (3.- 5.3) | - | 2.2(1.6 - 2.9) | - | _ |
| - | - | - | - | - | F | 5084 | - | _ | - | 17.2(16.1- 18.2) | - | 5.9(5.3 - 6.6) | - | 1.8(1.4 -2.1) | - | _ |
| 29 Burhan Abdullah Zaman,2021 [39] | Iraq | Duhok city | _ | FM | 24568 | - | _ | _ | 1.2(1.07 - 1.34) | _ | 94.8(94.5- 95.1) | _ | 0.31(0.25 - 0.39) | 4 | 2.2(2.02 - 2.39) | |
| - | - | - | - | - | M | 8063 | - | _ | - | 0.9 (0.7 - 1.1) | - | 95.1(94.6- 95.6) | - | 0.3(0.2 -0.4) | - | 2.2(1.8 - 2.5) |
| - | - | - | - | - | F | 16505 | - | _ | - | 1.3(1.1 - 1.5) | - | 94.7 (94.3 -95) | - | 0.3(0.2 - 0.4) | - | 2.2(1.9 - 2.4) |
| 30 Ahmed M Athab Al-Msari,2014 [40] | Iraq | Baquba City | _ | _ | FM | 2973 | - | _ | _ | 7.1 (6.2 - 8.1) | _ | _ | _ | 5(4.2 - 5.8) | _ | _ |
| - | - | - | - | - | M | 647 | - | _ | - | 3.5(2.2 - 5.2) | - | _ | - | 3.7(2.3 - 5.4) | - | _ |
| - | - | - | - | - | F | 2326 | - | _ | - | 8.1(7 - 9.3) | - | _ | - | 5.3(4.4 - 6.3) | - | _ |
| 31 Sabah Muhammed Salih,2021 [41] | Iraq | Kirkuk | 20-50 | _ | FM | 88 | - | _ | 9 | 22.7 (14.4- 32.8) | _ | _ | 1 | 23.8(15.4 - 34.1) | _ | _ |
| - | - | - | - | - | M | 20 | - | _ | - | 5(0.1 - 24.8) | - | _ | - | 20(5.7 - 43.6) | - | _ |
| - | - | - | - | - | F | 68 | - | _ | - | 27.9 (17.7 - 40.1) | - | _ | - | 25(15.2 -36.9) | - | _ |
| 32 Payman A. Hamasaeed,2019 [42] | Iraq | Erbil City | _ | _ | F | 433 | - | _ | _ | 3(1.6 - 5) | _ | _ | 1 | 9.4(6.8 -12.6) | _ | _ |
| 33 Fadhluddin Nasruddin Shakor,2019 [43] | Iraq | Sulaimaniyah city | _ | _ | FM | 115 | - | _ | 2 | 35.6(26.9 - 45.1) | _ | _ | _ | 44.3(35 -53.9) | _ | _ |
| - | - | - | - | - | M | 14 | - | _ | - | 28.5(8.3- 58.1) | - | _ | - | 64.2(35.1 - 87.2) | - | _ |
| - | - | - | - | - | F | 101 | - | _ | - | 36.6(27.2 - 46.8) | - | _ | - | 41.5(31.8 - 51.8) | - | _ |
| 37 Noor Thair Tahir,2021 [44] | Iraq | Baghdad | 12_62 | _ | FM | 1800 | - | _ | 10 | 3.2(2.4 - 4.1) | 1 | 14.1(12.5- 15.8) | 1 | 3(2.2 -3.9) | 3 | 4 (3 - 4.9) |
| - | - | - | - | - | M | 388 | - | _ | - | 3.3(1.8 - 5.6) | - | 12.8(9.7 - 16.6) | - | 3.8(2.1 -6.3) | - | 3.3 (1.8 - 5.6) |
| - | - | - | - | - | F | 1412 | - | _ | - | 3.1(2.3 - 4.2) | - | 14.4(12.6 - 16.3) | - | 2.7(1.9 – 3.7) | - | 4.1 (3.1 - 5.2) |
| 39 Hamed Abdel-Aziz Deraz,2019 [45] | Egypt | Zagazig city | 67.6± 7.1 | _ | FM | 126 | - | 13.3(8.3-19.8) | _ | 1.3(0.1 - 4.7) | _ | 7.3(3.7 - 12.7) | _ | 1.3 (0.1- 4.7) | _ | 3.3 (1 - 7.6) |
| - | - | - | - | - | M | 41 | - | 19.5(8.8-34.8) | - | _ | - | 7.3 (1.5 - 19.9) | - | 4.8(0.6 -16.5) | - | 7.3 (1.5 - 19.9) |
| - | - | - | - | - | F | 85 | - | 14.1(7.5-23.3) | - | 2.3(0.2 - 8.2) | - | 9.4 (4.1 - 17.7) | - | _ | - | 2.3 (0.2 - 8.2) |
| Amira H. Mahmoud,2005 [46] | Egypt | Qatif city | >60 | _ | FM | 100 | - | 20(12.6 -29.1) | 2 | 10(4.9 - 17.6) | 1 | 5 (1.6 - 11.2) | 1 | 3(0.6 -8.5) | 4 | 2 (0.2 - 7) |
| 22 Nearmeen M. Rashad,2019 [47] | Egypt | _ | _ | _ | FM | 430 | - | 29.3(25 -33.8) | 5 | 20.4(16.7 - 24.5) | 1 | 44.4(39.6 - 49.2) | 4 | 19.2(15.4 - 23.1) | 4 | 15.8(12.4- 19.6) |
** Subclinical Hypothyroidism: 1= TSH > 4 mIU/L and T4, T3, and fT4I were within the normal range/ 2= (TSH:↑, and fT4:N)/
# Overt Hyperthyroidism: 1= low TSH (<0.3 mIU/L)with high FT4I or T3 / 3= fT4 >1.7 ng dL−1 and TSH <0.1 mIU mL−1/ 4=FT3 > 3.71 pg/ml or FT4> 1.48 ng/dl or TSH < 4.94 μIU/ml / 5= Selfe report/ 6= TSH < 0.10 μIU/ml and FT4 >22 pmol/l./
$ Subclinical Hyperthyroidism: 1= Low TSH(<0.3 mIU/L) with normal FT4I and T3/ 2= TSH <0.1 mIU mL−1 with normal free hormone levels/ 3= TSH <0.2 with normal FT4/ 4= Low TSH and normal T3 and T4.
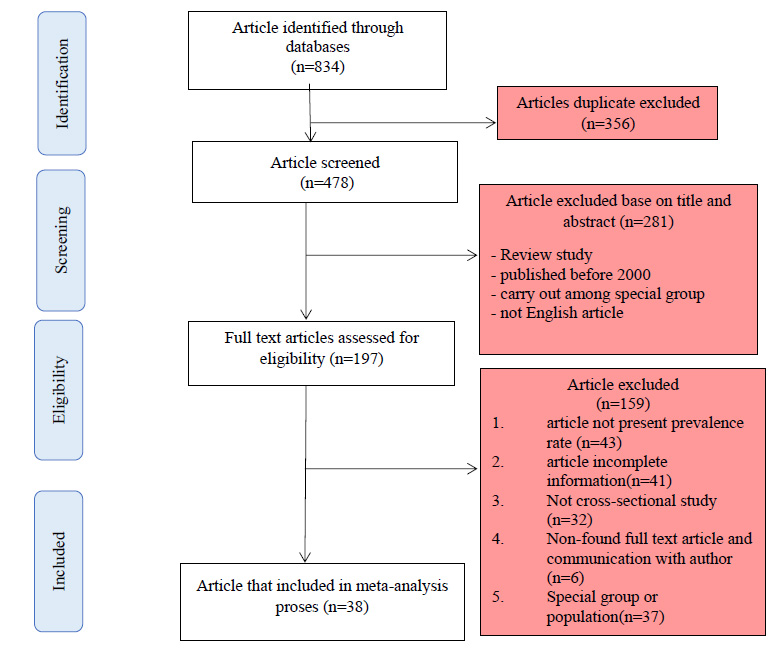
Flowchart of the present study selection.
3. RESULT
A total of 834 articles were identified from a database search, of which 356 articles were removed due to duplication. Next, 478 articles that were not duplicated were screened, and 281 articles were excluded based on irrelevant titles and abstracts. Then, 197 articles were selected to assess the full text. Of these, 159 articles were excluded for the following reasons: absence of prevalence rate, not providing sufficient information to calculate CI 95%, not finding the full-text article, not being cross-sectional, and being carried out among special groups. Eventually, 38 articles from Iran, Saudi Arabia, Egypt, Iraq, Turkey, and Jordan were eligible to be included in the meta-analysis (Fig. 1). We could not find eligible articles from other countries in the Middle East region.
Four thyroid disorders were described in this survey. The number of studies on the four thyroid disorders was 34 on overt hypothyroidism, 18 on subclinical hypo- thyroidism, 35 on overt hyperthyroidism, and 17 on subclinical hyperthyroidism.
The pooled prevalence of thyroid disorders was 19.2% (95% CI: 11.0 – 33.2) in the Middle East. The highest prevalence of thyroid disorders was observed in Saudi Arabia at 31.3% (95% CI: 17.9 – 54.9), and the lowest prevalence was in Iran at 12.7% (95% CI: 10.7 – 14.9). Some heterogeneity was seen among the study's findings (p< 0.001). In addition, the pooled prevalence of overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, and subclinical hyperthyroidism in the Middle East was 7.2% (95% CI: 3.6 – 14.3), 8.3% (95% CI: 5.3 – 13.0), 2.4% (95% CI: 1.4– 3.9), and 3.2% (95% CI: 2.1 – 4.7), respectively (Table 2).
Although analysis of the data by sex showed that the prevalence of thyroid disorders, overt hypothyroidism, subclinical hypothyroidism, and subclinical hyper-thyroidism was higher in women than in men, they were not statistically significant (p >0.5).
Analysis of the data by year of publication of articles illustrated an increasing trend in the prevalence of thyroid disorders between 2000 and 2022, which increased from 15.2% (95% CI: 9.8-23.6) during 2000 - 2010 to 31.5% (95% CI: 22.5- 44.2) during 2016 - 2022, overall (P < 0.001) (Figs. 2-4).
4. DISCUSSION
This study investigated the prevalence of thyroid disorders, including hypothyroidism and hyperthyroidism, in the Middle East region. The prevalence of thyroid disorders was high in this region [19.2% (95% CI: 11.0 – 33.2)]. Besides, the frequency of other non-communicable diseases, including hypertension, obesity, etc., was found to be considerable among the adult population in the Middle East [48, 49], which could explain the high prevalence of thyroid disorders.
Moreover, the overall prevalence of thyroid disorders was different among countries, ranging from 31.3 in Saudi Arabia to 12.7 in Iran. The high prevalence of thyroid disorders in Saudi Arabia can be attributed to the reasons mentioned in previous studies, including iodine deficiency and improper nutrition [8]. However, the differences observed in the prevalence of different thyroid lesions among countries in the Middle East may be due to diversity in the study methods, environmental factors, iodination status, and different cut-off values to identify thyroid disorders.
| Variables | N. of Studies (population) | Thyroid Dysfunction |
Test for Heterogeneity |
Overt Hypothyroidism |
Test for Heterogeneity |
Subclinical Hypothyroidism |
Test for Heterogeneity |
Overt Hyperthyroidism |
Test for Heterogeneity |
Subclinical Hyperthyroidism |
Test for Heterogeneity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (95% CI) | (P- value) | Prevalence (95% CI) | (P- value) | Prevalence (95% CI) | (P- value) | Prevalence (95% CI) | (P- value) | Prevalence (95% CI) | (P- value) | ||
| Country | - | - | - | - | - | - | - | - | - | - | - |
| Saudi Arabia | 14 (18520) | 31.3 (17.9 – 54.9) | P< 0.001 | 17.9 (10.5 – 30.3) | P< 0.001 | 18.9 (9.6 – 37.1) | P< 0.001 | 4.0 (2.1 – 7.6) | P< 0.001 | 4.6 (1.7 – 11.8) | P< 0.001 |
| Turkey | 6 (17700) | _ | 1.7 (0.8 -3.6) | 5.2 (3.2 – 8.6) | 1.05 (0.4 – 2.4) | 6.2 (2.1 – 17.9) | |||||
| Iran | 8 (20031) | 12.7 (10.7 – 14.9) | 4.2 (2.04 – 9.0) | 7.7 (4.5 – 13.09) | 2.05 (0.2 – 15.5) | 2.05 (1.06 – 3.9) | |||||
| Jordan | 1 (7085) | _ | 14.4 (14.0 -15.7) | 5.5 (5.0 -6.0) | 1.9 (1.6 – 2.2) | _ | |||||
| Egypt | 3 (656) | 20.4 (12.5 – 33.3) | 9.9 (3.8 – 25.9) | 12.2 (2.6 – 57.7) | 5.02 (0.8 – 29.2) | 5.5 (1.3 – 22.3) | |||||
| Iraq | 6 (29977) | _ | 6.3 (2.1 – 19.2) | 36.5 (5.6 – 236.8) | 5.9 (1.3 – 25.6) | 2.9 (1.6 – 5.2) | |||||
| Total (Middle-East) | 38 (93969) | 19.2 (11.0 – 33.2) | 7.2 (3.6 – 14.3) | 8.3 (5.3 – 13.0) | 2.4 (1.4 – 3.9) | 3.2 (2.1 – 4.7) | |||||
| sex | - | - | - | - | - | - | - | - | - | - | - |
| Female | 28 (54298) | 26.5 (18.4 – 38.04) | P = 0.5 | 8.9 (5.1 – 15.4) | P= 0.2 | 14.1 (9.6 – 20.8) | P= 0.2 | 3.6 (1.9– 6.6) | P= 0.6 | 4.2 (1.3– 13.7) | P= 0.7 |
| Male | 24 (29774) | 18.3 (17.5 – 19.07) | 5.4 (3.1 -9.5) | 8.1 (3.6 – 18.2) | 4.6 (2.3 – 9.07) | 3.07 (0.9 – 10.07) | |||||
| Year of publication | - | - | - | - | - | - | - | - | - | - | - |
| 2000 - 2010 | 4 (4298) | 15.2 (9.8 – 23.6) | P= 0.01 | 4.1 (1.8 – 9.0) | P= 0. 3 | 5.9 (4.7 – 7.4) | P= 0.02 | 1.6 (0.5 – 4.9) | P=0.4 | 1.6 (0.5 – 4.8) | P= 0.2 |
| 2011 - 2015 | 6 (17678) | 19.6 (16.5 – 23.0) | 5.6 (2.4 – 12.8) | 8.1 (4.9 – 13.4) | 1.6 (0.5 – 4.8) | 6.2 (2.1 – 18.4) | |||||
| 2016 - 2022 | 28 (71993) | 31.5 (22.5 – 44.2) | 8.0 (5.0 – 12.6) | 13.2 (7.7 – 22.6) | 3.7 (1.4 – 9.8) | 4.3 (2.6 – 7.2) |

The prevalence of overt hypothyroidism and subclinical hypothyroidism in the Middle East.
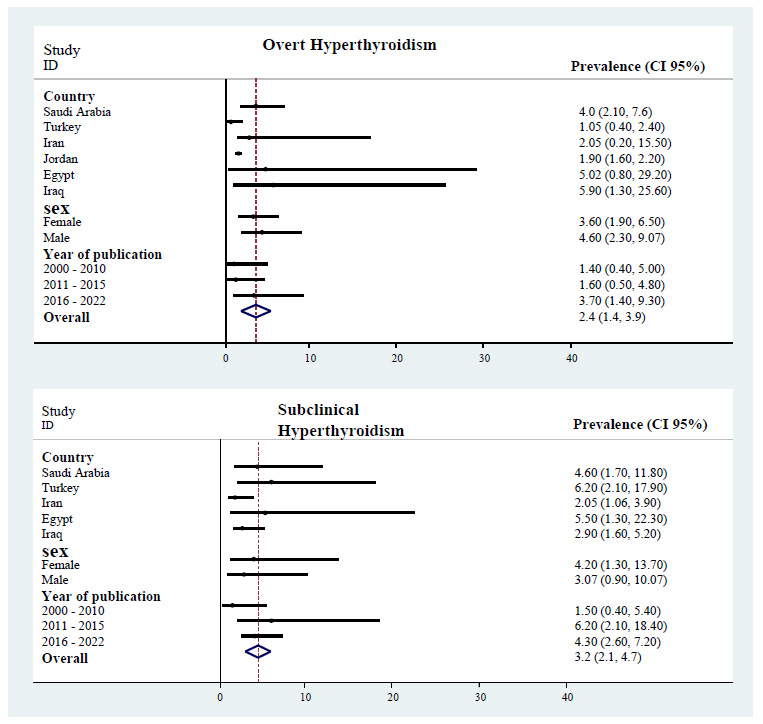
The prevalence of overt hyperthyroidism and subclinical hyperthyroidism in the Middle East.
The pooled prevalence of overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, and subclinical hyperthyroidism in the Middle East was 7.2% (95% CI: 3.6 – 14.3), 8.3% (95% CI: 5.3 – 13.0), 2.4% (95% CI: 1.4– 3.9), and 3.2% (95% CI: 2.1 – 4.7), respectively. A previous meta-analysis on thyroid dysfunctions estimated the prevalence of undiagnosed hyperthyroidism in Europe at 1.7% (95% CI: 1.66–1.88), with a clear predominance of the subclinical form of the disease: overt hyperthyroidism 0.35% (95% CI: 0.29–0.41) and subclinical hyper- thyroidism 0.50% (95% CI: 0.57-1.43) [50]. Moreover, overt hypothyroidism and subclinical hypothyroidism were found in 0.65% (95% CI 0.38–0.99) and 4.11% (95% CI 3.05–5.31) of Europe, respectively [51]. Comparing these results with the present meta-analysis results, the MiddleEast region exceeds Europe by 6.5% and 2% for overt hypothyroidism and hyperthyroidism and by 4.1% and 2.7% for subclinical hypothyroidism and hyperthyroidism, respectively. Moreover, the prevalence of hypothyroidism and hyperthyroidism among adults in the United States has been calculated at 9.47% and 1.19%, respectively [52]. Therefore, the prevalence of hypothyroidism and hyperthyroidism are roughly similar in the Middle East and the United States (0.7% versus 9.47%, 2.4% versus 1.9%).
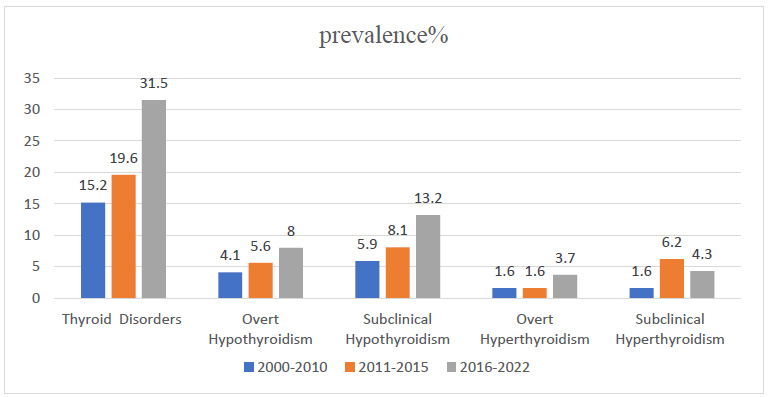
Trend and prevalence of thyroid disorders, overt hypothyroidism, subclinical hypothyroidism, overt hyperthyroidism, and subclinical hyperthyroidism from 2000 to 2022.
Non-communicable diseases have increased among adults in the Middle East during the last two decades. Moreover, the findings of this meta-analysis show an increasing trend in the prevalence of thyroid disorders between 2000 and 2022. Changing lifestyles, using an unhealthy diet (high-calorie and fat), obesity, using alcohol, and smoking in this regain could be possible reasons for an increasing trend in the prevalence of thyroid disorders [11, 48, 53].
The present results, like other studies [51], point out that the prevalence of subclinical hypothyroidism (8.3%) and subclinical hyperthyroidism (3.2%) is higher than overt hypothyroidism (7.2%) and overt hyperthyroidism (2.4%). This high prevalence sheds light on the potential for the underappreciation of subclinical thyroid diseases, which can have significant effects on the management and treatment of adult patients. Our findings suggested that additional thyroid function screening may be necessary among adults, as well as increased awareness of thyroid dysfunction.
The prevalence of thyroid dysfunction varies by age, sex, race/ethnicity, and iodine status of populations [54]. In this study, the prevalence of thyroid disorders, overt hypothyroidism, subclinical hypothyroidism, and subclinical hyperthyroidism was higher in women compared with men. Moreover, previous studies have shown that the prevalence of hypothyroidism has been higher in females [6, 30, 51]. The strongest support for screening comes from the 2005 AACE, American Thyroid Association (ATA), and Endocrine Society Consensus Statement, which suggested thyroid screening for all women starting at age 35 years and continuing every five years after that [50].
CONCLUSION
According to the results of the current meta-analysis, the prevalence of thyroid disorders was high among adults, especially women in the Middle East. Moreover, an increasing trend was observed in the prevalence of thyroid disorders during the last two decades. Therefore, this meta-analysis suggests the need for increased screening of thyroid disorders and prevention of disease at the primary level by educating the population.
LIMITATIONS
one of the limitations of this meta-analysis was that the cut-off value of serum thyrotropin (TSH), thyroxine (T4), and triiodothyronine (T3) to determine the four thyroid disorders was not identical between the studies. In addition, the years of publication of articles and the distribution of age were different between studies from different countries.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| TSH | = Serum Thyrotropin |
| T4 | = Thyroxine |
| T3 | = Triiodothyronine |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| ATA | = American Thyroid Association |


