All published articles of this journal are available on ScienceDirect.
Gut Microbiome Changes among Undernutrition and Stunting Infants and Children under 2 Years: A Scoping Review
Abstract
Background
Undernutrition and stunting are significant public health concerns globally, particularly in low- and middle-income countries. Nutritional intervention is the cornerstone of the management and prevention of these conditions. However, the gut microbiome has recently emerged as an essential modulator of the effects of nutritional interventions in undernutrition and stunting. This scoping review aims to examine the impact of nutrition intake (including if there is any intervention) over time on gut microbiome changes in infants and children under the age of 2 who experience undernutrition and stunting.
Methods
A literature search was conducted in PubMed and Cochrane Library, including studies from 2013 to 2023, using terms related to malnutrition, stunting, failure to thrive, and gut microbiome. Inclusion criteria were applied to select eligible studies for review. Five studies were chosen to be included in this review.
Results
The findings indicate that nutrition intake over time, including dietary supplementation and prebiotics, can influence the gut microbiome composition, diversity, and functionality in undernutrition and stunting infants and children. These may promote the growth of beneficial bacteria while reducing the abundance of harmful pathogens. Moreover, improvements in nutritional status, growth parameters, and immune function were observed in association with positive changes in the gut microbiota.
Conclusion
Nutrition intake can positively modulate the gut microbiome in undernutrition and stunting infants by supporting the growth of beneficial gut bacteria such as Actinobacteria, Bacteroides, Streptococcus, Bifidobacterium, Prevotella sp, and other bacterial taxa that vary with age, ultimately contributing to enhanced growth and development outcomes. Further studies are needed to better understand the mechanisms underlying these effects and to develop targeted nutritional interventions that optimize the gut microbiome in undernutrition and stunting infants and children under 2 years.
1. INTRODUCTION
Undernutrition and stunting are significant public health concerns that affect millions of infants and young children worldwide, particularly in low- and middle-income countries [1]. Undernutrition is characterized by the result of inadequate food consumption and recurrent infectious ailments.
The factors contributing to undernutrition are intricate, multifaceted, and not thoroughly comprehended. Nevertheless, the United Nations Children's Fund (UNICEF) has indicated that the primary reasons for malnutrition are insufficient dietary intake and recurrent illnesses [2]. Stunting is characterized as the proportion of children whose height-for-age falls below minus two standard deviations for moderate stunting and minus three standard deviations for severe stunting from the 2006 WHO Child Growth Standards median. Likewise, children are categorized as severely stunted if their length/height is below −3 SDs from the WHO child growth standards median for their specific age and gender [3].
These conditions have both short-term and long-term effects on health and development. Short-term effects include increased susceptibility to infections, delayed motor and cognitive development, poor academic performance, reduced productivity, and increased healthcare costs. Children who are undernourished or stunted have weakened immune systems, making them more vulnerable to infectious diseases such as pneumonia, diarrhea, and malaria [4, 5]. They may also experience delayed development of motor and cognitive skills, such as crawling, walking, problem-solving, and memory. These delays can impact academic performance, leading to poor grades and even school dropouts. Furthermore, under- nourished and stunted children are more likely to have reduced productivity in adulthood, which can impact national economic growth and development [4, 6, 7]. Finally, the increased risk of infections and other health problems associated with undernutrition and stunting, such as diabetes and cardiovascular disease in adulthood, can lead to increased healthcare costs [8].
Unfortunately, undernutrition is frequently under- diagnosed or underestimated in clinical practice, and preventing it to all degrees is thought to be an effective way of boosting child survival and reducing the significant financial burden placed on the healthcare system [9-14]. As a result, the WHO global targets for infant and young child nutrition call for a 40% reduction in the number of stunted children under the age of five by 2025, as well as a reduction and maintenance of childhood wasting (acute malnutrition) to a level of < 5% [15, 16].
Several factors can contribute to undernutrition and stunting in infants, including inadequate nutrient intake, poor feeding practices, and underlying medical conditions. Infants who are exclusively breastfed for the first six months of life are less likely to experience weight faltering and have a lower risk of stunting [4, 5]. However, if breastfeeding is insufficient or not possible, appropriate complementary feeding practices are crucial for meeting the infant's nutritional needs. Good nutrition intake, including nutritional interventions, is commonly used to prevent undernutrition and stunting in infants. This intervention may include supplementation with nutrients such as vitamins and minerals, provision of fortified foods, and nutritional counseling for mothers and caregivers [5, 8, 17-20]. While nutritional intervention has been shown to improve growth and reduce the risk of undernutrition and stunting, its impact on the gut microbiome is not well understood [21-23].
On the other hand, the gut microbiome, composed of trillions of microorganisms that inhabit the human gastrointestinal tract, plays a crucial role in nutrient absorption and metabolism, immune function, and host defense against pathogens. It has been noted that there are compositional differences in the gut microbiota between healthy and sick situations. Conditions of eubiosis are useful in managing a variety of microbial infections. Human health benefits from the development of eubiosis and the proper consumption of nutritious food [24]. The state of eubiosis is defined by a high abundance of potentially helpful species, mostly from the two bacterial phylums Firmicutes and Bacteroides, and a very low abundance of potentially harmful species, such as those from the phylum Proteobacteria (Enterobacteriaceae). When dysbiosis occurs, “bad bacteria” take over, and “good bacteria” are no longer in charge [25].
Alterations in the gut microbiome composition and function have been reported in undernourished and stunted children, suggesting a potential role in the pathophysiology of these conditions [26-29]. Recent studies have suggested that nutritional interventions may promote changes in the gut microbiome composition and diversity that could benefit undernourished and stunted infants. For example, probiotics, prebiotics, and syn- biotics, which are dietary supplements that contain live microorganisms or their substrates, have been shown to modulate the gut microbiome and improve nutrient absorption and utilization [26, 30].
This review aims to examine and explore the current literature on the microbiome change as an impact from effect of nutrition intake overtime from one time to another, including dietary supplementation among undernourished and stunted infants and children under two years old, and to provide insight into the potential of nutritional intervention to improve gut microbiome health and prevent undernutrition and stunting. The rationale for conducting a scoping review is that currently, there is very limited information or research available on this topic. For a systematic review, highly specific research questions are required, such as the impact of administering certain nutritional interventions on changes in the proportion of a specific bacteria, for example, lactobacillus. However, this method is not conducive to capturing variations in other bacteria or evaluating the effects of different nutritional interventions, given its narrow focus.
This review will systematically scope and map the published literature on various nutritional interventions used, including nutrient supplementation and dietary diversification, and their impact on the gut microbiome. Furthermore, this study will explore the potential mechanisms through which nutritional interventions may impact the gut microbiome and its subsequent effects on undernutrition and stunting. The findings of this review have important implications for policy-makers, health practitioners, and researchers working to combat under- nutrition and stunting in infants and children under two years old.
2. MATERIALS AND METHODS
This review was performed based on the methodological guidance for scoping review by the Joanna Briggs Institute (JBI) manual for evidence synthesis [29]. A literature search was conducted using electronic databases such as PubMed and Cochrane Library Scholar to identify relevant articles by T.S The search strategy used the following keywords and their combinations: “malnutrition,” “stunting” “failure to thrive,” “gut microbiome,” “children/toddler,” and “infants” with the exact keywords ((malnutrition OR malnourished OR growth impairment OR growth faltering OR severe acute malnutrition OR moderate acute malnutrition OR stunting OR undernourished) OR (weight gain OR body mass index)) AND (gut microbiota OR dysbiosis OR gut microbiome OR metagenomics OR gut microbiota immaturity OR intestinal microflora OR culturomics) AND (child OR children OR infant OR toddler) with age (birth-23 months old), human study and language (English) filter. The search was limited only to peer-reviewed journals, did not include grey literature, and was not done by hand searching from any reference list.
The inclusion criteria for the articles were as follows: (1) Study among infants up to the age of 2-year-old; (2) Quantitative Studies among children on the microbiome change as an impact from effect of nutrition intake over time (i.e., RCT, quasi experimental-pre/post study, prospective/retrospective cohort, and case-control); (3) evaluation of gut microbiome changes as an outcome measure; and (4) articles published from 2013 to 2023 in English.
The exclusion criteria were as follows: (1) VLBW (very low birth weight) infants/ premature/ preterm infants; (2) Intervention targeted among those above 2-year-old; (3) Focus among overnutrition/obesity; (4) Infectious/ other specific diseases (gestational diabetes mellitus, juvenile idiopathic arthritis, nonalcoholic fatty liver disease, cystic fibrosis, cirrhosis, fatty liver, anemia, lactose intolerance, etc.) related cases; (5) Only antibiotic intervention; (6) Intervention targeted among healthy infants/children; (6) Only study concept; (7) Only probiotic(s) intervention without other nutrients ; (8) Only from 1 case report; (9) On acute malnutrition infants/children (either severe, moderate, or mild); (10) Study/review without no specific microbiota parameter; (11) Cross-sectional study and (12) Study/review related to finding on maternal factors.
The results from the searches were screened in phases (Fig. 1) by T.S. and R.B. First, the sources were screened based on the information presented in the title and abstract. All duplicate articles (101 articles) were eliminated. Next, full-text articles were screened based on the set inclusion criteria, where 86 articles were removed. Then, the articles were assessed for eligibility to be included in the review based on exclusion criteria, and 17 articles were removed and 5 articles were included in this review. Discrepancies on the eligibility were resolved by consensus and discussion with a third author (R.D). We accepted all 5 studies regardless of their quality; hence, no critical appraisal was performed.
The data extracted from the articles included authors, year of publication, geographical setting, target popu- lation, study design, sample size, type of nutritional intervention, duration of intervention, and gut microbiome changes as an outcome measure. The data were analyzed qualitatively, and the results were synthesized to identify the key findings related to the effect of nutritional intervention on gut microbiome changes in undernutrition and stunting infants. The findings were then discussed in light of the current knowledge and the potential implications for future research and clinical practice.
This scooping review protocol has been registered on the Open Science Framework (https://osf.io/m2b9w).
3. RESULTS
In the search process, 209 articles were identified as potentially relevant. The selection process is detailed in Fig. (1), outlining the steps to identify the most relevant studies for inclusion in the scoping review. One hundred and one articles were removed due to duplication. Next, the processes involved screening the titles and abstracts of the articles to assess their alignment with the research focus. After this initial screening, 86 articles that did not meet the inclusion criteria were excluded. The full texts of the remaining articles were then carefully evaluated to determine their suitability for the review based on the set exclusion criteria, and 17 articles were removed. Five articles that met the predetermined criteria were included in the final analysis.
Table 1 presents a comprehensive summary of the selected articles, providing key information about each study, including the title, authors, year of publication, and primary findings related to the impact of nutritional interventions on the gut microbiome of undernourished and stunted infants and children under two years old. The table serves as a quick reference guide, facilitating a better understanding of the breadth and diversity of the reviewed studies.
As an overview, all participants in the study have the age of below 2 years old. Only in one study, we had participants starting from 6 months of age. There are 3 RCT and 2 prospective cohorts. Participants in the cohort studies may obtain an intervention as this is part of other studies. In the RCT studies, one of the nutrients contained in the active study product is zinc, in various formats of food such as complementary food, ready-to-use supplementary food, powders, and lipid-based supple- ments.
| Author, Year; Title | Location | Study Type | Participant (N), Age (mo) and Nutrition Status) | Intervention or Follow up Duration | Specific Nutrition for Intervention | Main Findings |
|---|---|---|---|---|---|---|
| Chen et al.,2021; A Microbiota-Directed Food Intervention for Undernourished Children | Slum area in Bangladesh | Randomized controlled trial (RCT) | N= 118, age 12-18 mo, WLZ <-2 to -3 SD |
3 months | Microbiota-directed complementary food (MDCF-2) or ready-to-use supplementary food (RUSF) | MDCF-2 consistently showed growth benefits in weight-for-length (higher by 0.011) and weight-for-age z scores (higher by 0.008), even at the 1-month follow-up (higher by 0.010 and 0.008 respectively). Changes in 70 plasma proteins and 21 associated bacterial taxa, identified through 16S rDNA sequencing, were positively correlated with weight-for-length z scores (P < 0.001). These proteins included mediators of bone growth and neurodevelopment. The findings suggest that targeted microbiota alterations may be linked to growth mechanisms. |
| Popovic et al., 2021; Micronutrient supplements can promote disruptive protozoan and fungal communities in the developing infant gut | Pakistan | Sub-analysis of c-RCT | N= 80, age 12-24 mo, two groups: a reference WLZ group with WLZ > −1 (mean 0.2, 95% CI [−0.1, 0.4]) and an undernourished group with WLZ < −2 (mean −2.9, 95% [CI −3.2, −2.7]) | 12 months | Micronutrient Supplementation without Zinc; Micronutrient Supplementation with Zinc, Nutritional Counselling and Education | Micronutrient powders (MNPs) affect infant microbiota, potentially disrupting early microbiome development. Key findings: − MNPs without zinc increase protozoa and mucormycetes carriage. − MNPs associate with specific bacterial communities and increased Actinobacteria. − Supplemented children show reduced bacterial diversity. − Vitamins, iron, and rural residence may promote protozoa and mucormycete colonization. − Adding zinc to supplements reduces Toxoplasma prevalence and protozoan richness. − Fungal diversity remains unaffected by supplements, age, residence, or nutritional status. − MNPs without zinc show a concerning predominance of Mucoromycota. − Zinc may reduce eukaryotic microbe infection and persistence. |
| Robertson et al., 2023; The gut microbiome and early-life growth in a population with high prevalence of stunting | Rural Zimbabwe | Sub- analysis from SHINE c-RCT | N=335 children aged 1-18 months with a prevalence of stunting (length-for-age Z-score (LAZ) < −2) varied from 18–34% across study time-points (a mean of 2.6 samples were analyzed each child) | 17 months | NA | Maternal HIV infection is linked to over-diversification and over-maturity of the early-life gut microbiome in uninfected children, with a lower abundance of Bifidobacterium species. Machine learning models (XGBoost) show that taxonomic microbiome features poorly predict child growth, while functional metagenomic features, especially B-vitamin and nucleotide biosynthesis pathways, moderately predict linear and ponderal growth and growth velocity. Targeting the early childhood gut microbiome could supplement efforts to reduce child malnutrition. |
| Rouhani et al. 2020; Gut Microbiota Features Associated With Campylobacter Burden and Postnatal Linear Growth Deficits in a Peruvian Birth Cohort | Place: Peruvian | Cohort study | N=928, 6-24 months | 24 months | NA | − Campylobacter was found in 93% (251) of asymptomatic fecal samples. − 10% increase in contaminated stools linked to a 0.02 reduction in length-for-age z scores (LAZ) at 3, 6, and 9 months (P<0.01). − Thirteen bacterial taxa indicative of cumulative Campylobacter burden identified. − Fourteen taxa were significantly associated with a high/low burden of enteroaggregative E. coli, norovirus, or Giardia. − Fecal microbiota disruptions may explain the effects of asymptomatic infections on early childhood growth. |
| Reyna et al., 2022; Longitudinal body mass index trajectories at preschool age: children with rapid growth have differential composition of the gut microbiota in the first year of life, that can be accessed at https://pubmed.ncbi.nlm.nih.gov/35428865/ | Canada | Cohort study | N=3059 children with various nutrition status | 5 years | NA | − Four BMI trajectories were identified: low, stable, normal, high stable, and rapid growth. − Gut microbiota analyzed at 3 months and 1 year using 16S RNA sequencing. − Rapid growth trajectory associated with: − Less likely to be breastfed − Lower microbiota diversity in the first year |
| - | - | - | - | - | - | − Decreased Akkermansia abundance over time − Higher Ruminococcus and Clostridium abundance at 1 year − Breastfed children at 6 months showed: − Higher Sutterella levels − Lower Ruminococcus and Clostridium levels |
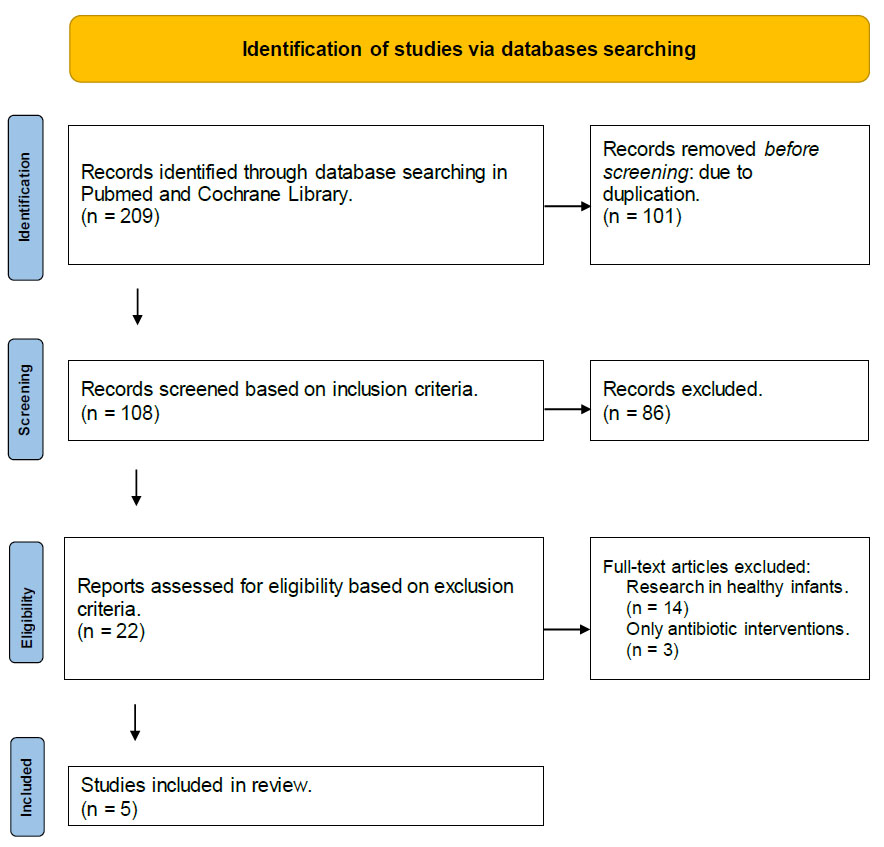
PRISMA Flow Diagram for the scoping review process.
The selected articles span a range of observational studies on various nutritional interventions, including dietary supplementation, prebiotics, fortified foods, and other interventions. Each study offers valuable insights into how the nutrition conditions over time, including the interventions, influencing the gut microbiome compo- sition, diversity, and functionality, shedding light on potential strategies to address undernutrition and stunting among young children.
Unfortunately, there are not enough insights or findings to be grouped into a diagram to show the initial condition of the child microbiota as the picture can be grouped into 1 group as it is known that all the studies were performed among children from various parts of the world with various conditions and consuming/ supplemented with various types of food or supplement.
These are the microbiota from the 5 studies that are linked to growth or better nutrition status: Faecalibacterium prausnitzii, Lachnoclostridium/Hunga- tella sp, Olsenella sp., Mitsuokella jajaludinii; Prevotella sp., Clostridium sp., Balutia sp., Ruminococcus sp., Mitsuokella multacida, Bifidobacterium sp, Coprococcus/ Eubacterium sp., Dorea formicigenerans, Faecali- bacterium prausnitzii, Blautia obeum, Akkermansia, and Sutterella. Whereas the following microbiota are linked to worse growth or worse nutrition status: protozoa; toxoplasma, mucormycetes, Campylobacter, E. coli, norovirus and Giardia.
This review was developed with the understanding that gut microbiome changes in undernourished infants and children under two years old due to a change in their nutrition status. Among the selected five clinical trial interventions investigated, dietary supplementation, prebiotics, fortified food, and other microbiota-directed approaches were assessed to understand their impact on gut microbiome changes and potential health implications.
The trials chosen present a diverse range of nutritional interventions explored to influence the gut microbiome in under- nourished and stunted infants and children under 2 years old. Dietary supplementation, prebiotics, fortified foods, and other microbiota-directed approaches offer promising avenues for optimizing gut microbiome changes and supporting health outcomes. Microbiota-directed foods were evaluated by Chen et al. (2021) in undernourished children. The formulation of MDCF used in this research was the MDCF-2 which resulted in changes in plasma protein levels. This intervention focused on providing specific foods or dietary components to promote the growth of beneficial gut bacteria while suppressing harmful pathogens [30].
Micronutrient supplementation was also explored in the study by Popovic et al. (2021). While micronutrients are crucial for addressing nutritional deficiencies, this study found that excessive micronutrient supplements may promote disruptive changes in protozoan and fungal communities in the developing infant gut, indicating the importance of carefully considering the dosage and type of micronutrients to minimize potential adverse effects on the gut microbiota [31].
A study by Reyna et al. (2022) explored longitudinal body mass index (BMI) trajectories in preschool-age children to identify potential links between rapid growth and gut microbiota composition during the first year of life. The study involved tracking the growth patterns and gut microbial composition of the children over time. Furthermore, by examining changes in the gut microbiome during early childhood growth spurts, the researchers aimed to uncover potential associations between gut microbial development and growth outcomes [32]. Next, a study by Rouhani et al. (2020) investigated gut microbiota features associated with Campylobacter burden and postnatal linear growth deficits in a Peruvian birth cohort. The study involved analyzing the gut microbial composition of infants and children with Campylobacter infection and comparing it with that of healthy controls. The researchers sought to understand how Campylobacter infection may disrupt the gut microbiome and impact postnatal linear growth [33].
Among the selected studies, Chen et al. (2021) conducted a microbiota-directed food intervention, which modified the gut microbiome of undernutrition children as an improvement in their nutrition status. This intervention promoted the growth of quite a number of prevotella sp and other species, which can be assumed to be beneficial bacteria while reducing harmful pathogens [30]. In contrast, Popovic et al. (2021) found that micronutrient supplementation could disrupt the gut microbiota composition, leading to the emergence of protozoan and fungal communities. Micronutrient powders have an impact on the developing infant microbiota, potentially leading to disruptions as certain organisms are promoted during early microbiome development. These findings have important implications for micronutrient supplementation strategies, especially when targeting vulnerable children in low-resource settings. Supplementing with micronutrients, excluding zinc, is linked to an increased presence of protozoa and mucormycetes in the gut. Micronutrient supplements are also associated with specific bacterial communities and a higher abundance of Actinobacteria. Children who receive supplementation tend to exhibit reduced bacterial diversity. Additionally, vitamins and iron, along with living in rural areas, may encourage colonization with distinct protozoa and mucormycetes. However, adding zinc to the supplements counteracts these increases, leading to a significant reduction in the prevalence of Toxoplasma and overall protozoan richness. Fungal diversity is not affected by age, supplementation, place of residence, or nutritional status. Nevertheless, the predominance of Mucoromycota, particularly in children receiving micronutrient powders without zinc, is concerning as these organisms have been associated with rare but serious invasive fungal infections reported in low-birth-weight infants and malnourished children [31].
While iron, vitamins, or both may foster the growth and survival of commensal and potentially pathogenic eukaryotes, resulting in changes to the eukaryotic community structure and disruption of the gut environment, the addition of zinc may limit the ability of some eukaryotic microbes to cause infection and persist. The study also found reduced bacterial diversity in 12-month-old infants receiving micronutrient supplements, along with elevated levels of Escherichia–Shigella and reduced beneficial Bifidobacterium [31].
Reyna et al. (2022) analyzed longitudinal BMI trajectories in preschool-age children and their association with gut microbiota composition. This provides insights into the links between gut microbial development and rapid growth during early childhood. In contrast, Rouhani et al. (2020) investigated gut microbiota features associated with Campylobacter burden and postnatal linear growth deficits in a Peruvian birth cohort. The intervention likely provided valuable information on the gut microbiota's role in growth and the impact of pathogenic infections [32].
Beyond the interventions and several observational, selected reviews also highlighted the impact of nutrition on the gut microbiome composition, diversity, and functionality in undernourished and stunted infants and children under 2 years old. Robertson et al. (2023) found that children with a high prevalence of stunting had distinct changes in their gut microbiome composition at different stages, including admission, discharge, and follow-up [34]. Beforehand, Robertson (2020) delved into the gut microbiome of children with malnutrition, providing insights into the relationship between nutrition and gut microbial composition. In addition to various essential factors for promoting healthy child development, the gut microbiota plays a vital role in controlling energy extraction from dietary intake, growth hormone signaling, colonization resistance, and immunological tolerance against infections. Consequently, any disturbance in the natural balance of the gut microbial community, especially during early life, can interfere with these critical pathways involved in infant growth and potentially lead to undernutrition [35, 36].
These studies collectively underscore the intricate interplay between nutrition and the gut microbiome in undernourished and stunted infants and children. Nutrition not only influences microbial composition and diversity but also impacts the functional capacity of the gut microbiota. This includes its role in nutrient metabolism, immune modulation, and protection against pathogens. Recognizing the pivotal role of nutrition in shaping the gut microbiome is essential for developing targeted interventions to combat malnutrition and stunting, ultimately improving the health and well-being of vulnerable populations.
The associations between improvements in the gut microbiome and changes in nutritional status, growth parameters, and developmental outcomes among undernourished and stunted infants and children under 2 years old were explored in this scoping review.
Chen et al. (2021) conducted a microbiota-directed food intervention for undernourished children and found that the intervention positively influenced the gut microbiome, leading to potential improvements in nutritional status and growth. Throughout the study, the weight-for-length and weight-for-age z scores showed consistent positive changes, indicating potential growth benefits associated with MDCF-2 intervention, including at the 1-month follow-up. The receipt of MDCF-2 was found to be associated with changes in the levels of 70 plasma proteins and 21 bacterial taxa, which were positively correlated with the weight-for-length z score (P<0.001 for both protein and bacterial taxa comparisons). Among these proteins were factors involved in bone growth and neurodevelopment, suggesting potential mechanisms by which the targeted manipulation of microbiota components may be linked to improved growth outcomes. Overall, these findings enlighten the potential pathways through which MDCF-2 intervention influences growth and provide valuable insights into the complex interactions between gut microbiota components and growth-related processes [30].
In the study by Popovic et al. (2021), the researchers investigated the impact of micronutrient supplements on the gut microbiome in developing infants. They found that such supplements could promote disruptive protozoan and fungal communities in the infant's gut. These disruptions in the gut microbiome composition have the potential to influence nutrient absorption and metabolism, which, in turn, can impact nutritional status and growth outcomes. This emphasizes the need for a nuanced approach when considering the use of supplements in the context of improving nutritional status in undernourished infants. [31] Meanwhile, Rouhani et al. (2020) conducted a comprehensive investigation into gut microbiota features associated with Campylobacter burden and postnatal linear growth deficits in a Peruvian birth cohort. Their research revealed a compelling relationship between the presence of Campylobacter, a bacterial pathogen often linked to gastrointestinal infections and linear growth deficits in infants. One of the notable findings was that infants who were exposed to Campylobacter exhibited impaired linear growth. After accounting for the length-for-age z-score (LAZ) at birth, each occurrence of Campylobacter-related diarrhea was linked to a decrease of 0.03 in the present LAZ, with a 95% confidence interval ranging from -0.04 to -0.01. This association was statistically significant, with a p-value of 0.002. The presence of Campylobacter in the gut microbiota appears to exacerbate these growth deficits, highlighting the significant role of the gut microbiome in affecting a child’s nutritional status [33].
Additionally, a longitudinal study by Reyna et al. (2022) that explored body mass index trajectories at preschool age reported different gut microbiome composition among those with rapid growth during the first year of life, potentially indicating associations with growth outcomes [32, 35].
4. DISCUSSION
The findings from this scoping review offer valuable insights with far-reaching implications for clinical practice, public health interventions, and policy-making, particularly in the context of undernourished and stunted infants and children.
Chen et al. (2021) explored a microbiota-directed food intervention for undernourished children and revealed the potential of targeted nutritional strategies to modulate the gut microbiome. These interventions have the potential to be translated into clinical practice, providing health professionals with effective tools to address undernutrition and stunting in early childhood. Moreover, the findings suggest that such interventions can be tailored to specific microbiota profiles, enabling personalized nutritional strategies for improved outcomes [30].
Popovic et al. (2021) investigated the impact of micronutrient supplements on the developing infant gut microbiome. While their research showed the potential for disruptions in microbial communities, it highlights the importance of careful consideration when administering supplements to infants. Future research should focus on optimizing supplement formulations to minimize adverse effects on the gut microbiota while maximizing their benefits for nutritional status [31].
Reyna et al. (2022) examined longitudinal BMI trajectories in preschool-age children and their gut microbiota compositions during the first year of life. Their findings hold implications for public health interventions, particularly in early childhood. Public health programs can consider incorporating microbiome assessments as part of routine child health check-ups. Identifying children with differential microbiota compositions early on could enable targeted interventions to support healthy growth trajectories [32].
Rouhani et al. (2020) highlighted the association between gut microbiota features and postnatal linear growth deficits in a Peruvian birth cohort. This research presents the importance of considering the gut microbiome in public health policy-making. Policies aimed at reducing childhood undernutrition and stunting should take into account the role of the microbiome, potentially leading to innovative strategies that leverage nutritional interventions to promote healthy growth [33].
The study by Robertson et al. (2023) on the gut microbiome and early-life growth in a population with a high prevalence of stunting emphasizes the need for comprehensive public health approaches. Policies should focus on addressing the root causes of undernutrition and stunting, including improving access to nutritious foods and promoting hygiene practices to minimize pathogen exposure [34].
In conclusion, the findings from these studies offer promising avenues for future research, clinical practice, and public health interventions. Targeted nutritional interventions, guided by a nuanced understanding of the gut microbiome, hold substantial potential for improving the health and well-being of undernourished and stunted infants and children. Policymakers and public health officials should consider these findings when designing strategies to combat childhood undernutrition and stunting, with the goal of breaking the cycle of poor health and improving long-term outcomes for vulnerable populations.
CONCLUSION
In conclusion, this scoping review highlights the potential of targeted nutritional interventions to positively influence the gut microbiome in undernourished and stunted infants and children under 2 years old. The findings suggest that these interventions can promote gut microbiota development such as Actinobacteria, Bacteroides, Streptococcus, Bifidobacterium, Prevotella sp and most-age discriminatory bacterial taxa, leading to improved growth and health outcomes. Further research is needed to identify specific microbial targets and mechanisms impacted by these interventions, enabling more personalized approaches to combat undernutrition and stunting.
The implications of these findings extend to clinical practice and public health interventions. Health professionals can use this knowledge to design targeted nutritional interventions that support optimal gut microbiome development in vulnerable populations. Integrating these interventions into national nutrition programs and community-based initiatives can lead to long-term health improvements and break the cycle of malnutrition and poverty in low-income and middle-income settings. Overall, this scoping review contributes valuable insights to the field and emphasizes the importance of evidence-based approaches to enhance the gut microbiome and health outcomes in vulnerable at-risk populations.
LIMITATIONS OF THE STUDY
The limitation of this scoping review stems from the inherent constraints of the included studies. The selected articles, though providing valuable insights into the gut microbiome changes among undernourished and stunted infants and children under two years, vary widely in methodologies, populations studied, and intervention types. This heterogeneity introduces challenges in synthesizing findings across studies and drawing compre- hensive conclusions. Additionally, the review was limited by its relatively short time span, focusing on studies published between 2013 and 2023 and the use of only two databases (PubMed and Cochrane Library) for the literature search. The limited number of articles included highlights the nascent state of research in this specific area, pointing to the broader issue of the scarcity of literature on gut microbiome changes in undernourished and stunted populations under two years of age. Consequently, while this scoping review serves as a foundational exploration, the diversity and paucity of available studies underscore the need for further research to establish more robust and generalizable insights into the relationship between nutritional interventions and gut microbiome changes in this vulnerable demographic.
AUTHOR’S CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.


