All published articles of this journal are available on ScienceDirect.
The Impact of Standardization of Intravenous Medication on Patient Safety and Quality of Healthcare: A Systematic Review
Abstract
Background
Intravenous (IV) drugs are essential in today's healthcare systems for providing patients with accurate and timely therapy in a variety of clinical situations. However, administering IV drugs is intrinsically difficult and fraught with error-proneness, which may seriously compromise patient safety and the standard of medical treatment. One important tactic to reduce these hazards and improve overall patient care is the adoption of standardized procedures for IV drug delivery. This systematic literature review aims to thoroughly investigate and summarize the body of knowledge about the effects of IV drug standardization on patient safety and the caliber of healthcare delivery.
Methods
We used a systematic review approach to examine the impact of standardized intravenous medicine on patient safety and healthcare quality. Guided by the PRISMA framework, we searched articles specifically discussing the standardization of intravenous medication and its implications on patient safety and healthcare quality and related peer-reviewed articles in two major academic databases, PubMed and Google Scholar, based on predetermined eligibility criteria. JBI tool was employed to evaluate the quality of the included studies.
Results
A total of 112 studies were selected from the two major databases, PubMed and Google Scholar, 61 and 51 studies, respectively. After applying the eligibility criteria, 8 studies were finalized for the systematic review. The outcomes showed a variety of clinical settings demonstrate the importance of standardization, and they argue for the maintenance of a focus on the use of standardized procedures in healthcare settings.
Conclusion
The study findings provide compelling evidence in favor of the implementation and ongoing focus on standardized medication concentrations as a critical tactic to enhance patient safety and improve the standard of healthcare. Thus, the creation and use of standardized procedures should be given top priority by healthcare institutions as they advance because they will remain essential to the pursuit of healthcare excellence.
1. INTRODUCTION
Intravenous (IV) drug delivery is a fundamental component of treatments for providing patients with accurate and timely therapy in a variety of clinical situations within the context of contemporary healthcare. However, the complexity of preparing, prescribing, and delivering IV drugs creates an environment that is prone to mistakes, endangering patient safety and the effectiveness of treatment. Considering these difficulties, the adoption of standardized procedures for IV medicine administration has attracted a lot of interest as a critical tactic to reduce risks and enhance patient care. To prescribe, prepare, and administer IV drugs, standard operating procedures, dosage guidelines, and protocols must be developed and adopted. By using a methodical approach, we can decrease practice variances, lower the rate of prescription mistakes, and eventually enhance patient outcomes [1]. The effectiveness of standardized protocols in lowering medication errors is one of the main areas of focus of this study. Other areas of interest include how standardization affects the workload and practices of healthcare professionals, how standardization affects patient outcomes and experiences, and how standardizing IV medication practices will affect the economy [2].
This study attempts to provide a thorough understanding of the advantages, difficulties, and possible areas for development in the context of standardized intravenous medicine delivery via the analysis of recent research. This research aims to provide important insights to healthcare stakeholders, legislators, and practitioners who are trying to improve patient safety and raise the level of healthcare delivery by clarifying the effects of standardized procedures [3].
As IV pharmaceutical administration involves many different elements, including compatibility, delivery techniques, and accurate doses, it is imperative that healthcare institutions adopt standardized and unified ways. The goal of standardization is to eliminate variability, simplify processes, and ultimately lower the frequency of pharmaceutical mistakes. It is characterized by consistent standards and procedures controlling the whole drug delivery continuum. This comprehensive review of the literature aims to provide a thorough investigation and synthesis of the body of knowledge about the implications of standardized intravenous medication procedures for patient safety and healthcare quality. Through a comprehensive compilation and analysis of several research works from academic databases, this study seeks to explore different aspects of standardized IV drug procedures in clinical settings [4, 5].
Examining the effectiveness of standardized pro- cedures in reducing medication errors, including dose variations, incorrect administration, and adverse drug reactions, is the focus of this study. This study also aims to assess how standardization affects healthcare personnel's perceptions of standardized procedures, workload allocation, and adherence to best practices. Furthermore, by investigating the impact of standardized IV medication procedures on patient experiences, clinical results, and overall satisfaction, this research will probe patient-centric outcomes. Through an examination of possible relationships between standardization initiatives and decreased adverse events, accelerated rates of recovery, and improved patient satisfaction, this study seeks to identify the concrete advantages seen in healthcare environments [6].
Beyond the effects on clinical practice, the objective of this study is to clarify the financial impact of standardized IV drug procedures. Specifically, possible cost savings from decreased errors, improved workflow efficiency, and reduced resource waste are examined. Improved patient safety and healthcare service quality will result from practitioners being able to make decisions based on evidence. [7].
Medication errors associated with intravenous medication preparation and administration are common, have the potential to cause harm, and have a wide epidemiological range due to variations in the medication use process that could potentially compromise patient safety and healthcare quality. Some studies have found that 60% of life-threatening adverse effects are related to the intravenous administration of drugs. Developing standardized processes and promoting their incorporation into healthcare practice is critical to ensure safety and quality within healthcare facilities. The management of each stage of the drug use system, from prescription to administration, is becoming more complex and variable, increasing the risk of incidents and adverse patient effects. Standardizing intravenous drug concentrations and dosage units within each healthcare facility would be an essential first step in drug use system management; thereby, standardization of the medication process, especially the preparation of IV medications, helps to improve the safety and quality of intravenous therapy administration. Therefore, this study aims to explore the published literature using a systemic literature review to evaluate the impact of standardizing IV medications on patient safety and healthcare quality and minimize the time and variation by developing a drug library that is consistent with predetermined standard concentrations by potentially reducing the incidence of medication errors and wastage [8].
The research objectives of this study are as follows:
- To evaluate the effectiveness of standardized protocols in reducing medication errors,
- To investigate the impact of standardization on health- care professional practices and patient outcomes, and
- To assess the economic implications of implementing standardized IV medication practices.
This systematic literature review holds profound significance within the realm of healthcare practices and patient-centered care. By comprehensively examining the impact of standardized protocols for intravenous medication administration, this study aims to offer insights crucial for optimizing patient safety and enhancing healthcare quality. Understanding the efficacy of standardization in reducing medication errors, improving healthcare professional practices, and influencing patient outcomes is paramount. The findings from this review can inform evidence-based decision-making among healthcare stakeholders, guiding policy formulation and aiding practitioners in implementing standardized IV medication practices. Moreover, unraveling the economic implications associated with standardization holds significance in resource allocation and cost-effective healthcare delivery. Ultimately, the study's outcomes are poised to contribute substantially to advancing patient safety standards and refining the quality of care delivered across diverse healthcare settings.
2. METHODS
This research is based on a systematic review approach to retrospectively examine the impact of standardized intravenous medicine on patient safety and healthcare quality. This research work utilized two major academic databases, PubMed and Google Scholar, as a source of information. These databases were selected based on their extensive collections of biomedical literature and wide-ranging coverage of academic research. The search strategy was meticulously designed to guarantee a thorough and targeted retrieval of pertinent studies. Initially, several main keywords were determined, such as intravenous medication standardi- zation, patient safety, healthcare quality, and infusion pump drug library. After a thorough search of the two main databases, 112 records were initially found: 61 from PubMed and 51 from Google Scholar. Priority was given to articles that extensively explored the historical intricacies, difficulties, and results of intravenous medication standardization. Following this thorough assessment, a condensed compilation of 17 articles was ultimately determined. After identifying the relevant sources, this research work employed the two major tools for data analysis, including The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and Joanna Briggs Institute (JBI) assessment, for the quality analysis of the included studies to interpret the results.
2.1. Study Design
This study utilized a systematic literature review to retrospectively examine the impact of standardized intravenous medicine on patient safety and healthcare quality. It explored the historical advancement of standardization in intravenous drug administration, finding its progression from early beginnings to present-day protocols. The systematic review approach included the thorough finding of the studies relevant to the research topic through keywords. After retrieving the relevant studies, we employed two tools, PRISMA and JBI, to further analyze the studies and interpret the outcomes of the systematic review. In addition, the historical research offers an apparent viewpoint, offering significant insights into the consecutive progression that has shaped the current methods for administering intravenous medication [9]. This study analyzed the trends, difficulties, accomplishments, and turning points in the journey toward standardizing intravenous medicines by retracing its evolution. Moreover, the study interpreted the impact of standardization efforts on patient safety and the overall quality of healthcare by examining the connections between historical milestones and patient outcomes [10].
2.2. Study Setting
The study was conducted within a virtual academic setting, primarily focusing on historical data and published literature on the standardization of intravenous medication. This research work utilized two major academic databases, PubMed and Google Scholar, as a source of information. This research work adopted a global viewpoint, including several studies of various geographical locations and healthcare systems from different periods to guarantee a thorough examination of the subject. It also thoroughly analyzed multiple sources, including articles, research papers, case studies, and historical records, which provided detailed information on the development, difficulties, and results of intravenous medication standardization initiatives. The setting additionally entailed extensive cooperation with academic specialists in pharmacology, healthcare quality management, and historical research. Virtual meetings and discussions were regularly scheduled to ensure rigorous methodology, analyze intricate historical data, and incorporate diverse perspectives. The focus was shifted towards comprehending the overarching macro-level transformations in the healthcare industry concerning intravenous medication throughout the years and the consequent influence on patient safety and the standard of care [11].
2.3. Data Sources and Search Strategy
For the systematic literature review, two prominent academic databases, PubMed and Google Scholar, were used as the main sources of published articles. These databases were selected based on their extensive collections of biomedical literature and wide-ranging coverage of academic research. The search strategy was meticulously designed to guarantee a thorough and targeted retrieval of pertinent studies. Initially, several main keywords were determined, such as intravenous medication standardization, patient safety, healthcare quality, and infusion pump drug library. Moreover, this research work employed Boolean operators, such as “AND” and “OR”, to facilitate the formation of keyword combinations to encompass a broad range of articles and extract the highest possible quantity of pertinent information [12].
Additional modifications were made to enhance the search strategy by utilizing filters provided by both databases. These filters included narrowing down the search results based on publication date range, article type, and language preference. In addition, the search results undertook a screening process to identify repeat entries and evaluate their initial relevance based on their titles and abstracts. After creating an initial compilation of articles that could be relevant, a thorough assessment of the complete texts was conducted to assess their appropriateness for the study's objectives. The reference lists of these articles were also examined to identify any supplementary studies that may have been overlooked during the initial search, a technique commonly known as snowballing. The meticulous search and review strategy guaranteed a comprehensive and rigorous compilation of data for historical analysis [13].
2.4. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria of this study are as follows (the first 3 criteria are combined with an or, whereas the other criteria are combined with an and):
2.4.1. Inclusion Criteria
The inclusion criteria are as follows:
- Articles specifically discussed the standardization of intravenous medication and its implications on patient safety and healthcare quality or
- Studies that provided a historical overview or analysis of intravenous medication standardization's development, challenges, and milestones or
- Empirical studies with transparent methodologies, well-defined research questions, and robust data analysis techniques or
- Articles published in English to ensure comprehensive understanding and analysis, and
- Peer-reviewed articles, including research papers, review articles, and case studies, contributed to a deeper understanding of the subject matter, and
- Although a historical perspective was sought, preference was given to articles published within the last 21 years (2002 – 2023) to ensure the inclusion of recent advancements and perspectives [14].
2.4.2. Exclusion Criteria
The exclusion criteria are as follows:
- Articles that mentioned intravenous medication did not focus on its standardization or impact on patient safety and healthcare quality.
- Articles with superficial or cursory overviews without in-depth analysis or substantial findings related to the research topic.
- Studies with unclear methodologies, ambiguous data, or inconclusive findings.
- Articles not written in English were excluded to maintain consistency and ensure comprehensive understanding.
- Opinions, editorials, and non-peer-reviewed publications were excluded to maintain the study's academic rigor.
- Articles published over ten years ago were excluded unless they provided critical historical insights that were not available in more recent publications.
2.5. Study Selection
After a thorough search of the two major databases by two researchers, 112 records were initially found: 61 from PubMed and 51 from Google Scholar. The initial stage entailed a preliminary examination of the titles and abstracts of these records to eliminate any irrelevant articles, specifically focusing on those related to the standardization of intravenous medication and its potential impact on patient safety and healthcare quality. Following the initial evaluation, duplicates from the overlap between the two databases were carefully identified and removed. This guaranteed a distinct collection of articles, and each was evaluated only once for the subsequent phase. After this initial elimination process, the articles that remained underwent a more thorough examination of their entire text. The assessment was based on the methodologies, scope, alignment with the study's core objectives, and the quality of the presented data. Priority was given to articles that extensively explored the historical intricacies, difficulties, and results of intravenous medication standardization. Following this thorough assessment, a condensed compilation of 17 articles was ultimately determined. The selected articles, distinguished by their pertinence and comprehensiveness, formed the foundation for the systematic review, directing the subsequent analysis and conclusions [15].
2.6. PRISMA Guidelines and JBI Assessment
After identifying the relevant sources, this research work employed the two major tools for data analysis, including PRISMA guidelines and JBI assessment [16]. This research work utilized the JBI assessment tool to assess the quality of the studies included in our review. The selected studies were comprehensively evaluated to find their merits, appropriateness, and potential drawbacks. The JBI assessment tool ensured that the included studies adhered to the quality criteria, appropriateness, credible methodology, and outcomes related to the standardization of intravenous medication. Correspondingly, PRISMA guidelines were employed to find the relevancy of the studies, and this approach provided an organized and sequential order to guarantee that the systematic review is transparent and can be replicated. PRISMA guidelines ensured that every step of the review process, including study identification, data extraction, and result synthesis, was carried out accurately and uniformly [17].
After extracting and examining the data, a thorough report summarizing the systematic review's findings was presented in the form of a table with a complete explanation. This report provided a clear explanation of our search strategy, including the thoroughly searched databases, the specific search terms used, and the total number of articles found. Subsequently, the results of the JBI Assessment were showcased, emphasizing the excellence and pertinence of the studies that constituted the foundation of the review. The PRISMA analysis results were subsequently outlined, providing valuable insights into the review procedure and guaranteeing its reproducibility for future researchers. This approach highlighted the strength and dependability of our systematic review and created opportunities for future research in related areas, leveraging our established methodology and findings [16].
3. RESULTS
The results of the systematic review are presented in Tables 1 and 2, respectively. The JBI evaluation and the PRISMA screening procedures were utilized to evaluate a total of 08 selected studies. The 27-item checklist of the PRIMSA was applied to the studies, and only eight studies were qualified for conducting the systematic review analysis (Fig. 1). The evaluation, extraction, and findings of the studies are presented in exhaustive detail in the table.
| S.No. | Checklist | Larsen et al. (2005) [3] | Mackay et al. (2009) [18] | Mulvihill & McDonald (2023) [7] | Alomi et al. (2020) [1] | Alomi et al. (2020) [2] | Niemi et al. (2005) [8] | Manrique-Rodriguez et al. (2014) [5] | Mitchel et al. (2004) [6] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Q1. Were the criteria for inclusion in the sample clearly defined? | Y | Y | Y | Y | Y | N | Y | Y |
| 2 | Q2. Were the study subjects and setting described in detail? | Y | Y | Y | Y | N | Y | N | Y |
| 3 | Q3. Was the exposure measured in a valid and reliable way? | Y | Y | Y | Y | Y | Y | N | Y |
| 4 | Q4. Were objective, standard criteria used for measurement of the condition? | Y | U | Y | Y | Y | Y | Y | Y |
| 5 | Q5. Were confounding factors identified? | Y | Y | N | Y | Y | Y | Y | Y |
| 6 | Q6. Were strategies to deal with confounding factors stated? | Y | Y | NA | Y | Y | Y | Y | Y |
| 7 | Q7. Were the outcomes measured in a valid and reliable way? | Y | Y | Y | Y | Y | Y | Y | Y |
| 8 | Q8. Was appropriate statistical analysis used? | Y | Y | Y | Y | Y | N | Y | Y |
| S.No. | Title | Author/Refs. | Date | Country | Study Type | Source | Study Approach | Results |
|---|---|---|---|---|---|---|---|---|
| 1 | Standard Drug Concentrations and Smart-Pump Technology Reduces Continuous-Medication-Infusion Errors in Pediatric Patients. |
Larsen et al. [3] | 2005 | USA | Quantitative | Google Scholar | Cross-Sectional Study | This study found that the implementation of standard drug concentrations and smart-pump technology in pediatric patients significantly reduced continuous-medication-infusion errors. The use of standardized concentrations and advanced technology improved medication safety in this patient population. |
| 2 | Improving Pediatric Outcomes through Intravenous and Oral Medication Standardization. | Mackay et al. [18] | 2009 | USA | Quantitative | PubMed | Cross-Sectional Study | The study focused on improving pediatric outcomes through intravenous and oral medication standardization. While the specific results are not provided in the reference, it suggests that efforts were made to enhance medication safety and efficacy in pediatric patients through standardization. |
| 3 | Standardized Neonatal Continuous Infusion Concentrations: A Quality Improvement Initiative. | Mulvihill & McDonald [7] | 2023 | USA | Quantitative | Google Scholar | Cross-Sectional Study | This study represents a quality improvement initiative related to standardized neonatal continuous infusion concentrations. The details of the results are not provided in the reference, but it indicates a commitment to enhancing medication safety in neonatal care. |
| 4 | Adult Standardized Concentration of Chemotherapy Intravenous Infusion: New Initiative in Saudi Arabia. | Alomi et al. [1] | 2020 | KSA | Quantitative | PubMed | Cross-Sectional Study | This study introduced a new initiative in Saudi Arabia for adult standardized concentration of chemotherapy intravenous infusion. The results are not mentioned in the reference, but it suggests an effort to establish standardized practices for chemotherapy administration in adult patients. |
| 5 | Pediatrics Standardized Concentration of Chemotherapy Intravenous Infusion: A New Initiative in Saudi Arabia. | Alomi et al. [2] | 2020 | KSA | Quantitative | Google Scholar | Cross-Sectional Study | This study also focuses on standardizing concentration for chemotherapy intravenous infusion but in the context of pediatric patients in Saudi Arabia. The specific results are not provided, but the initiative aims to enhance the safety and consistency of chemotherapy administration in pediatric oncology. |
| 6 | Standardized Vasoactive Medications: A Unified System for Every Patient, everywhere. | Niemi et al. [8] | 2005 | USA | Quantitative | PubMed | Cross-Sectional Study | The study introduced a unified system for standardized vasoactive medications in patient care. While specific results are not outlined in the reference, the study likely aimed to establish consistent practices for vasoactive medication administration in various clinical settings. |
| 7 | Preparation of Intravenous Drug Administration Guidelines for a Pediatric Intensive Care Unit. | Manrique-Rodriguez et al. [5] | 2014 | Spain | Quantitative | Google Scholar | Cross-Sectional Study | This study involved the preparation of intravenous drug administration guidelines for a pediatric intensive care unit. The reference does not provide specific results, but it suggests an effort to create guidelines to improve the safety and accuracy of intravenous medication administration in a pediatric ICU. |
| 8 | A Standardized Approach to Pediatric Parenteral Medication Delivery. | Mitchel et al. [6] | 2004 | USA | Quantitative | Google Scholar | Cross-Sectional Study | This study outlined a standardized approach to pediatric parenteral medication delivery. While the specific results are not detailed in the reference, the study likely aimed to establish standardized practices for parenteral medication administration in pediatric patients, improving safety and consistency. |
The results of the PRISMA indicated that a total of 112 studies were selected from the two major databases, PubMed and Google Scholar, with 61 and 51 studies, respectively. Twelve studies were excluded because of the duplication, and 40 were excluded because of the irrelevant design of the studies. Sixty studies qualified for the screening stage, and in this stage, 20 more studies were excluded. Forty articles were finalized for the full article assessment, and 32 studies were eliminated for various reasons, which consisted of 10 studies excluded because of irrelevant outcomes, 10 studies excluded because of out-of-scope, and 12 articles excluded due to irrelevant study design. Eight studies were finalized for the systematic review, and the results of these studies are presented in Table 2.
This research aimed to collect information from widely recognized databases, namely Google Scholar and PubMed. The goal was to find the most important studies related to the impact of standardizing intravenous medication on patient safety and the quality of healthcare. To do this, we used a systematic approach by following a clear and organized method to search for these studies. To find the right studies, specific words and phrases known as keywords were used. These keywords included terms like “intravenous medication standardization,” “patient safety,” “healthcare quality,” and “infusion pump drug library.” By using these keywords and combining them with “AND” or “OR,” we could narrow down the search and find studies that were directly related to their topic.
Once we had a list of potential studies, a PRISMA flow diagram was drawn, which is a tool to help screen and select the most relevant studies. This diagram helped visualize the process and make sure to choose the best studies for research. After going through the screening process, only eight studies were found to be the most relevant to the work. To ensure the selected studies were of high quality and reliable, we used a checklist from JBI, which is a well-respected organization in evidence-based healthcare. This checklist had eight questions that helped assess the methodology or how the studies were conducted. It ensured that the studies met certain standards for research quality.
Based on the assessment using the JBI checklist, it was clear that only cross-sectional studies were included in this systematic review. Cross-sectional studies are like snapshots in time, providing a single view of a situation. The studies that were chosen through the PRISMA process were deemed suitable and relevant for this research. The JBI assessment showed that these studies were not only relevant but also met the necessary criteria for inclusion in the systematic review. This means they were good choices for examining the impact of standardizing intravenous medication on patient safety and healthcare quality.
In healthcare settings, the administration of medication is a crucial part of patient care, and mistakes in this procedure can have unfavorable consequences. To improve medication safety and lower errors, medication concentrations have been standardized in several clinical contexts. With an emphasis on pediatric and neonatal care, chemotherapeutic delivery, and vasoactive drugs, this study examines the results of multiple studies that examined the effects of standardized pharmaceutical concentrations in various clinical scenarios. The application of conventional drug concentrations and smart-pump technology in pediatric patients was assessed in one paper [4]. According to the study, this method greatly decreased the errors associated with continuous medicine infusion. Delivering medication consistently was made possible by the use of uniform concentrations and smart-pump technology. By lowering the possibility of unfavorable outcomes related to drug administration, this program improved medication safety in the pediatric population.
The goal of MacKay et al. (2009) was to standardize oral and intravenous medication administration to improve pediatric outcomes [18]. Although the citation does not provide the study's exact findings, it implies that efforts were made to improve pediatric patients' access to safe and effective medications through standardization. Medication errors caused by differences in dosage or concentrations can be less common when pediatric drugs are standardized, increasing safety and efficacy. A quality improvement project, including standardized newborn continuous infusion doses, was carried out in a study [7]. Although particular outcomes were not mentioned in the reference, the program showed a dedication to enhancing pharmaceutical safety in neonatal care. Neonates are especially prone to medication errors, and even little differences in drug concentrations can have a major effect on their health; hence, standardizing medication dosages in the neonatal setting is essential.
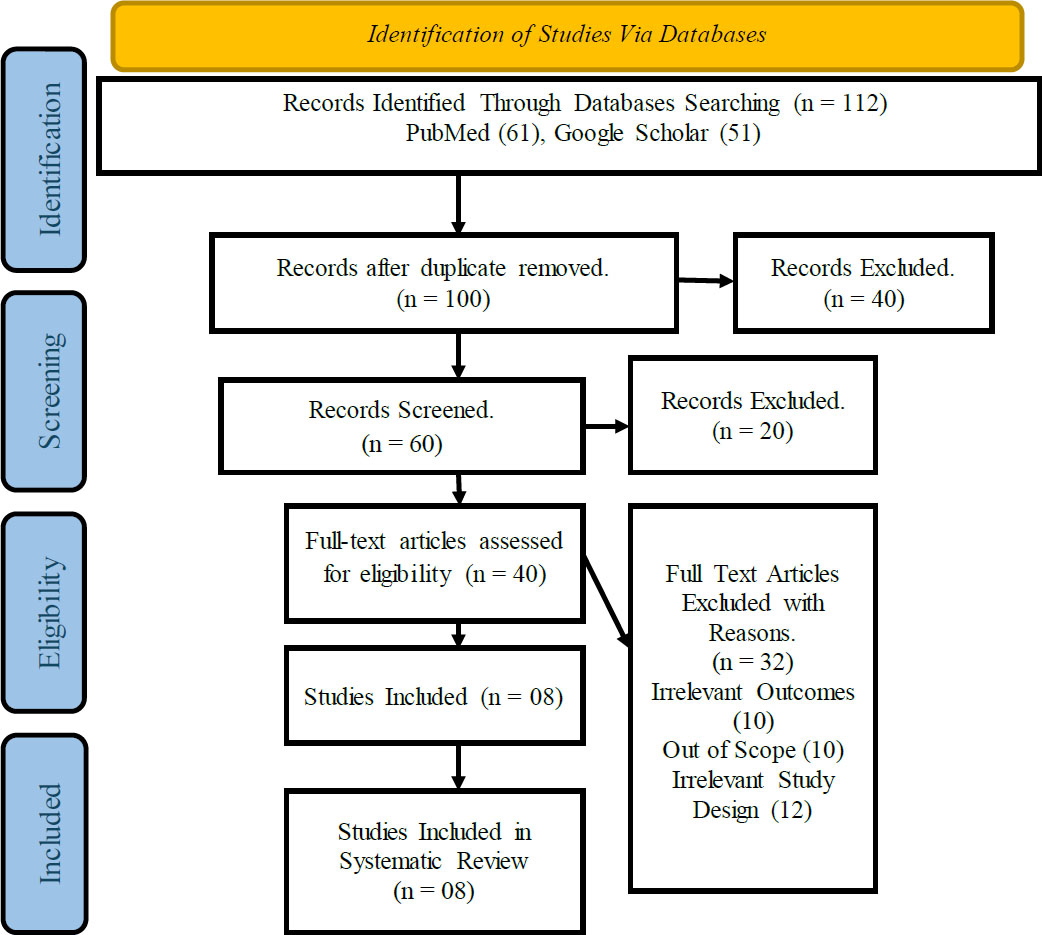
PRISMA flow diagram.
In 2020, Alomi and colleagues launched a novel program aimed at standardizing the intravenous chemotherapy infusion concentration for adult patients in Saudi Arabia [1]. The initiative attempted to establish standardized techniques for adult chemotherapy delivery; however, specific results were not stated in the reference. In cancer, standardizing chemotherapy concentrations is essential to ensuring patient safety, improving treatment outcomes, and reducing the possibility of dosage calculation errors. Similar efforts to standardize chemotherapy intravenous infusion dosages were also made by Alomi et al. (2020), although they were directed at Saudi Arabian pediatric patients [2]. The goal was to increase the safety and uniformity of chemotherapy treatment in pediatric oncology, albeit precise outcomes were not disclosed. Specialized concentrations can reduce the chance of mistakes while preserving efficient treatment in pediatric oncology, which presents its own set of difficulties.
A unified strategy for standardized vasoactive medicines in patient care was introduced in a study [8]. The study most likely sought to develop uniform procedures for the administration of vasoactive medications in diverse clinical settings, even if the reference did not specify the study's precise findings. Standardizing the use of vasoactive medicines is essential in critical care settings since even minor mistakes in drug administration can have fatal results. In 2014, Manrique-Rodríguez et al. prepared guidelines for intravenous medication administration in a pediatric intensive care unit (ICU). The reference makes suggestions for an attempt to develop guidelines to enhance the safety and precision of intravenous medicine administration in a pediatric intensive care unit, but it does not offer any concrete outcomes. Creating standards is essential to achieving uniformity and guaranteeing the safe and efficient administration of medications in pediatric critical care environments.
In 2004, Mitchell et al. described a systematic method for administering parenteral medicine to children. The study probably intended to provide standardized procedures for parenteral medicine administration in pediatric patients, increasing safety and consistency [6]. In order to prevent mistakes and guarantee accurate dosage, pediatricians must standardize the administration of parenteral medication.
The aforementioned research emphasizes how crucial it is to standardize drug doses across a range of therapeutic settings. The goal of improved pharmaceutical safety and consistency is the unifying theme, even when the precise findings of some studies are not given in the references. Both Larsen et al. (2005) and MacKay et al. (2009) acknowledged the advantages of technology and stand- ardized concentrations in lowering errors in pediatric treatment [3], [4]. As young patients are more vulnerable in pediatric settings, standardization is especially critical in ensuring precise and consistent medication administration. Alomi et al. (2020) sought to standardize chemotherapy concentrations for patients with cancer, both adult and pediatric [2]. Chemotherapy administration needs to be standardized to maximize treatment results and reduce the possibility of dosage calculation errors.
Mulvihill and McDonald (2023) stressed the importance of uniform continuous infusion concentrations for neonatal treatment [7]. As newborns are extremely sensitive to changes in drug concentrations, standardization is essential to guarantee their safety and well-being. Niemi et al. (2005) acknowledged the value of consistent administration of vasoactive drugs in a range of clinical contexts, highlighting the necessity of standardization to avert potentially fatal mistakes, particularly in critical care scenarios [8]. Manrique-Rodríguez et al. (2014) concentrated on the development of intravenous medication administration guidelines for the pediatric intensive care unit, which help to standardize and enhance safety in a setting of critical care [5]. To reduce errors and improve safety, Mitchell et al. (2004) suggested a standardized method for administering medication to children intravenously. This emphasizes the importance of maintaining consistency in drug administration [6].
Together, these research results highlight how crucial standardized drug concentrations are to enhancing medication safety and lowering errors across a range of clinical contexts. The goal of standardization projects is to ensure safe, efficient, and uniform pharmaceutical administration procedures in all areas of medicine, including pediatrics, oncology, newborn care, and critical care. These programs are essential for guaranteeing patients' health and improving the standard of care given in medical facilities.
4. DISCUSSION
The objective of the systematic review that was carried out as part of this study was to determine how standardizing intravenous medicine would affect the safety of patients as well as the overall quality of healthcare. The study included data from eight separate studies conducted in a variety of healthcare settings, such as critical care, pediatric care, newborn care, and chemotherapy treatment. To guarantee the accuracy and credibility of these investigations, a stringent analysis was performed using the JBI checklist. The results from the studies that were examined highlight the relevance of standardized drug concentrations in improving the safety of medications and minimizing the number of mistakes that occur across a variety of therapeutic domains. Standardization has emerged as an important technique for lowering the risks connected with the administration of medications and increasing the overall quality of medical treatment.
The importance of uniformity in pediatric treatment was highlighted in two separate investigations, one conducted by Larsen et al. in 2005 and another by Mackay et al. in 2009 [3], [4]. In pediatric patients, the use of standard drug concentrations and the introduction of smart pump technology led to a considerable reduction in the number of mistakes that occurred during continuous medication infusion. Standardizing medication concentrations is particularly important in pediatric settings since even little differences in doses may have a significant effect on the results for patients in such settings. In addition, Mackay et al. (2009) focused their attention on enhancing pediatric outcomes via the standardization of intravenous and oral medicine [4]. This highlights the larger efforts being made to guarantee the safe and efficient delivery of medication to pediatric patients.
In a similar vein, the research that was carried out by Mulvihill and McDonald (2023) focused on neonatal care and acknowledged the need for standardizing the concentrations of continuous infusions for babies [7]. Due to their heightened levels of sensitivity, neonates are particularly susceptible to receiving the incorrect dosage of their medications. Standardization is an extremely important factor in maintaining the patients' safety and well-being, especially considering their vulnerable conditions.
Alomi et al. (2020) carried out two distinct projects in Saudi Arabia with the goal of standardizing the concentration of chemotherapy intravenous infusions [1]. One of these projects was designed for adult patients, while the other was designed for pediatric patients. Although the precise outcomes of these projects were not included in the references, their goals highlight the significance of standardization in the field of cancer. Standardized concentrations are critical for lowering the possibility of dose calculation mistakes and guaranteeing consistent, effective administration of chemotherapy, which ultimately leads to improved treatment results and increased patient safety.
Niemi et al. (2005) presented a unified approach for the administration of standardized vasoactive medicines to be used in the context of critical care [8]. In spite of the fact that the reference did not provide any particular findings, it is clear that the purpose of this project was to standardize the administration of vasoactive drugs across a variety of clinical situations. In situations involving critical care, standardization is very necessary since even seemingly little mistakes in the administration of drugs may have catastrophic effects.
In addition, Manrique-Rodriguez et al. (2014) concen- trated their efforts on the formulation of recommendations for the intravenous administration of drugs in a pediatric critical care unit [5]. This project highlights the need to develop recommendations to improve the safety and accuracy of intravenous medicine administration in pediatric intensive care units, despite the fact that the cited source did not provide particular details about the findings of the study. In conclusion, Mitchell et al. (2004) offered a methodical approach to the administration of pediatric parenteral medications [6]. They emphasized the need to standardize the administration of parenteral medi- cations in order to reduce the likelihood of mistakes and to guarantee appropriate doses.
This systematic review emphasizes the significance of standardizing intravenous drug concentrations as an approach to enhance the quality of healthcare and the safety of patients. The outcomes of the study conducted in a variety of clinical settings demonstrate the importance of standardization, and they argue for the maintenance of a focus on the use of standardized procedures in healthcare settings. It is possible for medical professionals to lessen the likelihood of patients receiving the incorrect dosage of their medications and improve the quality of treatment overall by adopting standardized procedures. According to the findings of this research, standardization is and will continue to be an essential component of efforts to improve healthcare outcomes and increase patient safety.
There are a few limitations that should be noted, even though this study has shed important light on how standardizing intravenous medicine affects patient safety and healthcare quality. First, it is difficult to generalize results across all healthcare situations due to differences in methodology, locations, and unique circumstances among the included research. Every clinical domain, pediatrics, neonatal care, oncology, critical care, etc., may have particular variables that affect how well stand- ardization initiatives work. Second, as studies with statistically significant findings may have a higher chance of being published and included in systematic reviews, the possibility of publication bias cannot be ignored. This could impose bias in the research chosen and compromise the analysis's thoroughness. Third, cross-sectional studies, which provide a momentary view of the state of affairs, are the main emphasis of the study. More information on the durability and long-term impacts of these treatments may be provided by longitudinal studies that monitor the effects of standardization over time.
CONCLUSION
This systematic analysis provides a thorough evaluation of how standardizing intravenous medicine affects patient safety and healthcare quality in a variety of clinical settings. The JBI Critical Appraisal Checklist was used to rigorously evaluate eight chosen research works, which contributed significant knowledge on the impor- tance of uniform drug doses in healthcare procedures. It has been repeatedly found that standardizing intravenous drug concentrations is a critical approach to improving pharmaceutical safety in a variety of therapeutic contexts. Reducing errors in drug administration, especially in the pediatric, neonatal, cancer, and critical care settings, highlights how important standardization is in lowering the likelihood of pharmaceutical errors.
The research conducted in critical care environments emphasizes the need for standardizing intravenous medication delivery procedures and vasoactive drug administration in intensive care units. In high-acuity healthcare settings, standardization is essential for pre- venting potentially fatal mistakes and enhancing safety. According to some research, the creation of guidelines for the administration of intravenous drugs highlights the dedication to standardizing and enhancing the precision and security of drug delivery in specialized care environments, including pediatric critical care units.
The results of this comprehensive review confirm that the use of standardized drug concentrations is essential for improving medicine safety and healthcare quality. Healthcare facilities may drastically decrease medication mistakes, guarantee precise doses, and provide patients with consistent, efficient care by adopting standardized procedures. However, it is crucial to recognize the limits of the study. When extrapolating these results to larger healthcare settings, caution must be exercised due to variations in research techniques, possible publication bias, and the uniqueness of each clinical situation. Subsequent investigations must be conducted to provide more elaborate perspectives regarding the particular effects of standardization on patient outcomes, fortifying the argument in favor of its use in healthcare.
AUTHORS’ CONTRIBUTION
A.A.: Study conception and design; A.A.: Data collection; A.A. and S.A.: Analysis and interpretation of results.
LIST OF ABBREVIATIONS
| IV | = Intravenous |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| JBI | = Joanna Briggs Institute |
| ICU | = Intensive care unit |


