All published articles of this journal are available on ScienceDirect.
Epidemiological Study of Malta Fever (Brucellosis) in Northeastern Iran from 2016 to 2024
Abstract
Background
Control measures for infectious diseases based on evidence require health policymakers to have a solid understanding of the epidemiology surrounding these diseases.
Methods
This study is of the secondary data analysis type, which was conducted using the recorded data of patients with brucellosis in the health center of Torbat Heydarieh City (northeastern Iran) from 2016 to 2024. Data related to 2024 cases of brucellosis were analyzed using R software and Chi-2, Pearson, and independent t-statistical tests.
Results
The average age of patients suffering from Malta fever during the years under review was 37.64 years. The average frequency of the disease was 65.35 cases per hundred thousand people. 92% of patients lived in villages and 7.4% lived in cities. In terms of occupation, most cases of the disease were related to housewives (35.4%) and then to cattle farmers (34.95%). 88% of infected people had a history of contact with animals and 18% of people reported a history of the disease in other family members. 86% of infected people had a history of consuming non-pasteurized dairy products.
Conclusion
The findings underscore the importance of a multifaceted approach to preventing and controlling brucellosis. This includes targeted interventions in rural areas, improved hygiene and sanitation practices in high-risk settings, and education on proper dairy product handling and consumption. Monitoring and evaluating control measures are essential to ensure their effectiveness and adapt to changing trends and risk factors.
1. INTRODUCTION
The increasing interactions between humans and animals in the environmental context lay the groundwork for the emergence of transmissible diseases between these groups [1]. Such diseases pose significant threats to human health and diminish livestock productivity, particularly in developing nations. Unfortunately, zoonotic diseases often receive inadequate attention globally [2]. Notably, approximately 60% of identified diseases and over 75% of newly emerging diseases have zoonotic origins, underscoring the critical need to address these health issues [1, 2]. Among the most significant zoonotic diseases is brucellosis, recognized by the World Health Organization as a major neglected disease that raises serious public health concerns [3, 4].
The epidemiological landscape of brucellosis has evolved over recent decades, with its prevalence on the rise [5]. This disease is particularly widespread across Asia and Africa, affecting regions such as West Asia, Central Asia, and North Africa. Countries like Syria, Kyrgyzstan, Mongolia, Kenya, Algeria, and Iran report some of the highest rates of infection. Brucellosis lacks specific clinical signs and symptoms, often presenting similarly to other febrile infectious diseases [6, 7]. The incubation period for brucellosis ranges from 5 days to 2 months. Symptoms can manifest as a variety of clinical signs, including fever, chills, sweating, body aches, muscle pain, joint pain, and back pain [8, 9]. If the disease is not treated, it may lead to complications affecting the digestive, respiratory, and reproductive systems, as well as cardiovascular issues, neurological involvement, and even depression. Despite its low fatality rate, brucellosis has attracted significant attention due to the considerable financial burden it imposes on healthcare systems [10-12]. According to the World Health Organization's annual report, approximately 500,000 individuals globally contract brucellosis each year, with incidence rates in developed nations ranging from 4 to 10 percent [13]. The prevalence of brucellosis varies significantly across different regions. Areas such as the Channel Islands, Norway, Sweden, Finland, Denmark, Switzerland, Romania, Scotland, England, Japan, and Cyprus report no cases of brucellosis. In North America, Canada, and Australia, the disease is extremely uncommon, affecting fewer than 2 individuals per 100,000. Conversely, in certain regions like the Mediterranean and the Middle East, reported cases range from 1 to 78 per 100,000 people [14].
In Iran, despite having a comprehensive health care system and a notable decrease in disease incidence since the mid-1970s, brucellosis continues to be classified as an endemic disease. The distribution of brucellosis cases varies across different regions of Iran, with national incidence rates from 1991 to 2008 averaging approximately 43 per 100,000 individuals [15]. Razavi Khorasan province is particularly affected, attributed to its favorable climate for livestock farming and traditional animal husbandry practices [16]. A study conducted in Razavi Khorasan province between 2009 and 2013 reported an average incidence of brucellosis at 26 per 100,000 people, with over 70% of cases occurring in rural and urban areas combined [17].
Although human cases of brucellosis are infrequent, the disease imposes a significant economic burden on countries due to decreased fertility and productivity in livestock, which leads to lower milk production. Additionally, the expenses associated with prolonged and costly treatments for affected individuals contribute to disability and reduced productivity. Human-to-human transmission of brucellosis is rare; the disease primarily spreads to humans through the consumption of contaminated raw dairy products, meat, direct contact with infected animals, skin abrasions, and inhalation of particles contaminated with the urine and feces of infected animals [18-20]. A substantial portion of the Iranian population relies on agriculture, animal husbandry, and related professions, resulting in close interactions with livestock and a heightened prevalence of the disease [17, 21]. Effective control measures for brucellosis include continuous vaccination of both light and heavy livestock, enhancing animal living conditions, providing veterinary care, and educating at-risk populations. Furthermore, strategies such as preventing contact with infected animals, avoiding unpasteurized dairy products, and ensuring early diagnosis and treatment of patients are crucial in combating human brucellosis [22, 23]. In the initial phase of planning a control program for brucellosis, obtaining epidemiological data on infection prevalence within the local animal population is essential. Another critical finding from research is the correlation between various factors such as age, occupation, residence, seasonality, types of dairy products consumed, and history of exposure to the disease. Brucellosis poses an occupational risk for individuals who have direct contact with infected animals, as well as a non-occupational risk for those consuming unpasteurized dairy products. Given the economic impact of the disease, along with public unawareness regarding its transmission and management especially in rural regions these factors must be prioritized in health agendas across different areas [24]. The disease's prevalence is influenced by regional variables including climate conditions, livestock species, animal health standards, availability of pasteurized products, and diagnostic methodologies employed. Effective diagnosis of brucellosis requires a comprehensive approach that integrates epidemiological, clinical, and laboratory data [25, 26]. Understanding the incidence rates in both humans and animals, alongside the epidemiological profiles in affected regions through collaborative information sharing between health and veterinary sectors, is vital for successful disease prevention and control efforts [24, 27, 28].
Given that the northern and northeastern provinces of Iran exhibit high prevalence rates of brucellosis, Razavi Khorasan province, particularly Torbat Heydarieh City, has become a focal point for disease control and prevention efforts. To implement evidence-based control measures, it is essential to conduct a study that accurately delineates the disease's distribution by location. Therefore, this research was undertaken to examine the epidemiology of brucellosis in Torbat Heydarieh City.
2. METHODS AND MATERIALS
This study is a secondary analysis conducted to investigate the epidemiology of brucellosis in the cities covered by Torbat Heydarieh University of Medical Sciences (Northeastern Iran) from 2016 to 2024. Information related to 2024 cases of the disease in these cities was analyzed. Data on brucellosis patients from medical centers and private clinics in Torbat Heydarieh over the past 9 years were included in the study. The information was collected using a checklist with 7 items: age, place of residence, occupation, season, history of contact with infected animals, history of consuming unpasteurized dairy products, and history of vaccination. The criteria for inclusion in the study were based on national standard definitions: individuals with suspicious clinical symptoms and Wright's titer (≤1.80) or positive Coombs-Wright's titer and (2ME ≤1.40).
After collecting the information, the data were analyzed using R software. The analysis included chi-square tests for relationships between qualitative variables, Pearson's correlation tests for correlations between quantitative variables, and independent t-tests for comparing means of independent groups. Additionally, ArcGIS and GeoDa software were used for geographic analysis. Brucellosis patient data were entered into ArcGIS software for each region to display the spatial distribution of patients over the specified time period using density points.
3. RESULTS
In this study, information related to 2024 cases of disease in the cities covered by Torbat Heydarieh University of Medical Sciences was analyzed. In terms of the frequency of infected cases, the cities of Torbat Heydarieh with 965 cases (47.6%), Zaveh with 878 cases (43.4%), and Mah Velat with 181 cases (9%) had the lowest frequency. The average age of patients was 37.64 years (with a standard deviation of 18.38 years). The average age of affected men was 32.52 years (with a standard deviation of 19.65) and the average age of women was 38.83 years (with a standard deviation of 20.02 years). The cases reported in the age group of 31-40 had the highest frequency with 18.2%, followed by the age group of 50-41 years with 17.3% frequency. In general, it can be said that about a quarter of all cases were in the age group of 30-50 years.
The demographic characteristics of the patients are shown in Table 1. As can be seen, most of the disease cases were reported in people living in rural areas with 1874 (92.6%) cases, which was much higher than in urban areas with 150 (7.4%) cases. In terms of gender, men had the highest frequency with 1130 (56%) cases and women with 894 (44%) cases. In terms of occupation, the highest incidence of brucellosis was attributed to the number of 708 people, mainly to people with the job title of housekeeper (35%), and in the next category, the number of 706 people were livestock farmers (34.9%). The lowest cases were reported in butchers, less than 1%. As it is known, 88.19% of people have reported a history of contact with animals and 18.33% of people have reported a history of disease in other family members. 86.64% of the people who contracted the disease had a history of consuming non-pasteurized dairy products. Out of all the examined disease cases, 155 (7.66%) cases were reported to have failed treatment.
| Variable | Subset of Each Variable | Frequency | Frequency Percentage | Variable | Subset of Each Variable | Frequency | Frequency Percentage |
|---|---|---|---|---|---|---|---|
| Gender | Man | 1130 | 56 | Age | <10 | 253 | 12.5 |
| Woman | 894 | 44 | 20-11 | 269 | 13.3 | ||
| Job | Housekeeper | 708 | 35 | 30-21 | 308 | 15.2 | |
| Housekeeper - rancher | 22 | 1.1 | 40-31 | 369 | 18.2 | ||
| Rancher | 706 | 34.9 | 50-41 | 351 | 17.3 | ||
| Butcher | 10 | 0.5 | 51-60 | 247 | 12.2 | ||
| Child | 68 | 3.36 | >61 | 227 | 11.3 | ||
| Farmer | 78 | 3.9 | Residence | Rural | 1874 | 92.6 | |
| Employee | 13 | 0.6 | City | 150 | 7.4 | ||
| Worker | 57 | 2.8 | |||||
| Student | 193 | 9.5 | History of non-pasteurized dairy consumption | Yes | 1753 | 86.64 | |
| Other | 169 | 8.3 | No | 271 | 13.36 | ||
| Animal contact history | Yes | 1785 | 88.19 | Disease status | New patient | 1869 | 92.34 |
| Treatment failure | 155 | 7.66 | |||||
| No | 239 | 11.81 | Infecting other family members in the past 18 months | Yes | 371 | 18.33 | |
| No | 1653 | 81.66 |
In general, the incidence of disease in July is higher than in other months. Additionally, the incidence of disease in the cold seasons (autumn and winter) is lower than in the hot seasons (spring and summer). Fig. (1) displays the frequency of disease incidence in different months of the year during the 9-year period of the study.
The average frequency of disease in the cities covered by Torbat Heydarieh University of Medical Sciences for each year was 224.9 people, or in other words, 65.35 cases per 100,000 people. According to the population of the target cities (areas covered by Torbat Heydarieh University of Medical Sciences), the number of disease cases by City is shown in Table 2. Among the areas covered by Torbat Heydarieh University of Medical Sciences, Zaveh has the highest incidence rate with 144.09 cases per 100,000 people.
The spatial investigations of brucellosis incidence in the present study show that except in 2022, Zaveh City has the highest brucellosis incidence rate in 11 years compared to the other two cities covered by Torbat Heydarieh University of Medical Sciences. Also, this rate is always about 145 cases per hundred thousand people. The trend of disease occurrence in Torbat Heydarieh City has been decreasing, and Mah Velat City has also had an unstable trend with ups and downs. Fig. (2) shows the location of the areas covered by Torbat Heydarieh University of Medical Sciences. Additionally, Fig. (3) shows the spatial distribution of the incidence of brucellosis in the areas covered by Torbat Heydarieh Medical Sciences University.
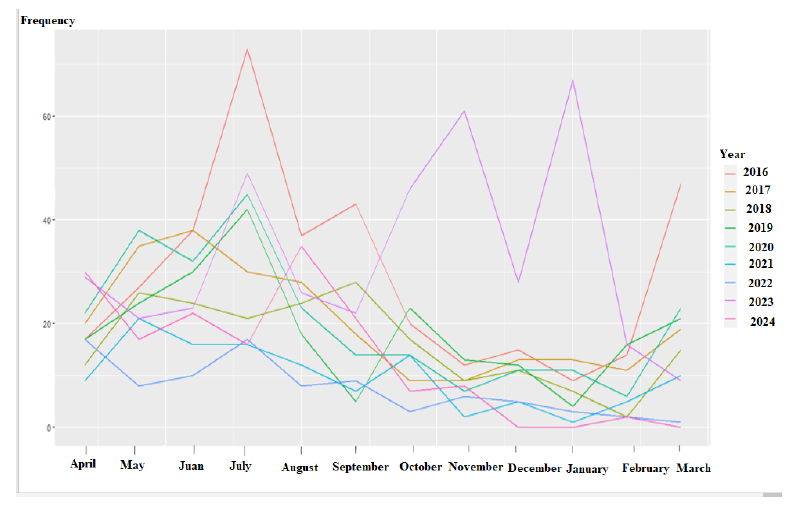
Frequency of disease in different months of the year during the 9-year period.
| City Name | City Population | Average Annual Frequency | Average Annual Incidence per 100,000 People |
|---|---|---|---|
| Torbat Heydarieh | 225,000 | 97.32 | 43.20 |
| Zaveh | 67,700 | 97.95 | 144.09 |
| Mah Velat | 51,400 | 30.1 | 58.58 |
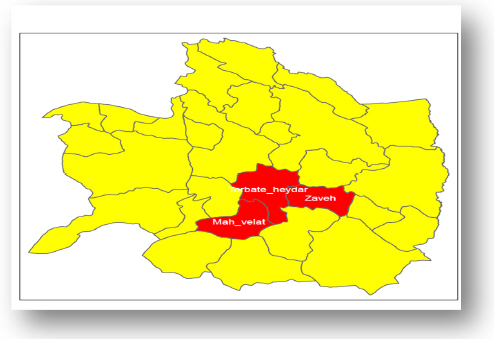
The location of the areas covered by Torbat Heydarieh University of Medical Sciences. The three study areas are shown in red.
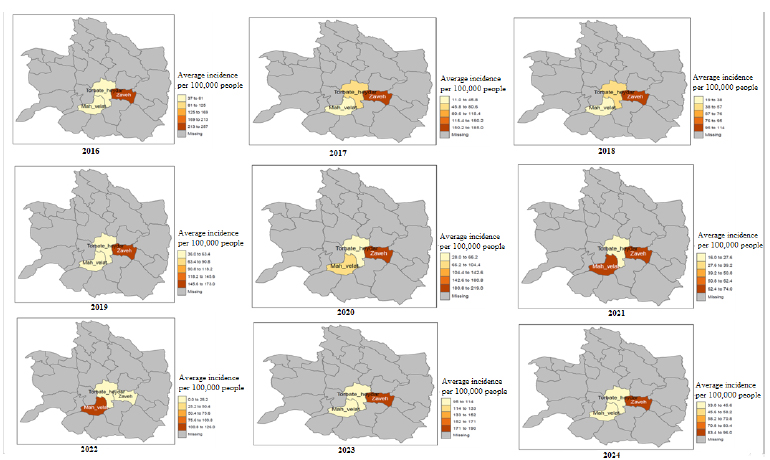
Spatial distribution of incidence rates of brucellosis in Areas covered by Torbat Heydarieh University of Medical Sciences (2016–2024). This map was created using R software by Tma and tydyvers package.
4. DISCUSSION
The current study aimed to assess the epidemiological status of brucellosis in cities affiliated with Torbat Heydarieh University of Medical Sciences in northeast Iran from 2016 to 2024. Findings revealed that the average age of patients diagnosed with brucellosis during this period was 37.64 years. Specifically, the average age for affected men was 32.52 years, while for women, it was 38.83 years. The highest frequency of cases occurred in the age group of 31-40 years, accounting for 18.2%, followed closely by the 41-50 age group at 17.3%. Overall, approximately 25% of all cases fell within the 30 to 50-year age range. This trend indicates that brucellosis is more prevalent among middle-aged individuals engaged in livestock and agricultural activities. Consequently, this economically and socially active age group requires targeted educational initiatives. Preventive measures should prioritize this demographic by focusing on evidence-based strategies aimed at reducing health risks and environmental contamination. Supporting this research finding, Gozidehkar et al. reported an average age of brucellosis patients at 36 years [29]. Similarly, Shirzadi et al. noted the highest disease frequency among individuals aged 30-59 [30], while studies by Bagheri and Bagherizadeh indicated an average age of 33 [31, 32]. Additionally, research conducted in northern and northwestern Iran found that the average patient age at diagnosis was 34 years [33], contrasting with a study in Mashhad that identified a predominance of cases among younger individuals aged 16-24 [24].
The findings of this study indicated that brucellosis was more prevalent in men than in women, with a distribution of 56% in men and 44% in women. This observation can be interpreted by noting that brucellosis is typically an occupational disease, resulting in higher rates among men who are more engaged in professions such as agriculture, shepherding, animal husbandry, butchery, and traditional slaughterhouse work. Conversely, the prevalence among housewives may stem from practices like hand milking and involvement in livestock care and agriculture. Supporting these findings, multiple studies have also reported a greater incidence of brucellosis in men compared to women [29-31, 34]. However, research conducted in Kurdistan and Lorestan found that housewives exhibited higher prevalence rates than men [35, 36]. The differences between these studies and the present research may be attributed to variations in geographical, cultural, and social contexts across different regions. The current research findings indicated that the average frequency of brucellosis in the cities associated with Torbat Heydarieh University of Medical Sciences was 224.9 cases annually or approximately 65.35 cases per 100,000 people. This suggests a notable prevalence of brucellosis in this region. With a population of 344,100, the estimated annual incidence in this area is 65.3 cases per 100,000, which is more than double the national average of 24.2 cases per 100,000. This highlights a significant incidence of brucellosis in this geographical area and categorizes it as one of the high-risk regions for the disease. In light of these findings, implementing educational programs focused on disease transmission and prevention is essential to reduce incidence rates among at-risk populations, particularly livestock farmers. Thus, there is a pressing need for health interventions and educational initiatives to address this public health concern effectively. The increase in disease cases in the regions associated with Torbat Heydarieh University of Medical Sciences may be attributed to enhanced access to laboratory services and improvements in diagnostic processes and statistical record-keeping. Additionally, the rise in disease prevalence among livestock could be linked to the illegal importation of unvaccinated and unhealthy animals from southern areas of the country. Supporting this research, findings from Gozidehkar et al. [29] indicated that brucellosis cases in South Khorasan rose steadily from 2015 to 2021. Furthermore, Shirzadi et al. reported an upward trend in brucellosis cases in Khorasan between 2009 and 2011, followed by a decline in 2012, before seeing another increase from 2013 to 2015 [30]. Conversely, Jafarnejad's study noted a decrease in initiatives such as vaccination programs and direct training for livestock farmers during the years 2009 to 2016 [34].
The results of the current study indicated that 92% (1,870 cases) of brucellosis patients resided in rural areas, while 7.4% (150 cases) lived in urban settings. This finding can be attributed to several factors. In rural regions, many individuals are involved in occupations such as animal husbandry and agriculture, which increases their exposure to infected livestock and dairy products, thereby heightening the risk of contracting brucellosis. Furthermore, awareness regarding transmission and prevention methods for brucellosis tends to be lower among rural inhabitants compared to their urban counterparts. This lack of knowledge may contribute to a higher prevalence of the disease in villages. Additionally, rural populations typically have less access to healthcare services than those in cities, which can delay diagnosis and treatment, further exacerbating the disease's prevalence in these areas. Environmental factors, such as the presence of disease-carrying animals and inadequate hygiene practices in livestock management, also facilitate the spread of the disease in rural settings. Ultimately, these findings underscore that brucellosis should be recognized as a significant health issue in rural communities. Implementing educational programs to enhance awareness, improving access to healthcare services, and establishing disease control initiatives for livestock could effectively reduce brucellosis prevalence in these areas. Supporting this research, studies by Gozidehkar et al. in Qaenat and other investigations in Khorasan and Kurdistan have similarly demonstrated that brucellosis prevalence is markedly higher in rural regions compared to urban areas [24, 29, 36]. The current research findings revealed that, regarding occupation, the highest number of cases were 708 individuals identified as housekeepers (35.4%), followed closely by 706 individuals categorized as livestock farmers (34.95%). This suggests that brucellosis is primarily a work-related illness, with increased prevalence among livestock farmers and those handling livestock products. The elevated infection rate among housewives can be linked to their involvement with livestock products and the traditional roles of men in agriculture, shepherding, animal husbandry, butchery, and slaughterhouse operations. Additionally, the transmission of brucellosis among housewives may result from practices such as hand milking and participation in agricultural activities. Supporting this observation, multiple studies indicate that brucellosis is more prevalent in men than in women [29-31, 34]. However, research conducted in Kurdistan and Lorestan has shown that housewives exhibit a higher prevalence of brucellosis compared to men [35, 36]. The differences between these findings and those of the current study may be attributed to varying geographical, cultural, and social contexts across different regions. The findings of the current study indicated that 88% of participants had a history of contact with livestock, while 18% reported a history of brucellosis in other family members. Additionally, 86% of individuals who contracted the disease had consumed non-pasteurized dairy products. These results highlight that contact with livestock and the consumption of non-pasteurized dairy products are the primary factors contributing to the spread of brucellosis. In this region, contact with livestock is a significant source of disease transmission, particularly in rural areas where people have close interactions with animals. Furthermore, consuming unpasteurized dairy products poses a risk as these items can be contaminated with the bacteria causing brucellosis. The data also suggest that brucellosis is prevalent as a familial disease, given that 18% of affected individuals reported a history of the illness among family members. This may be due to the transmission of the disease through close contact with infected individuals. These insights can assist health policymakers and researchers in this region to prioritize prevention and control measures for brucellosis, emphasizing the need to minimize contact with livestock and discourage the consumption of unpasteurized dairy products. Public education on safe dairy consumption practices is also crucial. Supporting these findings, research by Gozidehkar et al. [29] indicated that contact with livestock and non-pasteurized dairy products especially milk and cheese—was prevalent among brucellosis patients. Moreover, numerous studies have shown that most brucellosis patients had a history of consuming unpasteurized milk and dairy products and had direct contact with animals [34, 35, 37]. Despite previous studies confirming the link between unpasteurized dairy product consumption and brucellosis, these products remain commonly consumed, particularly local cheeses made from unboiled milk. Additionally, drinking milk directly from the udder of animals, especially in rural areas, is recognized as a significant risk factor for disease transmission. Increasing awareness about these risks among rural populations could be an effective strategy for reducing brucellosis cases. The findings of the current research indicated that the incidence of brucellosis in Torbat Heydarieh City is decreasing, while Mah Velat City exhibits an unstable trend. This improvement in Torbat Heydarieh can be attributed to effective measures implemented in recent years in Iran aimed at controlling and reducing the spread of brucellosis. These measures encompass disease prevention and control programs, public education initiatives, enhanced access to healthcare services, and improved animal health management. In contrast, Mah Velat City has experienced fluctuations in brucellosis incidence, suggesting difficulties in executing disease prevention and control programs effectively. Contributing factors to this instability include limited access to healthcare services, low levels of public awareness, and favorable environmental conditions for disease transmission. The insights from this study will aid health policymakers and researchers in identifying challenges faced in implementing brucellosis prevention and control programs in Mah Velat County. Additionally, examining the effective factors that contributed to the reduction of brucellosis cases in Torbat Heydarieh can inform the development of more effective strategies for other regions. Supporting this research, a study by Shirzadi et al. revealed an upward trend in brucellosis cases in Khorasan from 2009 to 2011, followed by a decrease in 2012 and another increase from 2013 to 2015 [30]. Other studies have demonstrated that implementing programs such as livestock vaccination and providing face-to-face training for livestock farmers has contributed to a decline in brucellosis cases [34, 36]. The findings of the current research revealed that the incidence of brucellosis is higher in July compared to other months. Generally, the disease is less prevalent during the colder seasons (autumn and winter) than in the warmer months (spring and summer). This trend can be explained by the increased number of livestock during the warmer seasons, as well as greater contact with aborted animal fetuses and higher consumption of non-pasteurized dairy products, which contribute to the rise in disease incidence. Supporting this observation, Gozidehkar et al. [29] found that brucellosis is more prevalent in the summer and spring, coinciding with the livestock breeding season. In their study, 62.9% of reported cases occurred in the first half of the year, while 37.1% were noted in the latter half. Additionally, these findings align with results from other similar studies [35, 36]. The overall pattern indicates that climatic factors and livestock management practices significantly influence brucellosis transmission dynamics throughout the year. The findings of the current study indicated that 92.34% of the patients were successfully treated for brucellosis, while 7.66% experienced treatment failures. This aligns with numerous studies that demonstrate a high success rate in treating brucellosis, with treatment failures occurring in a limited number of cases, underscoring the effectiveness of the treatment protocols [29, 34, 37, 38]. Certain factors have been identified as risk factors for relapse or treatment failure, including being male, occupational exposure (particularly in animal husbandry), a duration of illness of less than two weeks before starting treatment, and the presence of hepatosplenomegaly and thrombocytopenia . These insights can help healthcare providers tailor treatment strategies and improve outcomes for patients with brucellosis [38].
CONCLUSION AND RECOMMENDATIONS
The present study's findings provide important insights into the epidemiology of brucellosis, a significant public health issue. The average age of patients diagnosed with brucellosis over the studied years was approximately 38 years, indicating that the disease predominantly affects young adults. The average annual incidence in the cities was found to be 224.9 cases, translating to 65.35 cases per 100,000 people. This elevated incidence rate underscores the urgent need for effective prevention and control measures to curb the spread of the disease. Additionally, the study revealed that a substantial majority of patients with Malt fever, specifically 92%, lived in rural areas, while only 7.4% resided in urban settings. This disparity suggests that rural regions may require targeted interventions to address the specific challenges and risk factors associated with brucellosis. Occupationally, the most frequently affected groups were housekeepers (35.4%) and cattle farmers (34.95%), highlighting the necessity for implementing proper hygiene and sanitation practices in these environments to mitigate transmission risks. Furthermore, it was noted that 88% of patients had a history of contact with livestock, and 18% reported a history of brucellosis in other family members. This indicates that both direct and indirect exposure to the disease is possible, emphasizing the need for comprehensive prevention strategies. The study also found that 86% of patients who contracted brucellosis had consumed non-pasteurized dairy products, which underscores the importance of promoting safe handling and consumption practices for dairy products to reduce infection risks.
The research indicated a decreasing trend in the incidence of Malt fever in Torbat-e Heydariyeh City, suggesting that control measures implemented in this area have been effective. Conversely, Mah Velat City exhibited an unstable trend with fluctuations in incidence rates, indicating that more focused interventions are necessary to tackle the unique challenges and risk factors present in this region. These findings align with previous studies which have similarly highlighted the importance of addressing both environmental and occupational factors in controlling brucellosis outbreaks. In conclusion, the findings of this study underscore the importance of a multifaceted approach to preventing and controlling brucellosis. This includes targeted interventions in rural areas, improved hygiene and sanitation practices in high-risk settings, and education on proper dairy product handling and consumption. Additionally, monitoring and evaluating control measures are essential to ensure their effectiveness and adapt to changing trends and risk factors.
AUTHORS' CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article reports the results of a research project approved by Torbat Jam Faculty of Medical Sciences, Iran with the code of ethics IR.TRJUMS.REC.1401.007.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee, and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Considering that the data in this research is secondary data analysis, there was no need to obtain written and informed consent from the patients to collect the data.


