All published articles of this journal are available on ScienceDirect.
In Vitro Study of the Osterix and Osteopontin Genes Expression in Conditioned Medium- and Pulsed Electromagnetic Field-induced Bone Marrow Mesenchymal Stem Cells
Abstract
Background
Conditioned medium and electromagnetic field stimulate osteogenic gene expression and proliferation in rBMSCs for osteoblast differentiation in vitro.
Aim
This study aimed to determine the expression of Osterix and Osteopontin genes in bone marrow mesenchymal stem cells induced by medium and pulsed electromagnetic fields.
Methods
The experimental groups included rBMSCs cultured in α-MEM containing 10% FBS (negative control), in osteogenesis differentiation medium (positive control), and exposed to EMF (50 Hz, 1 mT), 30 min daily. The treatment groups were also exposed to CM (CM group), simultaneously exposed to osteogenesis differentiation medium and EMF (OD+50 Hz group), and also exposed to CM and EMF (CM+50 Hz group). Osterix (OSX) and Osteopontin (OPN) gene expression were evaluated by Real-time PCR, after 14 days.
Results
The OSX gene expression was significantly increased in the OD and CM+50HZ groups, as compared to the negative control (p˂0.05). These results demonstrated that CM+50HZ and OD promoted the expression of the OSX gene. This gene was also significantly decreased in the CM, 50HZ, and OD+5OHZ groups, compared to the OD group (p˂0.05). It was suggested that these treatments had an inhibitory effect on the expression of the OSX gene. The positive control group had a significantly higher level of OPN gene expression, than that of negative control. The CM, OD+50HZ, CM+50HZ, and 50HZ groups showed no significant difference of that gene expression, compared to the negative control.
Conclusion
In vitro osteogenic differentiation of rBMSCs occurs 14 days after induction, preparing osteoblasts for tissue engineering by combining CM and EMF for 30 minutes.
1. INTRODUCTION
Bone marrow mesenchymal stem cells (BMSCs) are often used in autologous transplants and have great potential for cell therapy, tissue engineering, and regenerative medicine. One objective of modern regenerative medicine is to produce a bone transplant using a population of live cells and paracrine factors [1]. According to a recent study from 2007 to 2018, the therapeutic use of mesenchymal stem cells (MSCs) in tissue engineering and regenerative medicine is connected to the paracrine pathways formed as a result of the secretion of various substances in the culture media. The secreted factors are referred to as secretome/conditioned medium (CM) [2]. Stem cells or osteoblasts-conditioned medium contains growth factors and cytokines including BMP2, TGF, basic fibroblast growth factor (bFGF), fibroblast growth factor (FGF-2), and insulin-like growth factor (IGF-1). In recent years, CM has been used to differentiate embryonic stem cells or MSCs into osteoblasts in vitro. Various secreted cytokines, growth factors, extracellular matrix proteins, matrix regeneration factors, as well as numerous extracellular vesicles play an important role in MSC therapy [3] According to Zhong et al. (2019), mouse osteoblast-derived CM can significantly improve osteogenic differentiation of iPSC-MSCs [4]. The migration, proliferation, and expression of bone-related genes, such as osteocalcin and Runx2 of rat MSCs were increased, using human BMSCs-CM under in vitro culture conditions [5]. Even if CM is obtained from the same kind of cells, its quality varies according to the number of cells, culture conditions, types of culture media, and preparation procedure [6]. In a research, it was shown that the human BMSC-CM can promote bone and tendon healing in a rat rotator cuff repair model by controlling the polarity of macrophages. Mesenchymal stem cell conditioned media (MSC-CM), is well known as a rich source of autologous cytokines, and is universally used in clinical medicine. [1]. MSC-CM delivered in gelatin sponges stimulates angiogenesis and promotes fracture healing in a diabetic rat model, according to research by Wang et al. MSC-CM demonstrated high levels of the angiogenic factors VEGF and IL-6. It can be a complementary treatment of non-union fracture in patients with diabetes, [2]. In a study, it was shown that MSC-CM has a very high potential to induce bone regeneration without stem cell transplantation. It was the first report to use CM for in vitro and in vivo bone regeneration [6]. Pulsed electromagnetic field (PEMF) has lately been known as a noninvasive, safe, without side effects, and prospective therapy strategy to accelerate bone repair in non-union and delayed fractures, and also osteoporosis and osteonecrosis of the femoral head. Studies have shown that PEMF exposure by stimulating Wnt/β catenin, Notch signaling pathways, and increasing intracellular ca2+ level, markedly increased the expression levels of osteogenesis markers (Col I, OCN, OPN, Runx2, ALP) in osteoblasts. PEMF-induced ERK/MEK signaling leads to the expression of OPN, RUNX2 genes, ALP activity, and calcification [7-11]. PEMF by stimulating MAPK/P38 plays a critical role in collagen synthesis in osteoblast-like cells [11]. It was reported that PEMF treatment increased the expression level of IGF-1 mRNA and induced osteogenesis [7]. Electrical stimulation significantly promotes osteoblast adhesion and proliferation, mineralized nodule formation, and bone extracellular matrix proteins. In addition, it stimulates the expression of bone-specific ALP, Runx2, type I collagen, OCN, and cytokines (BMP-2, IGF-1, and VEGF). Exogenous EMF has been found to affect cell behavior in vitro [8]. The expression of growth factors, nitric oxide signaling, cytokine regulation, and other activities are all affected by EMF. EMF at low (30-300 kHz) and very low (30-30 Hz) frequencies have been used to study the mentioned effects [9]. PEMF stimulation has been shown to promote osteoblast proliferation and differentiation as well as bone formation by increasing DNA content and AlPase activity, respectively [10]. De Matte et al. reported that PEMF can increase the expression of miR-26a, miR-29b, and miR-218, which induced osteogenesis and angiogenesis [11]. It was reported that exposure to PEMF promotes the synthesis of osteogenic markers including alkaline phosphatase (ALP) and osteocalcin (OC), in the presence of bone morphogenic protein (BMP2) [12]. It should be emphasized that electrical stimulation parameters such as duration, amplitude (voltage), type, and mode, effectively mediate cellular proliferation, gene expression, and differentiation [13]. The Runx2, Alp, Osterix (OSX), Osteopontin, and Osteocalcin genes regulate osteoblasts differentiation, respectively [14, 15]. RUNX2 expression is essential for direct differentiation of MSCs into osteoblastic lineages and require to maintain the immature state (pre-osteoblasts) [15]. While Osterix induces periosteoblast maturation and osteoblast differentiation [16]. Osteix is essential for osteoblast differentiation, bone mineralization, and formation during the embryonic and postnatal periods [17]. Runx2 regulates the expression of downstream OSX, which plays a key role in osteoblast differentiation. OSX is not expressed in Runx2-deficient mice, as stated by Nakashima et al [18]. OSX has been demonstrated to be expressed primarily in osteoblasts, osteocytes, prehypertrophic and hypertrophic chondrocytes, and not in osteoclasts. The production of osteoblast-specific matrix proteins, such as collagen type I (Col1), bone sialoprotein (BSP), osteonectin, osteopontin (OPN), and osteocalcin (OC), as well as osteoblast lineage commitment are both dependent on OSX [18]. Decreased limb length, delayed corticalization, and abnormal osteoblast distribution, caused by OSX disruption in osteoblasts. Postnatal inactivation of OSX in mice leads to multiple skeletal abnormalities, including deficits in osteocyte maturation and function, loss of new bone formation, and mineralized cartilage resorption [19]. It has been demonstrated that OSX overexpression induces osteocalcin expression and ALP activity in mesenchymal stem cells, as well as increases the calcification of primary mouse osteoblasts [20].
OPN is a key player in a number of biological processes, including matrix remodeling and tissue calcification, the proliferation, migration, and adhesion of several bone-related cells, including BMSCs, hema- topoietic stem cells, osteoclasts, and osteoblasts, as well as the inhibition of apoptotic activity and the production of different cytokines. Other studies have reported that OPN inhibits the proliferation and differentiation of osteoblasts and inhibits mineral deposition in bone. It is found that OPN plays an important role in mineralization, but has no effect on osteoblasts differentiation [21]. There are conflicting reports on OPN. The purpose of this study is to investigate the synergistic effect of the electromagnetic field and rBMSCs -conditioned medium (rBMSCs-CM) and osteogenesis differentiation medium on the expression of OSX and OPN genes to determine the role of the mentioned genes on osteogenesis in vitro.
2. METHODS AND MATERIALS
2.1. Isolation and Culture of Bone Marrow Mesenchymal Stem Cells
Adult Wistar rats (6-9 weeks, weighing 200–250 gr) were purchased from the Pasteur Institute of Tehran and transferred to the animal house of Damghan University for adaptation to the new environment. were sacrificed, and the femur and tibia bones. The bones were placed on a plate with phosphate buffer and penicillin under sterile conditions. The bone marrow was extracted using a 25-gauge syringe filled with α-MEM containing 10% FBS and incubated. The cultured cells were passaged at 70–80% confluency. For collecting the CM, cells at passage 3 were incubated in a serum-deprived medium for 72 h and then stored at -20°C.
2.2. The Experimental Groups were as follows
rBMSCs at passage four were divided into five groups: cultured cells in α-MEM containing 10% FBS (negative control), cultured cells in osteogenesis differentiation medium containing 7-10 M Dexamethason, 10 mM Beta-Glycerol-Phosphate, 50 ug/ml Ascorbic Acid bi-Phosphate, 15% FBS, 100 unit/ml Penicillin (Positive control). 50Hz group: cells exposed to FMF (50Hz, 1mT) for 30 min/day. CM group: cultured cells in BMSCs-CM. CM+50HZ group: cultured cells in BMSCs-CM and exposed simultaneously to 50 Hz, 1 mT EMF, for 30 min/day. OD+50HZ group: cultured cells in osteogenesis differentiation medium and exposed simultaneously to 50 Hz, 1 mT EMF for 30 min/day. After 14-day treatment, RNA extraction, cDNA synthesis, and real-time PCR were performed.
2.3. Real-time PCR
In this study, the expression level of genes was assessed using real-time PCR. The internal control gene was the GAPDH gene. Table 1 lists the details of the primers used in this study.
The q-PCR technique was used to evaluate Runx2 and Ocn genes in treated rBMSCs (39). Total RNA was extracted using RNX-plus reagent (Sinaclon, Iran) according to the manufacturer’s protocol. The purity of the extracted RNA was examined using a spectrophotometer (Eppendorf BiopHotometer). Samples with adsorbents of 260/230 ≥ 1.4 and 260/280 ≥ 1.8 were selected for analysis. PrimeScriptTM 1st strand cDNA Synthesis Kit (Takara, Japan) was used to synthesize cDNA. Real-Time PCR was done by Rotor-Gene 6000 PCR system, using RealQ Plus Master Mix Green (Amplicon, Denmark) and the primer sequences (Table 1) were designed using AlleleID software version 7.5 (Premierbiosoft, USA). In this study, the housekeeping gene Tbp was used as internal control and the expression level of each gene was compared with Tbp. The relative expression of genes in the treatment groups compared to the control group was calculated using -2ΔΔct and the final volume of the reaction solution was 10 μl. Finally, the RT-qPCR reaction with the amplicon amplification program includes initial denaturation: 95°C, 15minutes, denaturation: 95°C, 15 seconds, annealing and extension: 60°C, 45 seconds, melt: 65-94°C was performed.
| Gene | Primer Sequence | Product Size(bp) |
|---|---|---|
| Osterix | F:5’-CTCTCCATCTGCCTGACT-3’ R:5’-GCCATAGTGAGGCTTCTTCC-3’ |
84 |
| Osteopontin | F:5’-CTTGGCTTACGGACTGAG-3’ R:5’-AACTGGGATGACCTTGATAG-3’ |
136 |
| Tbp | F:5’ -TATCCTTCACCAATGACTCCTA-3’ R:5-’CAGTTGTTCGTGGCTCTC-3’ |
217 |
2.4. Statistical Analysis
Statistical analysis was performed using SPSS software version 16. In order to evaluate the significant differences between the experimental groups, a one-way ANOVA test followed by Tukey and LSD post-hoc tests were performed. The significant differences of treated cells on days 14 and 21 were evaluated using the Independent-sample T-test. p<0.05 was considered as a significant level and each experiment was repeated 3 times.
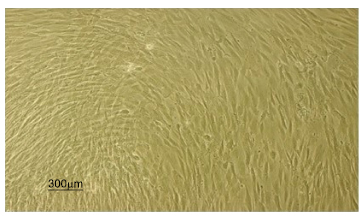
Shows the third passage of rat bone marrow stem cells.
3. RESULTS
3.1. Rat Bone Marrow Stem Cell Morphology
The cultured rat BMSCs were observed by an inverted microscope. Fresh cultured cells were round and floating. The cells at passage 3 appeared spindle, fibroblast-like, and elongated shape (Fig. 1).
3.2. Expression Levels of OSX mRNA
According to Fig. (2), after 14 days, OSX gene expression levels were significantly increased in the positive control group (9.03 ± 3.04) and CM+50HZ (8.13 ± 3.28), compared to the negative control (1.00 ± 0.00) p<0.05. There was no significant difference between the CM+50HZ and positive control groups, p>0.05. Additionally, the 50HZ (0.42 ± 0.16), OD+50HZ (0.30 ± 0.11) and CM (0.09 ± 0.03) groups showed no significant difference, as compared to the negative control group. There was a significant decrease in OSX gene expression in the CM, 50HZ, and OD+50HZ groups, compared to the CM+50HZ group, (p ≤ 0.05). CM and 50HZ groups did not differ significantly from OD+50HZ group (p>0.05). There was no significant difference between the 50HZ and CM groups, p>0.05.
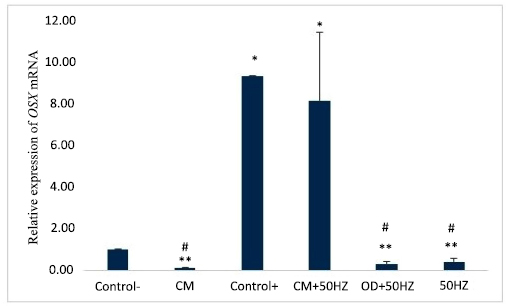
The average expression levels of the OSX gene in the experimental groups after 14 days. * p<0.05 versus negative control, ** p<0.05 versus positive control, and # p<0.05 versus CM+50HZ group.
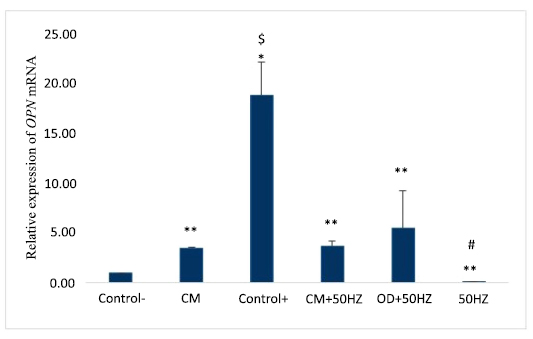
The average expression levels of the OPN gene in experimental groups, after 14 The symbols * p<0.05 versus negative control, ** p<0.05 versus positive control, # p<0.05 versus OD+50HZ group, and $ p<0.05 versus other groups.
3.3. Expression Levels of OPN mRNA
According to Fig. (3), after 14-day treatment, there was a significant increase of the OPN gene expression in the positive control (18.86 ± 3.34), compared to the negative control (1.00 ± 0.00), P <0.05. The 50HZ (0.09 ± 0.03), CM+50HZ (3.64 ± 0.61), OD+50HZ (5.54 ± 3.67), and CM (3.47 ± 0.14) groups did not vary significantly from the negative control group, p>0.05. The CM, 50HZ, and OD+50HZ groups did not show significant differences when compared to the CM+50HZ group, (p>0.05). The 50HZ group did not vary significantly from the OD+50HZ group (p>0.05). The CM group showed no significant difference in OPN gene expression, as compared to that of the OD+50HZ group (p>0.05), and also there was no significant difference between the 50HZ and CM groups, (p>0.05).
4. DISCUSSION
Mesenchymal stem cell research has recently taken a turn toward the paracrine pathway [2]. These cells secret a significant number of trophic molecules in the target tissue after transplantation, including growth factors, cytokines, and immune system regulatory molecules, which not only promote the implantation of new stem cells but also result in the best possible tissue healing. Moreover, mesenchymal stem cells are therefore regarded as the most crucial form of stem cells in tissue engineering and regenerative medicine [1, 2]. CM is the term for factors that cells secret into their culture media [2]. CM is crucial for many physiological processes and carries out the immune system's regulatory function and have the capacity to promote or repress a large variety of particular kinds of T cells [5]. The trans-differentiation of iPSC-MSCs toward osteoblasts can be enhanced by osteoblast- CM. The research will assist in improving the conditions under which inducing osteogenesis of iPSC-MSCs and will support the prospective use of iPSC-MSCs for bone tissue engineering [4]. Vascular smooth muscle cells (VSMCs) undergo osteoblastic differentiation and mineralization, which is inhibited by MSC-CM, according to research by Shuangshuang Wang et al. This is accompanied by a drop in calcium levels, an increase in ALP activity, and a decrease in the expression of BMP-2, Runx2, Msx2, and OC. Wang et al. demonstrated that MSC-CM includes high amounts of the angiogenic factors VEGF and IL-6, which are released in gelatin sponges, stimulated angiogenesis, and improved fracture repair in a diabetic rat model. Maybe, it could be a different approach to treat fracture non-union in diabetic patients [2]. Additionally, it has been noted that BMSCs exposed to the same PEMF have varying proliferation capacities depending on the different stages of ossification. It is unknown whether PEMF stimulation has conflicting effects on the proliferation rate of osteoblast-like cells. PEMF electrical impulses may control the cell cycle by changing the type or quantity of cytokine receptors or ion channels in the cell membrane. As a result, the cells may react to PEMF stimulation differently, depending on the type, maturation stage, and density. Following FDA approval in 1979, PEMF has been known as a safe adjuvant biophysical therapy in bone fracture healing, bone grafting, congenital pseudarthrosis, and other bone diseases. Tingting Wang demonstrated the synergistic effect of BMP9 and PEMF (1.8 or 2.4 mT) on the expression of osteogenic genes in the early and middle stages of ossification, as well as the expression of Runx2, ALP, OPN transcription factor protein, and extracellular matrix formation in human periodontal ligament stem cells (hPDLSCs) [22]. According to Gabriele Ceccarelli, exposure to PEMF could enhance the initial cell proliferation in BM-MSC-mediated osteogenesis and fasten osteogenesis [23]. In the current study, a considerable increase in OSX gene expression was observed in OD and CM+50HZ groups compared to the negative control, after 14 days of treatment. These findings demonstrated that co-treatment with CM and EMF 50HZ, or OD treatment promotes OSX gene expression in rat BMSCs. While, the expression of the mentioned gene was significantly decreased in the CM, 50HZ, and OD+5OHZ groups compared to the OD group, suggesting that such treatments had an inhibitory effect on the expression of the OSX gene. The expression of the OSX gene in BMSCs co-treated with EMF 50HZ and hADSCs-CM was similar to that of the positive control. The results of Jing Sun et al. are in conflict with our results. They demonstrated that Runx2 gene expression decreased in osteoblasts treated with MSC-CM (from 3 to 6 days), whereas Osx gene expression did not change. They also demonstrated that, by the seventh day of treatment, gene expression had been restored to normal levels [24]. After 14 days, the OPN gene expression in the positive control group (OD) was significantly higher than that in the negative control group. While there was no discernible difference in OPN gene expression in CM, OD+50HZ, CM+50HZ, and 50HZ groups, as compared to the negative control group. The significant decrease of this gene in the CM, OD+50HZ, CM+50HZ, and 50HZ groups relative to the positive control demonstrates the inhibitory effect of the aforementioned treatments on OPN gene expression. According to the findings of Jing Sun et al., osteoblasts treated with the MSC-CM group showed decreased cell proliferation from the third to the sixth day, as well as decreased osteocalcin and osteopontin gene expression, and also ALP activity on the third day [24]. In this study, the significant decrease of OPN gene expression in the 50HZ group, as compared to that of the OD+50HZ group, indicated the negative effect of EMF on gene expression. Kaivasojoa, has previously reported that [23], when hBMSCs were treated with PEMF (75 Hz, 1 mT), the expression of osteogenic markers ALP, SMAD1, Runx2, Osteopontin, and Osteocalcin increased in comparison to the control group. Of course, conflicting results have also been reported. As in the current study, PEMF may cause a disorder of osteogenic gene expression or have no effect on that. According to research by KIM et al human BMSCs treated with magnetic nanoparticles and exposed to EMF exhibit significant expression levels of osteoblast markers osteocalcin, osteopontin, and osteonectin [25, 26].
CONCLUSION AND RECOMMENDATIONS
According to the results of the current study, electromagnetic waves or conditioned medium could not affect the expression of the OSX gene; but co-treatment was more effective. Osteogenesis differentiation medium stimulated the expression of OSX and OPN genes. While both EMF and CM concurrently had no effect on the expression of the OPN gene. In this study, the cells differentiated into osteoblasts. The results demonstrated that the OSX gene may be expressed by cells exposed to EMF and CM. The differentiation of immature to mature osteoblasts is caused by the expression of this gene, which is involved in the mineralization (calcification) of osteoblasts. This gene is expressed in both immature and mature osteoblasts. Early phases of osteoblast mineralization (calcification) are influenced by the OPN gene. While EMF and CM had no effect on the expression of the OPN gene. Therefore, it may be a resulted that the differentiated osteoblasts do have not the ability to produce extracellular matrix minerals failed to initiate mineralization, and merely underwent the differentiation stage. Our study showed that simultaneous treatment of EMF and CM has synergistic effects on osteogenic differentiation of rBMSCs. Therefore, the synergistic effect of EMF and CM by the acceleration of bone maturation can be used for improving bone repair. The limitation of this study was the in vitro nature of the research and how it may not fully replicate in vivo conditions.
AUTHORS’ CONTRIBUTION
R.R.: Study conception and design; M.H.G.K.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BMSCs | = Bone Marrow Mesenchymal Stem Cells |
| MSCs | = Mesenchymal Stem Cells |
| IGF-1 | = Insulin-like Growth Factor |
| CM | = Conditioned Medium |
| bFGF | = Basic Fibroblast Growth Factor |
| MSC-CM | = Mesenchymal Stem Cell Conditioned Media |
| PEMF | = Pulsed Electromagnetic Field |
| ALP | = Alkaline Phosphatase |
| OC | = Osteocalcin |
| rBMSCs-CM | = rBMSCs-Conditioned Medium |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research project was approved by ethics committee of the Faculty of Biology, Damghan University, Iran with the code of ethics (IR.DU.REC.1401.003).
HUMAN AND ANIMAL RIGHTS
All the animal experiments were performed in accordance with the Guide for the Care and use of laboratory animals.
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The NC3Rs ARRIVE Guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [M.K] upon reasonable request.


