All published articles of this journal are available on ScienceDirect.
Investigating the Apoptosis Status of Peripheral Lymphocytes in COVID-19 Patients in the Post-COVID-19 Era: A Cross-sectional Study
Abstract
Background
Although COVID-19 has been contained and the world is now in the post-corona era, evidence shows that the coronavirus can still cause hospitalization and even death of patients by inducing cell apoptosis.
Aim
The present study was conducted to investigate the apoptosis status of peripheral lymphocytes in patients with COVID-19 in the post-corona era.
Methods
This cross-sectional study was conducted using a simple random sampling method in December, 2023, by examining the apoptosis level of peripheral lymphocytes in samples taken from 54 COVID-19 patients hospitalized in Velayat Damghan Hospital. Using an RT-PCR test, the nucleic acid of the SARS-CoV-2 virus was identified in all COVID-19 patients. Apoptosis assay was performed using the Annexin V/Propidium Iodide technique. Statistical analysis was performed using GraphPad Prism and inferential statistics tests.
Results
A total of 54 patients with COVID-19 in the age range of 21 to 59 years, of whom 44.4% were hospitalized in the non-ICU department and 55.5% in the ICU department. Among them, 27.7% required intubation, while 27.7% did not need intubation. The highest level of apoptosis in peripheral lymphocytes was observed in the intubated ICU (4.28%) and non-intubated ICU (1.89%) groups. The intubation group showed a significant difference from the non-intubation group (p < 0.01). In all groups, there was a significant increase in the level of apoptosis compared to the healthy control group (p < 0.01).
Conclusion
The increased apoptosis in COVID-19 patients raises concerns about the potential impact on immune function and the development of long-term health complications.
1. INTRODUCTION
Severe Acute Respiratory Syndromes (SARS) in 2002–2003, Middle East Respiratory Syndromes (MERS) in 2012, and unique human illness, COVID-19, in December, 2019, were all caused by coronaviruses, which are RNA viruses [1, 2]. On January 12th, 2020, the World Health Organization (WHO) announced SARS-CoV-2 as the agent of the new coronavirus infectious illness (COVID-2019) [3]. Pathogenic agents can impact more than one organ, and the disease can present as a multisystem, multifactorial, or multiorgan condition [4]. Acute Respiratory Distress Syndrome (ARDS) in COVID-19 patients may present with extra clinical signs [5]. A flow cytometry study may yield crucial information regarding the inflammation or immune system suppression of the patient as well as the disease's progression since the severity of COVID-19 worsens over time and can lead to Acute Respiratory Distress Syndrome (ARDS) in certain extreme cases [6]. People with post-COVID-19 syndrome have reported chronic symptoms, such as weariness, widespread myalgia, and musculoskeletal and joint discomfort, in surveys [7, 8]. All of these symptoms are connected to decreased antioxidant levels, oxidative stress, and dysfunctional mitochondria [9, 10]. The pathogenesis and management of fatigue associated with the COVID-19 pandemic may benefit from a better understanding of the mitochondria's function [11]. Anaerobic glycolysis may occur as a result of defective oxidative phosphorylation in the mitochondria [12]. Cellular damage results from increased glycolysis, which can also impede other metabolic processes and lactate levels [13, 14]. The ability of the virus to avoid the host's immune system by specifically infecting neurons may be the cause of weariness and muscular aches [15, 16]. When infecting neurons, SARS-CoV-2 can enter a state of latency with low replication and infiltrate the host immune system [17]. A cytokine storm and hypercoagulopathy in the infected host may be connected to the activation of inflammasomes, which are innate immune system receptors [18, 19]. As a result, the individual who has post-COVID-19 syndrome may have persistent infections that can recur anytime. Moreover, stress impairs the host's immune system [20]. In order to eradicate the virus, mostly in the respiratory system, the body responds to SARS-CoV-2 with a prolonged, exaggerated inflammatory response [21, 22]. Depending on how long the infection has been present, this inflammation might be advantageous or detrimental [23]. Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) are two important classes of inflammatory stimulation that trigger immune responses [24]. When the SARS-CoV-2 PAMP is detected, antiviral genes are triggered, creating a cellular antiviral state that helps cells contain and eradicate the infection [25]. When SARS-CoV-2 is introduced, neutrophils become activated and release extracellular neutrophil traps, which, in turn, trigger DAMPs [26, 27]. Cells generate DAMPs, especially in the lungs, to signal the innate immune system to initiate cell death [28, 29]. Furthermore, to initiate the inflammatory response, heat shock proteins and high-mobility group Box 1 activate mitogen-activated protein kinases and the nuclear factor kappa light polypeptide gene enhancer in B-cells (NF-κB) [30]. PAMPs and DAMPs attach to receptors in the central nervous system upon activation, stimulating intracellular inflammasomes and the CNS defense system [30, 31]. Inflammasomes cause inflammation in response to pathogenic microorganisms, but persistent or systemic inflammatory disorders can arise from excessive inflammation [32]. According to the hypothesis by Eze and Starkweather (2021) [31], the pathophysiological events that underlie the onset and maintenance of pain, such as the release of proinflammatory mediators by microglia and the activation of inflammasomes, are linked to the symptoms of post-COVID-19 syndrome. In response to different stimuli, such as viral infection, single cells of multicellular organisms engage in systematic self-destruction that ultimately results in apoptosis, a genetically controlled, preprogrammed occurrence. Numerous viruses with various viral gene products have been shown to cause apoptosis both in vivo and in vitro [33, 34]. In this study, we investigated the levels of peripheral blood lymphocyte apoptosis of COVID-19 patients in post-COVID-19 to find out whether it still poses a threat to us, given the reduced mortality rate of this epidemic.
2. METHODS AND MATERIALS
2.1. Controls and Patients
Blood samples were collected from 54 patients (33 men and 21 women) with COVID-19 who were treated in the Velayat Damghan Hospital, affiliated with Semnan University of Medical Sciences, in December, 2023. These patients were classified into 3 different groups: 30 patients admitted to the intensive care unit (15 of them intubated and 15 not intubated), and 24 patients admitted to other departments of the hospital. Moreover, 15 healthy people without COVID-19 disease were controlled. The terms for their arrival and departure are listed below. The observation of clinical symptoms, followed by the collection and analysis of laboratory samples in accordance with the guidelines set forth by Iran's Ministry of Health, Medicine, and Medical Education, proved that individuals were infected with the COVID-19 virus. The following requirements must be met in order to be eligible for participation in the study: receiving written consent to engage in research, being older than eighteen, not having contracted an infectious disease during the previous thirty days of joining the healthy individuals' group, and not being pregnant. The Ministry of Health's new coronavirus guidelines were used to categorize the mild and severe clinical grades. Table 1 compiles the clinical and demographic information of the gathered verified COVID-19 patients. All medical laboratory data was gathered by Velayat Hospital's clinical laboratory. Using an RT-PCR test, the nucleic acid of the SARS-CoV-2 virus was identified in all COVID-19 patients. An earlier description of the comprehensive protocol can be found in the literature [35]. Acute respiratory viral infections, such as COVID-19, were diagnosed using patient-provided oropharyngeal and nasopharyngeal (NP/OP) swabs. RNA from clinical samples was extracted using Chemagic 360 technology (PerkinElmer Inc.). The N1 and N2 genes of the virus linked to COVID-19 were identified using the Viasure SARS-CoV-2 RT-PCR Detection Kit (Certest Biotec SL, Spain) [36].
2.2. Blood Sample Collection
Six milliliters of the patient's blood were drawn and placed in sterile blood tubes using EDTA. From this blood, 200–400 µl was extracted and put into 1.5 ml centrifuge tubes. After ten minutes, the residual EDTA blood was centrifuged at 3000xg.
2.3. Flow Cytometric Analysis
Using an Attune NxT flow cytometer (Thermo Fisher Scientific), two-color flow cytometry was examined. Two rounds of washings were performed on peripheral blood mononuclear cells (PBMCs) using 4-(2-Hydro-xyethyl) piperazine-1-ethane-sulfonic acid) (HEPES buffer (Gibco)). After adding 3 x 105 cells to 50 μl of rebinding buffer, 1 μl of Annexin V-FITC was added, and the mixture was incubated for 15 minutes at room temperature in the dark. After adding and analyzing PI (0.25 μg/ml), the analysis was completed. A total of 40,000 occurrences were gathered. It is believed that late apoptosis is the cause of the population's doubling. Annexin V and PI-positive populations were identified as early necrosis cells and early apoptosis cells, respectively. Data from every sample was examined using Flowjo software (treestar). To stain and identify dead cells, late apoptotic/necrotic cells, early apoptotic cells, and non-apoptotic cells, fluorescein Annexin V-FITC and PI were combined.
2.4. Analytical Statistics
For the statistical analysis and graphs in the study, GraphPad Prism version 8.0 was employed. To characterize the continuous variables with interquartile ranges (IQRs), the mean ± standard deviation, or median, was used. Either a percentage or frequency was used to explain the categorization factors. Based on quantitative data, the Kolmogorov-Smirnov test revealed that it was normal. The Kruskal-Wallis test was used to compare more than two independent parameters. The categorical data was assessed using the chi-square test. To compare the variables, Fisher's exact test was used. A p-value of < 0.01 was regarded as significant. The means of the quantitative variables were compared between groups using an analysis of variance (ANOVA) test.
3. RESULTS
3.1. Results of Clinical and Analytical Examinations of COVID-19 Patients
With a mean age of 56 years, 54 patients with COVID-19 in December, 2023, were examined in this study (Table 1).
In mild and severe instances, clinical symptoms did not differ considerably (Table 1). Fatigue is the most common symptom across all COVID-19 patients (79.6%). Chest pain ranks the highest in the non-ICU group (95.8%), whereas sputum output ranks the lowest (12.5%). Fever (100%) was found to be the most common symptom in the non-intubated ICU group, while diarrhea (6.66%) was the least common symptom. In the group receiving intubation and admitted to the intensive care unit, fatigue accounted for the largest percentage of symptoms (100%) and anorexia (0%). When comparing individuals with COVID-19 in the severe clinical stage to those in the moderate stage, there was a significant rise in the apoptosis percentage of peripheral blood lymphocytes (p < 0.01). Fig. (1) presents the comparison of the parameters of peripheral blood lymphocyte apoptosis across several populations. The rate of apoptosis in peripheral blood lymphocytes was higher in ICU patients, a difference that was statistically significant (p < 0.01) from the healthy control group. In mild cases of COVID-19, the average number of apoptotic cells was significantly lower than in severe cases; this significant difference indicates severe lymphopenia in severe cases (p < 0.01). At several flow cytometry stages, the control group had much lower levels of apoptosis than the intubation group (Fig. 1), and the results demonstrated a significant difference (p < 0.01). The percentage of Annexin V-positive cells in each sample was measured to determine the frequency of apoptosis; Q2 was taken into account for late apoptosis and Q3 for early apoptosis. The comparison graphs (Fig. 1) indicate the proportion of apoptotic cells in patients with COVID-19 in both severe and mild clinical stages in relation to the control group. The findings indicated that as the illness worsens, the proportion of apoptotic cells rises. The fluorescence intensity of Annexin V-FITC/PI in COVID-19 patients in 2023 across various hospital departments is displayed in Fig. (1).
| Number (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | All COVID‐19 Patients (n = 54) | Non-ICU (n = 24) | Non-intubated -ICU (n = 15) | Intubated-ICU (n = 15) | Control (n = 15) | p-value* |
| Age, median (IQR), y Sex Male Female |
56 (21-59) 33 (61.1%) 21 (38.8%) |
47 (21-52) 10 (41.6%) 14 (58.3%) |
53 (41-57) 8 (53.3%) 7 (46.6%) |
58 (38-59) 9 (60%) 6 (40%) |
44 (25-53) 10 (66.6%) 5 (33.3%) |
0.001 0.001 |
| Signs/symptoms Dry cough Chest pain Fatigue Fever Sputum production Diarrhea Vomiting Dyspnea Nausea Headache Anorexia |
35 (64.8%) 41 (75.9%) 43 (79.6%) 22 (40.7%) 17 (31.4%) 23 (42.5%) 32 (59.2%) 20 (37.0%) 33 (61.1%) 40 (74.0%) 10 (18.5%) |
19 (79.1%) 23(95.8%) 15 (62.5%) 5 (20.8%) 3 (12.5%) 20 (83.3%) 18 (75%) 9 (37.5%) 11 (45.8%) 20 (83.3%) 3 (12.5%) |
5 (33.3%) 5 (33.3%) 13 (86.6%) 15 (100%) 6 (40%) 1 (6.66%) 6 (40%) 4 (26.6%) 13 (86.6%) 14 (93.3%) 3 (20%) |
11 (73.3%) 13 (86.6%) 15 (100%) 2 (13.3%) 8 (53.3%) 2 (13.3%) 8 (53.3%) 7 (46.6%) 9 (60%) 6 (40%) 0 (0%) |
0 (0%) 1 (6.66%) 2 (13.3%) 3 (20%) 1 (6.66%) 2 (13.3%) 0 (0%) 0 (0%) 0 (0%) 1 (6.66%) 1 (6.66%) |
0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 |
4. DISCUSSION
The coronavirus family of viruses, which mostly affects bats, has a wide range of hosts. The majority of the time, coronavirus symptoms are minor respiratory illnesses that resemble the common cold. These viruses are called after the Latin word for crown (corona), which refers to their surface spikes that resemble crowns. As SARS-CoV-2 severely damages several organs, it has caused appalling rates of morbidity and mortality worldwide. Although a large number of people are surviving the acute stage of COVID-19, there is growing evidence that the long-term consequences of SARS-CoV-2 infection can have an impact on an individual's quality of life and capacity to resume work. A constellation of chronic symptoms, such as dyspnea, exhaustion, taste and smell loss, cognitive decline, chest discomfort, and arthralgia, is frequently present [37]. By starting the innate immune response and producing inflammatory cytokines and a procoagulant state, the SARS-CoV-2 infection damages cells. These symptoms may appear more than four weeks after the infection's start and may be new, persistent, or recurrent. These symptoms can appear in people with post-COVID-19 illnesses at different stages of acute infection (Centers for Disease Control and Prevention, 2021). Many names for post-COVID-19 problems have been proposed in the literature, including post-acute COVID-19 syndrome, chronic COVID-19, long-term COVID-19, long-term COVID-19, and post-COVID-19 syndrome. These phrases, along with others, signify that the individual has not recovered to their pre-COVID-19 state of health. In this article, “post-COVID-19 syndrome” is used for consistency and clarity. Numerous researchers are looking at the origins of these symptoms, as well as the reasons behind, timing, and methods of treating them [34, 37]. Scientists and physicians have put out a wide range of general pathophysiological theories for post-COVID-19 syndrome. The intensity of the sickness and the affected organs are among these processes, along with the immunologic abnormalities and inflammatory damage, pathophysio- logical alterations unique to the virus, and oxidative stress. Notwithstanding numerous theories and research indicating mechanisms of action, the precise pathophysio- logical mechanism of post-COVID-19 syndrome remains unknown. Understanding the demands for patient care after the acute phase of the COVID-19 pandemic is crucial, especially in light of the mechanisms resulting in persistent symptoms more than three or four weeks after the onset of severe disease [37, 38]. The processes of lymphocyte cell pathogenesis and apoptosis caused by harmful infectious viruses like COVID-19 are still mostly unclear.
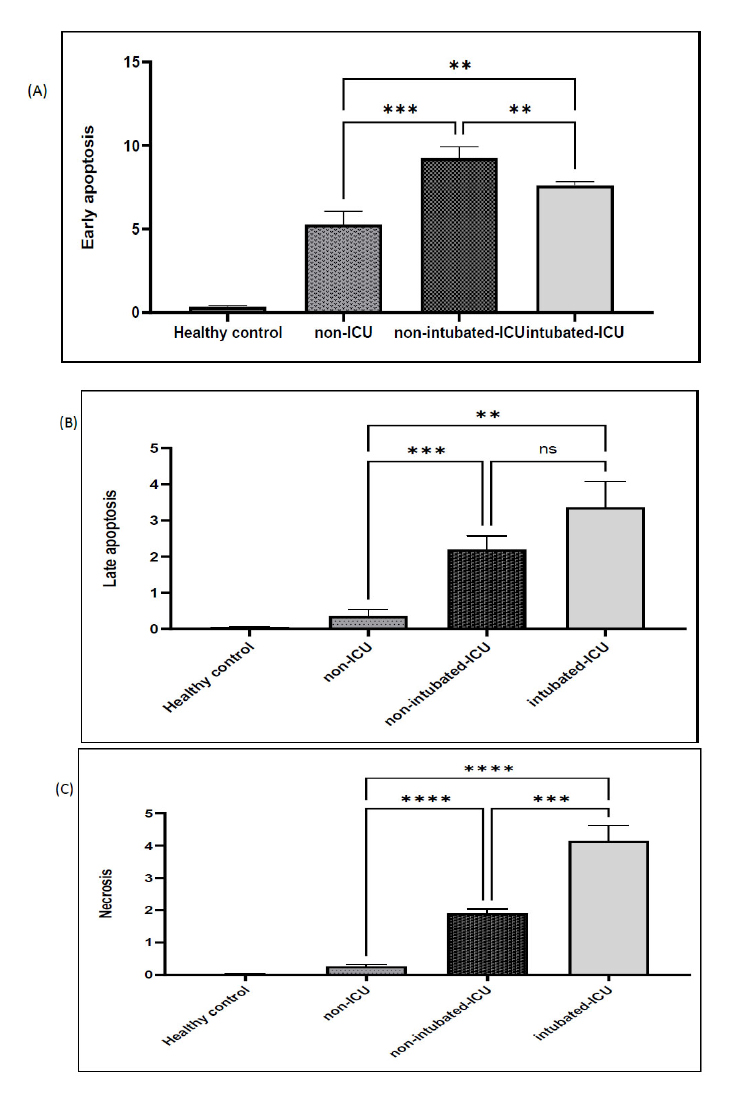
The average proportion of apoptotic cells found in all COVID-19 patients in 2023 is displayed. Significant differences (p < 0.01) between mild and severe cases are indicated by an asterisk (*). (Col: one-way ANOVA).
CONCLUSION AND RECOMMENDATIONS
This study found that COVID-19 patients, especially those requiring intubation, exhibited significantly higher apoptosis levels compared to healthy controls. This suggests that severe infection triggers excessive cell death, contributing to immune dysregulation and disease severity. Elevated apoptosis may impair immune function, increasing vulnerability to infections and long-term health issues like chronic inflammation. The findings highlighted the need for targeted therapies to regulate apoptosis, early identification of high-risk patients, and further research into the underlying mechanisms to improve treatment strategies and outcomes.
RECOMMENDATIONS
A few recommendations are as follows:
1. Further research: Investigate the specific pathways and mechanisms involved in the increased apoptosis observed in COVID-19 patients. This will help to develop targeted therapies to modulate the immune response and potentially reduce the severity of the disease.
2. Develop diagnostic tools: Develop diagnostic tools that can accurately assess the level of apoptosis in peripheral blood lymphocytes in COVID-19 patients. This will enable early detection of immune dysregulation and facilitate timely interventions.
3. Implement clinical trials: Conduct clinical trials to evaluate the efficacy of potential therapies aimed at reducing apoptosis and improving immune function in COVID-19 patients.
4. Promote public health measures: Continue to promote public health measures, such as vaccination, mask-wearing, and social distancing, to mitigate the spread of COVID-19 and reduce the burden on healthcare systems.
5. Monitor long-term health consequences: Conduct long-term follow-up studies to assess the long-term health consequences of COVID-19, including the potential impact of immune dysregulation on long-term health outcomes.
LIMITATIONS AND STRENGTHS OF THE STUDY
This study only included patients hospitalized in the Velayat Damghan Hospital. As a result, caution should be exercised when extrapolating the findings. To improve the precision and accuracy of COVID-19 disease charac- terization, conducting comprehensive and prolonged studies is advisable. This research was carried out cross-sectionally, which makes it difficult to draw conclusions about causality. Considering the mentioned limitations, it is suggested that future studies be conducted with a larger sample size and in different hospitals so that the findings are more reliable.
AUTHORS’ CONTRIBUTION
E.A.: Study conception and design; M.H.G.: Data collection; R.R.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SARS | = Severe Acute Respiratory Syndromes |
| WHO | = World Health Organization |
| ARDS | = Acute Respiratory Distress Syndrome |
| PAMPs | = Pathogen-Associated Molecular Patterns |
| DAMPs | = Damage-Associated Molecular Patterns |
| PBMCs | = Peripheral Blood Mononuclear Cells |
| IQRs | = Interquartile Ranges |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Biomedical Research Ethics Committee of Damghan Islamic Azad University, Iran which issued the study’s code of ethics (IR.IAU. DAMGHAN.REC.1400.005).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.


