All published articles of this journal are available on ScienceDirect.
Pesticide Exposure and Cancer Risk: A Cross-sectional Study on Farmers in Nakhon Sawan, Thailand
Abstract
Introduction
Cancer is a leading cause of disease burden worldwide. Although much of the existing literature, primarily from Western countries, suggests an association between pesticide exposure and cancer risk, these findings may not be directly applicable to the Thai population. This is due to differences in social, economic, and public health contexts, as well as variations in health status, pesticide use patterns, and self-protection behaviors. This study aimed to investigate the relationship between pesticide exposure and cancer risk among Thai farmers. The findings are expected to contribute valuable insights for public health prevention and control programs, as well as enhance the existing body of literature.
Methods
In this cross-sectional study, data were collected from 10,646 farmers aged 20 years and older through in-person interviews using a structured questionnaire. The association between pesticide exposure and cancer was analyzed using logistic regression, adjusting for potential confounding factors.
Results
The study identified a significant association between the historical use of pesticides, including insecticides, herbicides, and fungicides, and cancer risk. Among 39 individual pesticides examined, sixteen were found to have a significant odds ratio. The study’s results aligned with existing literature regarding the potential effects of glyphosate, paraquat, 2,4-D, folidol, chlorpyrifos, EPN, mevinphos, dichlorvos, endosulfan, dieldrin, mancozeb, maneb, and copper sulfate. Additionally, this study newly identified a significant association with propineb (OR = 2.41, 95% CI: 1.39–4.17), carbendazim (OR = 1.77, 95% CI: 1.11–2.81), and benomyl (OR = 4.04, 95% CI: 1.69–9.63).
Conclusion
The study found cancer prevalence among farmers in Nakhon Sawan, Thailand, to be associated with historical pesticide use. These findings aligned with existing literature, underscoring the potential effects of long-term pesticide exposure on cancer risk. This issue warrants increased public attention and stricter regulation of pesticide use. An effective exposure prevention program should be urgently implemented, particularly for Thai farmers.
1. INTRODUCTION
In 2023, global pesticide use averaged 3.7 million tons, with a significant increase observed since 2000, both in Thailand and worldwide [1]. Thailand, an agricultural nation, has 12 million individuals registered as farmers [2]. To boost agricultural productivity, a substantial number of chemical pesticides have been employed. In 2023, the country imported approximately 140 million tons of agricultural hazardous substances, primarily herbicides, insecticides, and fungicides [3]. Research has shown that pesticides affect humans, animals, and the environment through both direct and indirect exposure. The health impacts of pesticide exposure can be both short-term and long-term, with risks including neurological disorders and various types of cancer, such as lung cancer, liver cancer, breast cancer, and leukemia [4, 5].
Studies conducted in the United States, Canada, and Sweden have found an association between glyphosate use and an increased incidence of lymphoma, immune system cancers [6], and acute myeloid leukemia (AML) [7]. The Agricultural Health Study (AHS) in Iowa and North Carolina reported that the fungicide metalaxyl and the organochlorine insecticide lindane were linked to an increased risk of thyroid cancer [8]. In North India, a retrospective study reported a higher prevalence of bladder cancer among farmers who used chlorpyrifos and cypermethrin [9]. In China, researchers found a link between organochlorine pesticides and breast cancer [10]. Additionally, DDT, a widely used organochlorine pesticide, has been associated with hepatocellular carcinoma [11].
Given the differences in socioeconomic status, health services, pesticide use, and preventive behaviors among farmers, more studies, particularly from developing nations, are needed. Cancer is a leading cause of disease burden in Thailand, with over ten thousand new cases reported annually [12]. This study aimed to investigate the relationship between pesticide exposure and cancer risk. The study participants were farmers from Nakhon Sawan, one of the provinces in Thailand with the highest cancer incidence and pesticide usage rates. This study analyzed both groups of chemicals and individual substances for potential associations. The results will provide valuable insights into cancer prevention efforts in Thailand and contribute to the global body of literature on this important issue.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
This cross-sectional study was conducted among farmers in Nakhon Sawan, Thailand. The province is located approximately 250 km north of Bangkok and has a population of 1,066,455 people and 401,432 households distributed across 15 districts (data from 2016). The majority of the population are farmers, with rice, sugarcane, and cassava being the main crops. In 2017, the province had a gross domestic product (GDP) of 21,852 THB (716 USD) [13].
2.2. Study Participants and Sampling Procedure
The study collected data from 10,646 participants who were farmers aged 20 years or older and had been working as farmers for at least five years. Participants were selected using a multistage sampling procedure. First, three districts were purposively selected based on the number of farmers and the types of crops grown. These districts represent areas where all three of the province’s main crops, rice, sugarcane, and cassava, are cultivated.
Within each district, at least three sub-districts were randomly selected to recruit approximately 3,000 participants. In each sub-district, 1,000 to 1,200 farming families, representing 30-100% of all farmer families in the area, were included in the study. With the support of local authorities, hospitals, and village health volunteers (VHVs), farming families were identified and contacted for data collection. In each family, only one adult farmer was interviewed, with the family head or their spouse given priority as the participant.
Data on pesticide use and diseases were collected through face-to-face interviews conducted by village health volunteers (VHVs). These VHVs attended a one-day training session on how to properly interview participants and use the online questionnaires. Most of the interviews were conducted in the participants' homes. Data collection took place between October 2020 and February 2021.
The minimum sample size was calculated to be 9,847 based on the following assumptions: a significance level of 95%, a power of 80%, an unexposed-to-exposed ratio of 1:1, a 5% outcome rate among the unexposed, and an odds ratio of 1.2. In this context, ‘exposed’ refers to individuals who have ever used pesticides for agricultural purposes in their lifetime, while ‘unexposed’ refers to those who have never used pesticides.
2.3. Data Collection and Questionnaire
In this study, data were collected using a questionnaire developed in our previous research [14]. The questionnaire consisted of three major sections. Part I gathered demographic information, including gender, age, marital status, completed education, and monthly income, as well as details on cigarette smoking and alcohol consumption. The second part focused on pesticide use, which is defined as the mixing or spraying of any pesticide for agricultural purposes. Pesticides were categorized into insecticides, herbicides, and fungicides. The study collected data on 39 specific pesticides, including 22 insecticides, 8 herbicides, and 9 fungicides. These chemicals are commonly used in Thailand and have been previously reported in the literature as being linked to cancer. The questions were of the 'yes' or 'no' type. Additionally, information on the number of days per year and the number of years pesticides were used was collected to assess the frequency of exposure.
Exposure was quantified by multiplying the number of days per year by the number of years pesticides were used. The total lifetime exposure days were calculated as (number of days per year) × (number of years of pesticide use). To reduce recall bias, all specific pesticide names, including scientific, common, and trade names, were read to participants during the interview process.
In the third section, participants were asked whether they had received a medical diagnosis of cancer, using a ‘yes’ or ‘no’ question. This information was then verified against disease records coded according to ICD-10 (C00-D48) from local hospitals. Only cases confirmed through these records were included in the data analysis. Confirmation of data was performed by health staff from the local hospitals.
2.4. Statistical Analysis
Demographic data were summarized using fre- quencies, percentages, means, and standard deviations, and comparisons were made using the Chi-square test. The associations between pesticide exposure and cancer risk were analyzed using multivariable logistic regression, adjusting for potential confounding factors. The adjusted variables included in the model were gender (male, female), age (continuous), marital status (married, single, divorced/widowed/separated), education completed (none, primary school, secondary school, college degree or higher), monthly income (<5000 THB, 5001-10000 THB, 10001-30000 THB, >30000 THB), cigarette smoking (non-smoker, ex-smoker, current smoker), and alcohol consumption (non-drinker, ex-drinker, regular drinker). Both crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Due to the limited number of cancer cases, environmental pesticide exposure was not included in the analysis. For comparative analysis, p-values less than 0.05 were considered statistically significant. All data were analyzed using SPSS version 19.
3. RESULTS
The number of female participants slightly out- numbered the male participants. Among those diagnosed with cancer, approximately 74% were women (Table 1). Over 80% of the study participants were aged over 40, with a median age (IQR) of 55 years. The cancer group was slightly older than the non-cancer group. This is typical for diseases with long latent periods, such as cancer. Most participants were married, had completed primary school, and had a monthly income of less than 10,000 THB. The cancer group also had slightly lower rates of cigarette smoking and alcohol consumption. This may be due to increased health awareness among individuals with the disease. Among 10,646 participants, the study found 173 cancer cases (1.63%). Most of them had breast cancer (51 cases, 27.4%), lung/bronchial cancer (35 cases, 20.2%), and cervical/uterine/ovarian cancer (25 cases, 14.5%) (Table 2).
| Characteristics |
Non-cancer, N (%) N=10473 |
Cancer, N (%) N=173 |
p-value |
|---|---|---|---|
| Gender | - | - | <0.001* |
| Male | 4879 (46.6) | 49 (28.3) | - |
| Female | 5594 (53.4) | 124 (73.7) | - |
| Age, years | - | - | 0.012* |
| < 40 | 1420 (13.6) | 10 (5.8) | - |
| 41-60 | 5510 (52.6) | 100 (57.8) | - |
| >60 | 3543 (33.8) | 63 (36.4) | - |
| Median (IQR) 55 (47- 64) | - | - | - |
| Marital Status | - | - | 0.114 |
| Married | 7883 (75.3) | 132 (76.3) | - |
| Single | 984 (9.4) | 9 (5.2) | - |
| Divorce/widow/separate | 1606 (15.3) | 32 (18.5) | - |
| Education | - | - | 0.143 |
| Non-education | 426 (4.1) | 11 (6.4) | - |
| Primary school | 7775 (74.2) | 135 (78.0) | - |
| Secondary school | 2100 (20.1) | 25 (14.5) | - |
| College degree or higher | 172 (1.6) | 2 (1.1) | - |
| Income (THB per month) | - | - | 0.097 |
| <5000 | 4123 (39.4) | 64 (37.0) | - |
| 5001-10000 | 5098 (48.7) | 79 (45.7) | - |
| >10000 | 12523 (11.9) | 30 (17.3) | - |
| Cigarette smoking | - | - | <0.001* |
| Non-smoker | 8453 (80.7) | 157 (90.8) | - |
| Ex-smoker | 615 (5.9) | 10 (5.8) | - |
| Current smoker | 1405 (13.4) | 6 (3.4) | - |
| Alcohol consumption | - | - | <0.002* |
| Non-drinker | 7606 (72.6) | 145 (83.8) | - |
| Ex-drinker | 993 (9.5) | 14 (8.1) | - |
| Regular-drinker | 1874 (17.9) | 14 (8.1) | - |
| Cancer type | Number (%), N= 173 |
|---|---|
| Breast cancer | 51 (27.4) |
| Lung/bronchial cancer | 35 (18.8) |
| Cervical/uterine/ovarian cancer | 25 (13.4) |
| Colon/stomach/esophagus/pancreas cancer | 24 (12.9) |
| Liver/bile duct cancer | 11 (5.9) |
| Leukemia | 7 (3.8) |
| Brain cancer | 7 (3.8) |
| Lymphoma | 6 (3.2) |
| Other types (prostate, bladder, thyroid, skin, soft tissue) | 20 (10.8) |
| Pesticide |
Non-cancer N=10473 (%) |
Cancer N=173 (%) |
OR (Crude) (95% CI) |
OR (Adjusted)* (95% CI) |
|---|---|---|---|---|
| Pesticide | 9003 (86.0) | 160 (92.5) | 2.00 (1.13-3.54) | 2.05 (1.16-3.64) |
| Insecticide | 5902 (56.4) | 123 (71.1) | 1.90 (1.36-2.65) | 1.94 (1.39-2.72) |
| Herbicide | 8710 (83.2) | 155 (89.6) | 1.74 (1.06-2.84) | 1.82 (1.11-2.98) |
| Fungicide | 3272 (31.2) | 61 (35.3) | 1.19 (0.87-1.64) | 1.22 (0.89-1.69) |
| Rodenticide | 2043 (19.5) | 35 (20.2) | 1.04 (0.71-1.52) | 1.03 (0.71-1.51) |
| Molluscicides | 2479 (23.7) | 45 (26.0) | 1.13 (0.80-1.59) | 1.09 (0.77-1.56) |
The study found that cancer prevalence among the participants was significantly associated with their historical use of various groups of pesticides, including any pesticide (OR = 2.05, 95% CI 1.16-3.64), insecticide (OR = 1.94, 95% CI 1.39-2.72), and herbicide (OR = 1.82, 95% CI 1.11-2.98) (Table 3).
For individual pesticides, significant adjusted associations were observed for most of the pesticides surveyed in this study. Among insecticides, significant ORs were observed for the organophosphates folidol, chlorpyrifos, EPN, mevinphos, and dichlorvos, as well as for the organochlorines endosulfan and dieldrin (Table 4). Fig. 1 shows the adjusted odds ratios (OR) with 95% confidence intervals (CI) for all individual pesticides analyzed in this study. Significant odds ratios (OR) were found for the herbicides glyphosate, paraquat, and 2,4-D. Significant associations were also noted for most of the fungicides analyzed, including mancozeb, maneb, propineb, carbendazim, benomyl, and copper sulfate.
4. DISCUSSION
The results of this study indicate a strong association between pesticide exposure and cancer. Specifically, the risk of cancer is significantly predicted by the historical use of various types of pesticides, including insecticides and herbicides. Of the 39 individual pesticides surveyed, sixteen also showed a significant odds ratio (OR). These findings support existing literature on the cancer risk associated with pesticide exposure. A report by the Toxics Action Center and Pesticide Watch highlighted that pesticide exposure can lead to various types of human cancers [15]. A study on Brazilian farmers found that those exposed to pesticides were 2.9 times more likely to develop cancer compared to non-exposed farmers [16]. Similarly, a study from Iowa, USA, found that farmers using insecticides had a 1.37 times higher risk of developing cancer [17]. Another study found that agricultural workers in Italy and Brazil who had used any pesticide were at a higher risk of developing cutaneous melanoma [18].
For organophosphate (OP) insecticides, the study found significant odds ratios (ORs) for folidol, chlorpyrifos, EPN, mevinphos, and dichlorvos. These findings are consistent with a study from North India, which reported a higher prevalence of bladder cancer among farmers using OP insecticides [9]. Chlorpyrifos exposure has also been linked to an increased risk of breast cancer, kidney cancer, and prostate cancer [8, 19, 20]. Previous studies have found that dichlorvos exposure is associated with various health effects, including cancer [21, 22]. OP insecticides can cause cancer through various mechanisms. They induce the production of free radicals, promoting lipid peroxidation, which subsequently causes DNA damage [23, 24]. Additionally, these pesticides exhibit endocrine-disrupting properties, significantly increasing the likelihood of hormone-related cancers such as breast and prostate cancer [8, 25]. Among the specific pesticides, folidol has been shown to induce cellular abnormalities at the molecular level, while chlorpyrifos is both mutagenic and carcinogenic in various organs [26, 27].
| Pesticide |
Non-cancer N (%) (N=10473) |
Cancer N(%) (N=173) |
OR (Crude) (95% CI) |
OR (Adjusted) * (95% CI) |
|---|---|---|---|---|
| HERBICIDE | - | - | - | - |
| Non-selective herbicides | - | - | - | - |
| Glyphosate | 8710 (83.2) | 155 (89.6) | 1.74 (1.06-2.84) | 1.82 (1.11-2.98) |
| Paraquat | 3459 (33.0) | 70 (40.5) | 1.37 (1.01-1.87) | 1.38 (1.01-1.90) |
| Diuron | 249 (2.4) | 6 (3.5) | 1.47 (0.64-3.36) | 1.46 (0.63-3.37) |
| Selective herbicides | - | - | - | - |
| 2,4 D | 4690 (44.8) | 95 (54.9) | 1.50 (1.11-2.03) | 1.56 (1.15-2.12) |
| Butachlor | 1762 (16.8) | 32 (18.5) | 1.12 (0.76-1.65) | 1.12 (0.75-1.66) |
| Alachlor | 633 (6.0) | 15 (8.7) | 1.47 (0.86-2.52) | 1.54 (0.90-2.65) |
| Acetochlor | 228 (2.2) | 5 (2.9) | 1.33 (0.54-3.28) | 1.40 (0.56-3.47) |
| Ametryn | 315 (3.0) | 7 (4.0) | 1.35 (0.63-2.92) | 1.34 (0.62-2.91) |
| INSECTICIDE | - | - | - | - |
| Organophosphates | - | - | - | - |
| Folidol | 720 (6.9) | 30 (1.3) | 2.84 (1.90-4.24) | 2.87 (1.91-4.32) |
| Methomyl | 587 (5.6) | 11 (6.4) | 1.14 (0.61-2.11) | 1.10 (0.58-2.07) |
| Methamidophos | 228 (2.2) | 5 (2.9) | 1.33 (0.54-3.28) | 1.36 (0.55-3.38) |
| Monocrotophos | 211 (2.0) | 4 (2.3) | 1.15 (0.42-3.13) | 1.15 (0.42-3.17) |
| Chlorpyrifos | 1909 (18.2) | 47 (27.2) | 1.67 (1.19-2.34) | 1.75 (1.24-2.47) |
| EPN | 151 (1.4) | 6 (3.5) | 2.45 (1.07-5.63) | 2.41 (1.04-5.58) |
| Mevinphos | 84 (0.8) | 5(2.9) | 3.68 (1.47-9.19) | 4.00 (1.57-10.13) |
| Dicrotophos | 235 (2.2) | 5 (2.9) | 1.29 (0.52-3.18) | 1.33 (0.54-3.32) |
| Dichlorvos | 82 (0.8) | 4 (2.3) | 2.99 (1.08-8.27) | 3.31 (1.18-9.28) |
| Organochlorine | - | - | - | - |
| Endosulfan | 1263 (12.1) | 35 (20.2) | 1.84 (1.27-2.69) | 1.84 (1.24-2.71) |
| Dieldrin | 70 (0.7) | 4 (2.3) | 3.51 (1.26-9.74) | 3.72 (1.32-10.47) |
| Aldrin | 15 (0.1) | 1 (0.6) | 4.05 (0.53-30.85) | 3.80 (0.60-38.13) |
| DDT | 500 (4.8) | 9 (5.2) | 1.09 (0.55-2.15) | 1.02 (0.51-2.02) |
| Chlordane | 139 (1.3) | 3 (1.7) | 1.31 (0.41-4.15) | 1.52 (0.47-4.86) |
| Heptachlor | 2413 (23.0) | 40 (23.1) | 1.00 (0.70-1.43) | 1.00 (0.70-1.44) |
| Carbamates | - | - | - | - |
| Carbaryl | 355 (3.4) | 10 (5.8) | 1.74 (0.91-3.34) | 1.83 (0.95-3.51) |
| Carbosulfan | 1003 (9.6) | 18 (10.4) | 1.09 (0.67-1.79) | 1.10 (0.67-1.81) |
| Carbofuran | 936 (8.9) | 21 (12.1) | 1.40 (0.88-2.23) | 1.35 (0.84-2.18) |
| Pyrethroids | - | - | - | - |
| Permethrin | 1057 (10.1) | 23 (13.3) | 1.36 (0.87-2.12) | 1.37 (0.87-2.15) |
| Avermectins | - | - | - | - |
| Abamectin | 4586 (43.8) | 88 (40.9) | 1.32 (0.98-1.79) | 1.35 (0.99-1.83) |
| Neonicotinoids | - | - | - | - |
| Imidacloprid | 217 (2.1) | 6 (3.5) | 1.69 (0.74-3.87) | 1.60 (0.69-3.69) |
| Mixed Organophosphates | - | - | - | - |
| Profenofos | 218 (2.1) | 5 (2.89) | 1.40 (0.56-3.44) | 1.44 (0.58-3.59) |
| FUNGICIDE | - | - | - | - |
| Dithiocarbamate Fungicide | - | - | - | - |
| Mancozeb | 506 (4.8) | 17 (9.8) | 2.14 (1.29-3.56) | 2.28 (1.34-3.87) |
| Maneb | 393 (3.7) | 16 (9.2) | 2.61 (1.54-4.41) | 2.77 (1.61-4.76) |
| Propineb | 417 (4.0) | 15 (8.7) | 2.28 (1.33-3.92) | 2.41 (1.39-4.17) |
| Thiophanate | 105 (1.0) | 2 (1.2) | 1.15 (0.28-4.71) | 1.15 (0.27-4.76) |
| Benzimidazole Fungicide | - | - | - | - |
| Metalaxyl | 3272 (31.2) | 61 (35.3) | 1.19 (0.87-1.64) | 1.22 (0.89-1.69) |
| Benomyl | 85 (0.8) | 6 (3.5) | 4.39 (1.89-10.19) | 4.04 (1.69-9.63) |
| Carbendazim | 820 (7.8) | 22 (12.7) | 1.71 (1.09-2.69) | 1.77 (1.11-2.81) |
| Inorganic Fungicide | - | - | - | - |
| Copper Sulphate | 543 (5.2) | 17 (9.8) | 1.99 (1.19-3.31) | 1.94 (1.14-3.29) |
| Bordeaux Mixture | 37 (0.3) | 2 (1.2) | 3.29 (0.78-13.79) | 3.22 (0.75-13.69) |
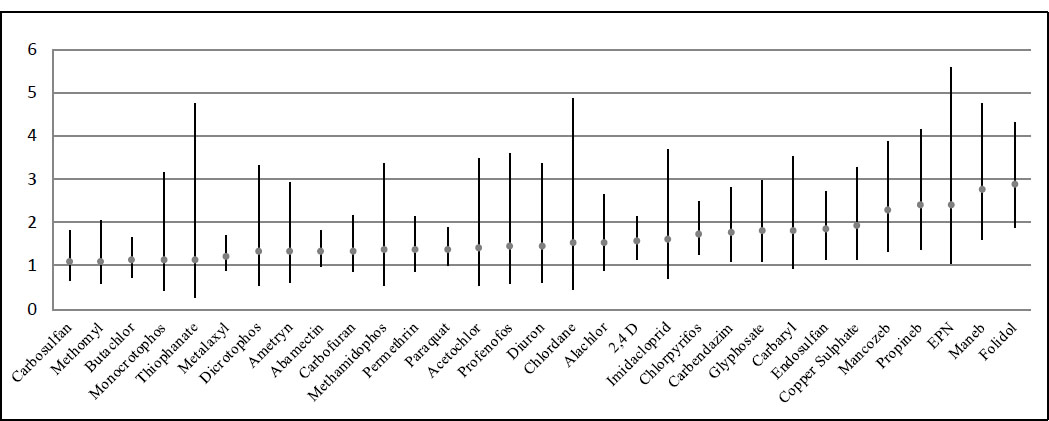
Adjusted odds ratio with 95% confident intervals for individual pesticides.
In this study, two organochlorine (OC) insecticides, endosulfan and dieldrin, showed a significant odds ratio (OR) with cancer. Previous research has found that endosulfan exposure increases the risk of thyroid cancer by 1.84 times [28]. In the United States, the upward trend in thyroid cancer incidence from 1999 to 2019 was significantly correlated with endosulfan use [28]. Regarding dieldrin, prior studies have linked its exposure to an increased risk of breast cancer [10, 29]. These findings may be explained by the fact that OC pesticides can induce the production of free radicals, leading to cellular DNA damage and contributing to the formation and growth of cancer cells [22, 30, 31]. Furthermore, OC exposure can cause chronic inflammation, a key factor in cancer development. OC pesticides also function as endocrine-disrupting chemicals (EDCs), which can further facilitate cancer progression [32]. They competitively bind to thyroid transport proteins, disrupt hormone signaling and transport, and contribute to thyroid gland abnormalities and tumorigenesis [33-36]. Endosulfan, in particular, has been associated with various organ abnormalities, including carcinoma and liver cancer [11, 37-39].
This study found significant associations between the herbicides glyphosate, paraquat, and 2,4-D and cancer (Table 4). In the United States, Gabriella Andreotti (2020) reported that farmers exposed to paraquat had a higher risk of developing renal cell carcinoma, a type of kidney cancer, and thyroid cancer [19]. Additionally, 2,4-D was identified as a potential carcinogen. A study using data from North America, the European Union, and Australia found that 2,4-D exposure was linked to specific subtypes of non-Hodgkin lymphoma (NHL), with those exposed for over six years at an increased risk for T-cell lymphoma [40]. Glyphosate has consistently been associated with various cancers, particularly non-Hodgkin lymphoma (NHL) [41, 42]. Paraquat is known to cause tissue irritation and is classified as a carcinogenic substance due to its ability to induce the proliferation of cancer cells [19, 43].
This study also found significant odds ratios (ORs) for most of the fungicides analyzed, including mancozeb, maneb, propineb, carbendazim, benomyl, and copper sulfate (Table 3). These findings are consistent with, and extend, the existing literature that reports carcinogenic risks associated with mancozeb, maneb, propineb, benomyl, and copper sulfate [18, 36, 44-46]. Laboratory studies have shown that dithiocarbamate fungicides can break down into ethylenethiourea (ETU), a mutagenic agent capable of damaging DNA and causing genetic alterations in cells [47]. Exposure to mancozeb has been linked to an increased risk of thyroid cancer and cutaneous melanoma [18, 36]. Studies have also found that maneb significantly increases the risk of cutaneous melanoma [18, 44, 48]. Previous studies have found that benomyl exposure increases the risk of breast cancer among female farmers [45]. Additionally, copper sulfate has been associated with increased mortality from all cancers, particularly stomach cancer [46]. In conclusion, exposure to fungicides can significantly increase cancer risk.
This study has several strengths, particularly its focus on a developing country, which allows for comparisons with existing literature, most of which originates from Western countries. The use of a large sample size, with a high number of participants who have used pesticides, enabled the identification of additional individual chemicals that are significantly associated with cancer. However, the study also has limitations. The cross-sectional design means that exposure and disease data were collected simultaneously, making it impossible to establish causality. Despite the large sample size, the number of cancer cases was still relatively small, which limits the study's power to detect true associations. Additionally, the use of lifetime historical exposure data may introduce recall bias. However, this type of information bias, if it occurs, will only dilute the observed effects. Nevertheless, in a population study, a questionnaire method might be the most practical approach. The study is also subject to biases from other potential confounding factors that were not controlled for, including genetic predispositions, exposure to other sources of pollution, or other pesticides. With limited data, further analysis is not possible.
CONCLUSION
In summary, the findings from this study support the existing literature on the potential effects of pesticides on cancer development. The study found that insecticides, herbicides, fungicides, and sixteen individual pesticides were significantly associated with cancer prevalence among farmers in Thailand. These results highlight the urgent need for stringent pesticide regulations and effective exposure protection measures for farmers, particularly in Thailand. Further research on this issue, along with the development of health- and environmentally-friendly pesticides, is essential.
AUTHORS’ CONTRIBUTION
A.V., C.J., and C.S.: Made substantial contributions to the conception and design of the study. K.J.: Supported data analysis. A.V., C.J., and C.S.: Wrote the first draft. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| 2,4-D | = 2,4-dichlorophenoxyacetic acid |
| AML | = Acute myeloid leukemia |
| EDCs | = Endocrine-disrupting chemicals |
| EPN | = Ethyl p-nitrophenyl thionobenzenephosphonate |
| ICD-10 | = International Classification of Diseases, Tenth Revision |
| NHL | = Non-Hodgkin Lymphoma |
| OC | = Organochlorine insecticides |
| OP | = Organophosphate insecticides |
| VHV | = Village health volunteer |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Board of Naresuan University, Thailand (project number 550/60).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from each participant prior to the interview process.
AVAILABILITY OF DATA AND MATERIAL
We preferred not to deposit the raw data. The data will be made available upon request through the corresponding author [C.S].
FUNDING
The study received funding support by The National Science Research and Innovation Fund (NSRF) of Thailand, Awards/Grant number: R2563B009.
ACKNOWLEDGEMENTS
First, the authors extend their gratitude to the study participants for their invaluable information and contributions. They also thank the village health volunteers for their assistance with data collection. The authors greatly appreciate the support provided by the local hospitals and the Nakhon Sawan Provincial Public Health Office.


