All published articles of this journal are available on ScienceDirect.
A Challenging Late Transcatheter Patent Ductus Arteriosus (PDA) Closure in Pediatric Populations: A Case Report
Abstract
Background
The treatment of Patent Ductus Arteriosus (PDA) in adults and adolescents can be challenging. However, percutaneous transcatheter closure has been proven to be a safe and effective approach in these populations. Devices, such as the Amplatzer Duct Occluder, are routinely used despite the limited number of related studies. This study aims to analyze the success of transcatheter PDA closure and post-procedure follow-up in two case reports. We also review our experience with this technique.
Case Presentation
We managed two patients with large PDA: one was a newly diagnosed case presenting with chest pain and dyspnea with normal pulmonary pressure (12/7 mmHg), while the other one was a chronic case with Eisenmenger syndrome that had been medically managed for years as it was inoperable. This patient was recently admitted to our department with palpitations and hyperdynamic heart, showing subsystemic pulmonary artery pressure (91/53 mmHg).
The successful transcatheter closure of a significant PDA in such cases may effectively eliminate the dangerous combination of large PDA shunt and pulmonary hypertension in this high-mortality, high-morbidity risk population.
Discussion
Immediate post-closure pulmonary artery pressure measurements, performed using specialized devices (8-10mm Lifetech and 12mm VSD muscular Memoporr), showed no residual PDA. Serial follow-up examinations demonstrated complete resolution of symptoms. Thus, while PDA progression varies between cases, each patient requires individualized evaluation and management.
Conclusion
Transcatheter PDA closure (TCPC) in adults presents technical challenges; however, both patients in our series successfully underwent the procedure without complications and demonstrated sustained reductions in pulmonary artery pressure during follow-up monitoring.
1. INTRODUCTION
The Patent Ductus Arteriosus (PDA) represents an abnormal vascular condition that is typically diagnosed and managed during early childhood [1, 2]. Echocardiography serves as a valuable predictive tool for assessing the hemodynamic significance of PDA [3, 4], while clinical judgment remains essential in management decisions. Transcatheter PDA closure (TCPC) has emerged as the preferred treatment approach for both pediatric and adult populations [5, 6]. Nevertheless, uncertainties persist regarding optimal patient selection and procedural timing due to conflicting safety data [7, 8]. Treatment strategies, including observation for spontaneous closure, COX inhibitor therapy, surgical ligation, or TCPC, are primarily determined by shunt volume [9, 10]. Adult PDA cases present unique technical challenges due to potential calcification, tissue friability, tortuosity, aneurysm formation, and ductal shortening [11, 12]. While percutaneous transcatheter closure has demonstrated safety and efficacy in infants, current evidence regarding its application in adolescent and adult populations remains limited [13, 14]. Transcatheter closure has emerged as the treatment of choice for Patent Ductus Arteriosus (PDA) in pediatric patients, demonstrating excellent success rates and favorable safety profiles [15, 16]. While generally safe, late complications may rarely manifest years post-procedure [17]. This case report illustrates a complex late transcatheter PDA closure in a child, underscoring the technical challenges that may develop in such situations [17, 18]. As one of the most prevalent congenital heart defects, PDA constitutes 5-10% of all congenital cardiac anomalies [19, 20]. The transcatheter approach has become the gold standard for PDA closure across all age groups, achieving complete occlusion rates surpassing 98% at one-year follow-up [3, 21]. The procedure maintains a low complication rate (<1%), with potential adverse events, including device embolization, hemolysis, arrhythmias, and endarteritis [22-24]. Although luminal stenosis of the left pulmonary artery or aorta occurs more frequently in young children, this complication remains uncommon in adult populations [25, 26].
Most complications arise either during device deployment or in the immediate post-procedural period, with delayed complications representing a clinical rarity [13]. Nevertheless, case reports have documented serious late sequelae, such as aortic dissection developing years after initial implantation [27, 28]. These delayed complications present unique management dilemmas for interventional cardiologists, as they must address both altered anatomy and the presence of previously deployed devices during subsequent interventions [13, 22, 29]. The existing literature provides limited documentation of late complications following transcatheter PDA closure, with only sporadic case reports addressing these rare events [22]. This case contributes valuable insights to the evolving understanding of potential long-term risks associated with the procedure, emphasizing the critical need for judicious patient selection, precise procedural execution, and comprehensive long-term monitoring to promptly detect and address any delayed complications [30, 31]. While transcatheter PDA closure demonstrates an excellent safety profile and efficacy in the short term, the interventional cardiology community must maintain awareness of possible late-onset complications [3, 13]. Implementation of systematic echocardiographic surveillance and regular clinical evaluation remains paramount for early identification of device-related issues, such as migration or vascular obstruction, enabling timely therapeutic intervention when required [32, 33]. The favorable risk-benefit ratio of the procedure should be carefully balanced against these potential, albeit uncommon, long-term considerations in clinical decision-making. This case series highlights the importance of ongoing research and long-term monitoring to fully characterize late outcomes following transcatheter PDA closure in pediatric populations. With the increasing number of procedures being performed, establishing robust surveillance protocols becomes essential for early detection and management of rare delayed complications. While adult catheter-based PDA closure remains understudied, we present two successful cases of percutaneous closure in patients with normal pulmonary pressures, demonstrating complete symptom resolution without complications during follow-up. These outcomes represent a previously underreported clinical course in our regional experience with PDA management.
2. CASE PRESENTATION
2.1. Case Presentation 1
A previously healthy 10-year-old female (45 kg) presented to our center with complaints of chest pain and exertional dyspnea. Physical examination revealed a characteristic continuous murmur at the left upper sternal border. Transthoracic echocardiography demonstrated left atrial and ventricular dilation with preserved systolic function (LVEF 65%), along with a large PDA (4mm pulmonary end, systolic pressure gradient 100 mmHg, diastolic gradient 40 mmHg) and trace mitral regurgitation. Notably, pulmonary artery pressures were within normal limits. Cardiac catheterization via femoral access confirmed normal hemodynamics (PA pressure 12/7 mmHg [mean 10], aortic pressure 95/50 mmHg [mean 67]). The anatomical configuration permitted successful transcatheter closure using an 8-10mm Lifetech device. Immediate post-procedural angiography demonstrated complete occlusion without residual shunt. Surveillance imaging at two-month intervals showed no evidence of device-related complications, including coarctation or left pulmonary artery stenosis. During follow-up, the patient remained asymptomatic with normal pulmonary pressures, requiring no further medical therapy (Fig. 1).
2.2. Case Presentation 2
A 5-year-old girl (14 kg) with a known history of large PDA (3.5–4 mm) and systemic pulmonary hypertension (PH) since infancy was admitted to our department. Initial evaluation at one year of age had revealed a dilated Main Pulmonary Artery (MPA), enlarged Pulmonary Artery Branches (PABs), Bi-Directional (BD) shunt, and chamber dilation, with catheterization confirming severe PH suggestive of Eisenmenger syndrome, precluding surgical intervention. She was managed medically but recently presented with palpitations, hyperdynamic precordium, and a systolic murmur at the apex and left lower sternal border. Electrocardiography showed normal sinus rhythm with left conduction delay. Repeat angiography demonstrated subsystemic pulmonary artery pressures (PAP 91/53 mmHg [mean 67]) and aortic pressures of 110/64 mmHg (mean 85), permitting successful transcatheter PDA closure using a 12 mm muscular Memoporr VSD occluder (Fig. 2). Post-procedural assessment revealed mild Mitral Regurgitation (MR) and Tricuspid Regurgitation (TR), mild Left Atrial (LA) and Left ventricular (LV) dilation (LVIDD 41 mm, LVIDS 26 mm), mild Pulmonary Insufficiency (PI), and residual mild PH (PAP 55/20 mmHg [mean 34]), with no residual shunt or coarctation.
During follow-up, the patient remained asymptomatic. Medical therapy, including sildenafil (1 mg/kg/dose q6h), captopril, and furosemide (1 mg/kg/day divided q12h), was continued for residual PH management.
3. DISCUSSION
PDA is an abnormal vascular connection between the descending thoracic aorta and the main pulmonary artery, accounting for 6-11% of all congenital heart defects. Clinical presentation in adults ranges from asymptomatic cases to severe manifestations, including heart failure and pulmonary arterial hypertension (PAH), with dyspnea, palpitations, and chest pain being the most common symptoms [34]. While most PDAs are diagnosed and treated during infancy - resulting in lower prevalence among adults [1] - management of persistent PDAs becomes increasingly complex due to the potential development of PAH. Echocardiographic assessment of shunt direction remains crucial for determining candidacy for transcatheter closure [35]. Although percutaneous closure represents the treatment of choice for infant PDAs, adult cases present unique challenges. In elderly populations, untreated PDA carries an estimated annual mortality rate of 1.8%, with significant risks of bacterial endocarditis, progressive pulmonary vascular disease, and congestive heart failure necessitating intervention [34]. Since Porstmann's pioneering percutaneous PDA closure in 1967 [1], device technology has advanced significantly. The current armamentarium includes various occlusion devices, with the Amplatzer duct occluder (ADO I and II, AGA Medical Corporation) now representing the standard for adult PDA closure [34]. While previous studies have documented successful device deployment with complete occlusion and minimal complications in adults [1, 4, 34], regional outcomes - particularly regarding detailed PDA progression patterns - remain unreported (Figs. 1 and 2). Our immediate and long-term occlusion results align with established literature, though we employed an antegrade pulmonary arterial approach rather than the more conventional retrograde arterial technique. Notably, our cases demonstrated no anatomical variations, such as interrupted inferior vena cava or initial venous access failure [34].
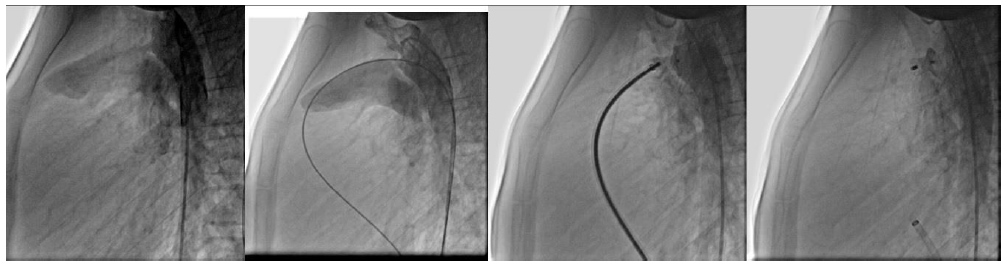
Pre-, intra-, and post-transcatheter PDA closure process of the first patient.
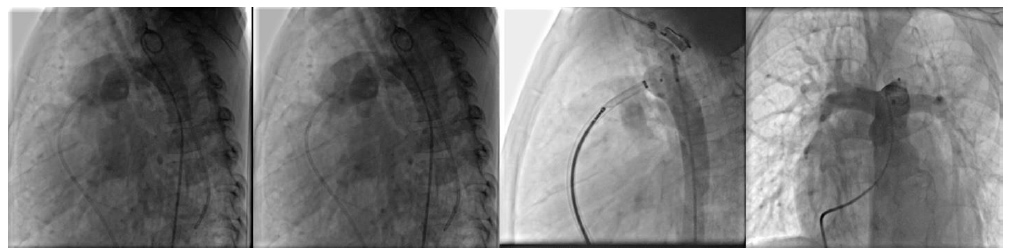
Pre-, intra, post transcatheter PDA closure process of the second patient.
Prior studies have reported unsuccessful percutaneous PDA closure in patients with severe Pulmonary Hypertension (PAH), often necessitating surgical intervention [4, 34]. In contrast, both of our cases demonstrated an immediate and sustained reduction in PA pressure following device occlusion. Several factors influence procedural success, including vascular accessibility, ductal size, morphological stability, and patient-specific anatomical challenges. Advanced age contributes to increased ductal fragility, tortuosity, and calcification, complicating percutaneous closure [1, 34, 36]. Additionally, comorbidities, such as congestive heart failure, atrial fibrillation, and pulmonary vascular disease, may impact outcomes. Patient selection remains critical; those with a pulmonary-to-systemic blood pressure (PA: SBP) ratio <0.8 are considered optimal candidates for closure, while test occlusion helps avoid right heart failure or hemodynamic instability in borderline cases [35]. Device selection also plays a key role; while the Amplatzer Duct Occluder (ADO) is most commonly reported, alternatives, such as the Cera occluder (up to 24–26 mm), Cardi-O-Fix, Cocoon, and Occlutech devices, have been utilized. Notably, our use of the Lifetech 8–10 mm and muscular Memoporr 12 mm devices represents a novel approach in the literature. Beyond technical feasibility, cost-effectiveness remains an important consideration in device selection [1, 34]. Several complications were considered after percutaneous PDA occlusion, including aortic isthmus and pulmonary stenosis, thrombosis and embolization, LV systolic dysfunction, high PA pressure, residual shunts, mitral regurgitation, infections, etc. However, no complications at any stage were observed in the current report [34, 36]. The progression of PDA is significantly challenging and may vary case by case. It seems necessary to evaluate each patient and all interfering factors, with special attention to pulmonary artery pressure, to determine precisely whether percutaneous occlusion is optimal or not.
4. LEARNING POINTS
The management of PDA requires individualized patient assessment, as each case presents unique characteristics and symptoms. Immediate monitoring of Pulmonary Artery Pressure (PAP) post-closure is critical to evaluate procedural success and detect residual shunting. Serial follow-up is essential to identify late complications and ensure long-term patient stability. While transcatheter closure is generally safe, complications may arise, particularly in high-risk patients, necessitating prompt intervention. Device selection and deployment techniques significantly influence outcomes, emphasizing the need for expertise in procedural execution. High-risk patients, including those with Eisenmenger syndrome, require tailored strategies to reduce morbidity and mortality. Long-term monitoring is crucial, as complications such as pulmonary hypertension may develop later. A multidisciplinary approach involving pediatric and interventional cardiologists optimizes patient care. Advances in transcatheter techniques continue to improve outcomes, highlighting the importance of ongoing research. This case underscores the educational value of understanding PDA management complexities and staying updated on evolving practices in pediatric cardiology.
CONCLUSION
The progression of PDA is highly variable and unpredictable, necessitating greater clinical focus. While transcatheter closure in adolescents and adults presents complexities, it remains a safe approach with minimal complications when performed carefully. Further research and expanded studies on PDA in adult populations are strongly recommended to refine management strategies.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: S.S.G.: Data Collection; H.B.: Study Concept/Design; M.N.: Data analysis or Interpretation; R.R.: Conceptualization; F.R.: Writing—Original Draft Preparation; All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| PDA | = Patent Ductus Arteriosus |
| TCPC | = Transcatheter PDA Closure |
| MPA | = Main Pulmonary Artery |
| BD | = Bi-Directional |
| LA | = Left Atrial |
| LV | = Left Ventricular |
| PAH | = Pulmonary Hypertension |
| ADO | = Amplatzer Duct Occluder |
| PAP | = Pulmonary Artery Pressure |
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author [F.R] upon request.
ACKNOWLEDGEMENTS
Declared none.


