All published articles of this journal are available on ScienceDirect.
The Efficacy of Betahistine in Reducing Postoperative Nausea and Vomiting (PONV) Following Laparoscopic Cholecystectomy
Abstract
Introduction
Patients undergoing laparoscopic cholecystectomy are at high risk of postoperative nausea and vomiting (PONV). In this study, we investigated the effect of oral betahistine on the reduction of incidence and severity of PONV in patients undergoing laparoscopic cholecystectomy.
Methods
This is a randomized, double-blind clinical trial; 132 patients were randomly assigned into two groups, who received betahistine 16 mg orally or placebo 3 hours before surgery, respectively. The severity and incidence of PONV were evaluated using the NRS (Numerical Rating Scale). Side effects of the drug were reported as a secondary outcome of the study.
Results
The rate of PONV absence and the need for rescue anti-nausea medication in the betahistine group were higher than those in the placebo group (66.7% vs. 39.4%, p = 0.008). The severity of postoperative nausea in the betahistine group was significantly lower than in the placebo group from the time of recovery to 48 hours after surgery (p<0.001). Moreover, the need for rescue anti-nausea medication in the placebo group was significantly higher than in the betahistine group (p = 0.002). Finally, there was no statistically significant difference between the two groups in terms of drug side effects.
Discussion
Betahistine significantly reduced the incidence and severity of postoperative nausea and vomiting compared to placebo (p = 0.008 and p < 0.001, respectively), required less rescue medication (p = 0.002), and showed no difference in side effects
Conclusion
Betahistine significantly prevented PONV in patients undergoing laparoscopic cholecystectomy.
Clinical Trial Registration Number
The study was reported under the Iranian Clinical Trial Center (N:lRCT2020 1225049829N1).
1. INTRODUCTION
Postoperative Nausea and Vomiting (PONV) is one of the most common complications after surgical procedures [1]. PONV is associated with greater postoperative pain, confusion, duration of hospitalization, treatment cost, and reduced patient satisfaction [2]. Despite numerous methods used to prevent and control PONV, its incidence ranges from 20% to 30% and in high-risk patients, it is 80% [3].
Laparoscopy is an appropriate method in cholecystectomy surgery because it reduces hospitalization time and postoperative complications, but PONV can lead to stretching of the peritoneal membrane and diaphragmatic irritation [4, 5]. Therefore, methods for preventing nausea and vomiting should be selected based on their safety, effectiveness, patient satisfaction, and cost-efficiency [6]. Ondansetron has been widely used as the established drug for PONV prevention [7]. A study in the field reported that 66.7% of patients undergoing laparoscopic gynecologic surgery experienced PONV within 48 hours of surgery [8].
Due to the complex physiopathology involved in PONV and the multifactorial nature of this complication, it has been seen that anti-nausea medications with different mechanisms reduce PONV in high-risk patients [9]. Recently, 5-hydroxytryptamine (5-HT3) receptor antagonists have been introduced as the first and second risk preventive anti-nausea medicines in PONV [10, 11]. The vestibular system stimulates the vomiting center and increases PONV through H1 receptors. Anticholinergic drugs, which lead to antiemetic effects by reducing vestibular sensitivity, also act through these receptors [12].
Betahistine is a histamine analog that acts as an H1 receptor agonist and H3 receptor antagonist, modulating histaminergic activity in the vestibular system and Central Nervous System (CNS) [13]. By activating H1 receptors, it suppresses nausea signaling to the vomiting center, while H3 antagonism enhances histamine’s inhibitory effects, reducing vestibular hyperactivity. This dual action underlies its efficacy in mitigating motion sickness and PONV [14].
Recent studies have suggested that betahistine, a histamine H1 receptor agonist, may offer distinct advantages over ondansetron in preventing PONV [15, 16]. Considering this, we conducted a comprehensive study to investigate the effects of betahistine as a potential alternative for PONV.
PONV is a significant concern due to its potential for harm and annoyance, and also because of the lack of a proper study on betahistine in patients undergoing laparoscopic cholecystectomy [17]. This study therefore investigated the effect of oral betahistine on reducing the incidence and severity of nausea and vomiting after cholecystectomy.
2. PATIENTS AND METHODS
This prospective, double-blinded clinical trial was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS. IMAMHOSPITAL.REC.1397.058), and written consent forms were obtained from all patients before participation. For participants under 18-20, their parents or legal guardians provided informed consent on their behalf. The study was reported under the Iranian Clinical Trial Center (N:lRCT20201225049829N1).
The inclusion criteria were: patients aged 15 to 60 years, candidates for elective laparoscopic cholecystectomy, and individuals with a history of motion sickness. A total of 132 patients were enrolled in the study between August 2019 and October 2020, of whom 108 were classified as ASA I or II. Exclusion criteria included uncontrolled diabetes, history of nausea and vomiting, consumption of anti-nausea and vomiting drugs, systemic steroids in the 24 hours before surgery, middle ear disease, history of betahistine allergy, no vestibular symptoms, pregnancy, obese people with Body mass index (BMI)> 35 kg/m2, and cases where laparoscopic surgery becomes open cholecystectomy.
Patients who met the inclusion criteria were randomly allocated into two groups: B (betahistine) and P (placebo) using a blocking method with the random allocation software. The color of the placebo tablets was made identical to that of betahistine tablets, ensuring a double-blinded setup where neither the patients nor the investigators were aware of the assigned treatment. Before surgery, patients received sufficient explanations and training on how to assess the severity of postoperative nausea. Patients were requested to indicate their pain intensity on a Numerical Rating Scale (NRS) by selecting the number that corresponded to their perceived level of pain. The NRS consisted of a range of numbers, typically between 0 and 10, 0 and 20, or 0 and 100, from which patients circled the number that best represented their pain intensity. Also, vomiting episodes were counted separately.
The first group (B) received 16 mg oral betahistine tablets (manufactured by Aktoverco). In contrast, the second group (P) received a placebo tablet (manufactured by the Sari School of Pharmacy) composed of starch and identical in size, shape, color, and structure to the betahistine tablets. Both medications were administered orally, 3 hours before surgery.
Surgeons, patients, inpatient nurses, and researchers who collected postoperative data were all blinded regarding the therapy.
Patients were under an 8-hour fast for solid foods and a 2-hour fast for clear liquids before surgery. General anesthesia was started with midazolam 0.05 mg/kg, fentanyl 2µ/kg, and thiopental at a dose of 3-5 mg/kg, followed by 0.5 mg/kg atracurium for intubation. Anesthesia was continued with isoflurane (C3H2ClF5O) and oxygen. During the operation, 50 μg of fentanyl was injected intravenously every 1 h. After anesthesia induction, patients received 50 μg of intravenous fentanyl and were scheduled to receive 30 mg of ketorolac every 8 hours during the first 24 hours after surgery for pain control. The severity of nausea and vomiting, the amount of ondansetron and opioids used by patients as well as other possible drug side effects, including dry mouth, headache, itching, and skin rash in the two study groups after consciousness in the recovery ward and the surgical ward were evaluated and recorded at 1, 3, 6, 12, 24, 48 h after surgery. The incidence and severity of PONV at 24 h were evaluated based on the NRS scoring system. If a patient experienced nausea with an NRS score ≥ 4, vomiting, or required anti-nausea medication, they were administered 8 mg of intravenous ondansetron. If a patient experienced pain with an NRS score ≥ 4 or required analgesic medication, they were administered 2 mg of intravenous morphine, and the event was recorded.
The 48-hour follow-up was selected because PONV peaks within 24–48 hours [8], aligning with our significant findings. This timeframe aligns with standard post-operative hospitalization for consistent monitoring and fully captures betahistine’s pharmacokinetics (peak: 3–4 h; clearance: 24 h), while avoiding potential confounders that may arise later.
3. STATISTICAL ANALYSIS
SPSS 24 was used to analyze the data, which was described using percentages, means, medians, minimums, and maximums. Normality of quantitative variables was assessed using the Shapiro-Wilk test. Fisher's exact test was utilized to compare the distribution of pain intensity between the two groups, specifically the Placebo group and the Betahistin group.
The Mann-Whitney U test was used to assess the statistical significance of the comparison of severity scores between the Placebo group and the Betahistin group at each time point.
Additionally, the Friedman test was employed to evaluate the statistical significance of intra-case comparisons within each group over time.
Generalized estimating equations (GEE) models with Wald Chi-Square tests were used to compare the effect source and model parameters between the case and control groups over time. The significance level was less than 0.05.
The work has been reported in line with the CONSORT flowchart and checklist criteria (Fig. 1).
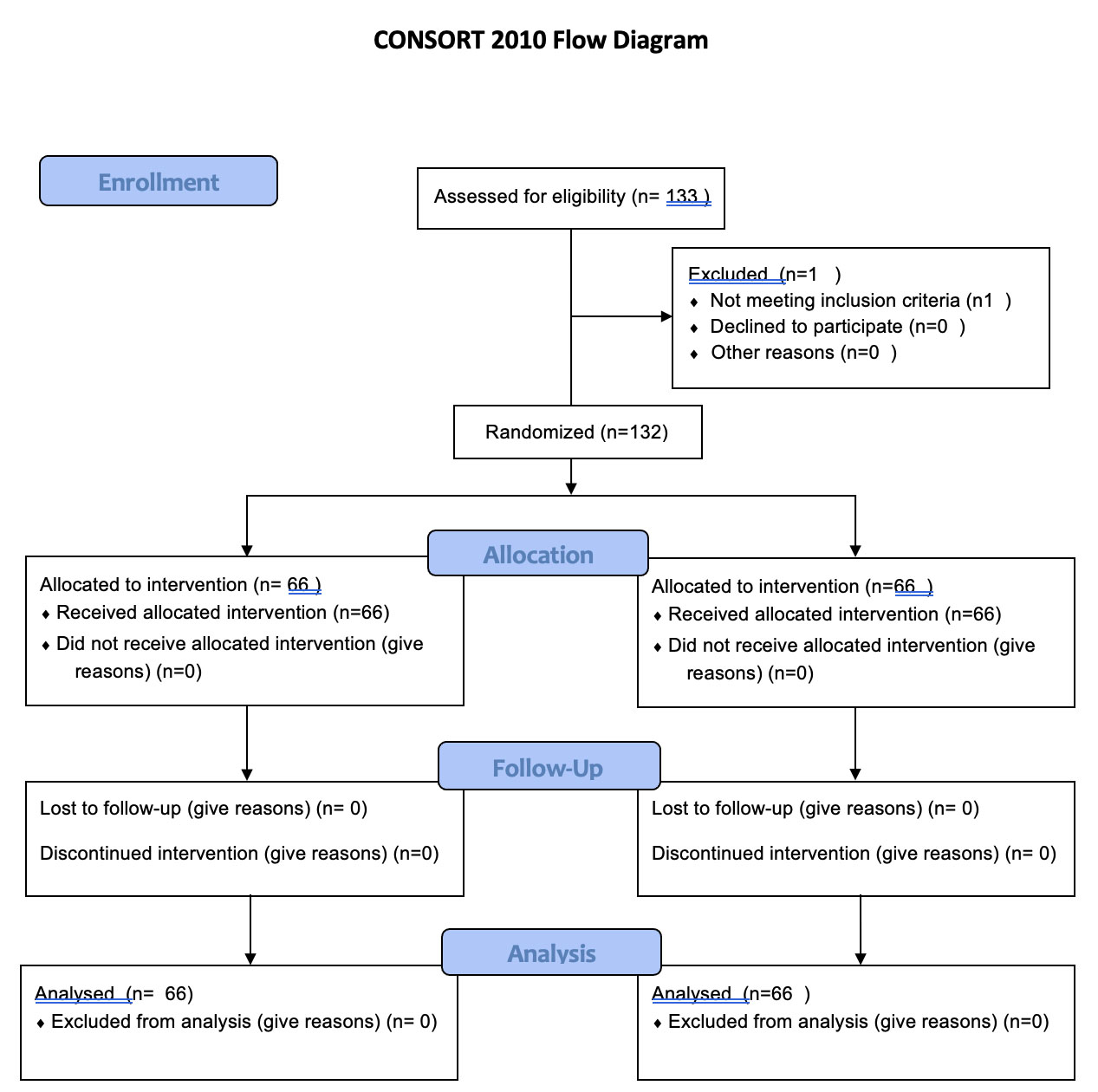
Consort flow diagram.
4. RESULTS
A total of 133 patients were included in the study. One patient in the B group was excluded due to the need for open surgery caused by severe visceral adhesions. Therefore, data were analyzed from the remaining 132 patients (66 patients in group B and 66 patients in group P).
Table 1 presents the results of comparing the Betahistine group and the Placebo group in terms of various factors. The analysis reveals interesting findings. Firstly, there is a marginally significant difference in gender distribution between the two groups, with the Betahistine group having a higher percentage of males compared to the Placebo group. However, there are no significant differences observed in age, height, weight, motion sickness frequency, PONV frequency, operation time, and anesthesia time between the two groups. These results suggest that the demographic and clinical characteristics of the participants were well-balanced between the Betahistine and Placebo groups, minimizing potential confounding factors. Therefore, any observed differences in the outcome variable can be attributed to the treatment effect with greater confidence rather than to other variables.
4.1. Postoperative Nausea and Vomiting
Table 2 provides important information on the severity of symptoms and the response to treatment in the Betahistine group compared to the Placebo group at different time intervals. The results demonstrate significant differences between the two groups in terms of recovery time. At time zero, the Betahistine group showed a lower percentage of patients experiencing symptoms compared to the Placebo group (48.5% vs. 21.2%, p < 0.001). Similar patterns were observed at subsequent time points, with the Betahistine group consistently showing lower symptom frequencies compared to the Placebo group (p < 0.001). These findings indicate that Betahistine may have a beneficial effect in reducing the severity and duration of symptoms in the study population.
| P-value | Betahistin Group (N=66) | Placebo Group (N=66) |
|
|---|---|---|---|
| 0.064 | (42.4%)28 | (21.2%)14 | Male |
| (57.6%)38 | (78.8%)52 | Female | |
| 0.167 | 71/12±24/41 | 3169/11±69/39 | Age (year) |
| 0.456 | ± 161.35.7 | 160.5± 4.3 | Height (cm) |
| 0.921 | 63.12±7.5 | 60.2±5.3 | Weight (kg) |
| 0.445 | 13 | 16 | Motion sickness (Frequency) |
| 0.253 | 4 | 5 | PONV (frequency) past |
| 0.782 | 60.3±5.7 | 58.5±4.3 | Operation time(min) |
| 0.325 | 73.52±5.2 | 78.85±5.4 | Anesthesia time (min) |
| Time | Severity | Placebo Group | Betahistin Group | P-value* | Betahistin Group | Placebo Group | P-value# | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Mean | Standard deviation | Mean | Standard deviation | ||||
| Time of recovery | 0 | 32 | 48.5% | 14 | 21.2% | 0.000 | 1.848 | 1.970 | 5.091 | 3.404 | 0.000 |
| 1 | 0 | 0.0% | 0 | 0.0% | |||||||
| 2 | 8 | 12.1% | 0 | 0.0% | |||||||
| 3 | 4 | 6.1% | 14 | 21.2% | |||||||
| 4 | 16 | 24.2% | 2 | 3.0% | |||||||
| 5 | 6 | 9.1% | 0 | 0.0% | |||||||
| 6 | 0 | 0.0% | 0 | 0.0% | |||||||
| 7 | 0 | 0.0% | 12 | 18.2% | |||||||
| 8 | 0 | 0.0% | 14 | 21.2% | |||||||
| 9 | 0 | 0.0% | 10 | 15.2% | |||||||
| After 1 hour | 0 | 34 | 51.5% | 24 | 36.4% | 0.000 | 1.212 | 1.409 | 3.818 | 3.066 | 0.000 |
| 1 | 6 | 9.1% | 0 | 0.0% | |||||||
| 2 | 6 | 9.1% | 0 | 0.0% | |||||||
| 3 | 18 | 27.3% | 2 | 3.0% | |||||||
| 4 | 2 | 3.0% | 0 | 0.0% | |||||||
| 5 | 0 | 0.0% | 12 | 18.2% | |||||||
| 6 | 0 | 0.0% | 12 | 18.2% | |||||||
| 7 | 0 | 0.0% | 14 | 21.2% | |||||||
| 8 | 0 | 0.0% | 2 | 3.0% | |||||||
| After 3 hours | 0 | 24 | 63.6% | 24 | 36.4% | 0.000 | 0.909 | 1.308 | 3.394 | 2.738 | 0.000 |
| 1 | 4 | 6.1% | 0 | 0.0% | |||||||
| 2 | 4 | 6.1% | 2 | 3.0% | |||||||
| 3 | 16 | 24.2% | 0 | 0.0% | |||||||
| 4 | 0 | 0.0% | 2 | 3.0% | |||||||
| 5 | 0 | 0.0% | 20 | 30.3% | |||||||
| 6 | 0 | 0.0% | 14 | 21.2% | |||||||
| 7 | 0 | 0.0% | 4 | 6.1% | |||||||
| After 6 hours | 0 | 44 | 66.7% | 24 | 36.4% | 0.000 | 0.758 | 1.146 | 2.939 | 2.384 | 0.000 |
| 1 | 2 | 3.0% | 0 | 0.0% | |||||||
| 2 | 12 | 18.2% | 2 | 3.0% | |||||||
| 3 | 8 | 12.1% | 0 | 0.0% | |||||||
| 4 | 0 | 0.0% | 16 | 24.2% | |||||||
| 5 | 0 | 0.0% | 20 | 30.3% | |||||||
| 6 | 0 | 0.0% | 2 | 3.0% | |||||||
| 7 | 0 | 0.0% | 2 | 3.0% | |||||||
| After 12 hours | 0 | 48 | 72.7% | 26 | 39.4% | 0.000 | 0.576 | 1.001 | 2.455 | 2.093 | 0.000 |
| 1 | 2 | 3.0% | 0 | 0.0% | |||||||
| 2 | 12 | 18.2% | 0 | 0.0% | |||||||
| 3 | 4 | 6.1% | 8 | 12.1% | |||||||
| 4 | 0 | 0.0% | 24 | 36.4% | |||||||
| 5 | 0 | 0.0% | 6 | 9.1% | |||||||
| 6 | 0 | 0.0% | 2 | 3.0% | |||||||
| After 24 hours | 0 | 56 | 84.8% | 26 | 39.4% | 0.000 | 0.212 | 0.545 | 1.909 | 1.646 | 0.000 |
| 1 | 6 | 9.1% | 0 | 0.0% | |||||||
| 2 | 4 | 6.1% | 6 | 9.1% | |||||||
| 3 | 0 | 0.0% | 22 | 33.3% | |||||||
| 4 | 0 | 0.0% | 12 | 18.2% | |||||||
| After 48 hours | 0 | 64 | 97.0% | 26 | 39.4% | 0.000 | 0.030 | 0.174 | 1.152 | 1.064 | 0.000 |
| 1 | 2 | 3.0% | 10 | 15.2% | |||||||
| 2 | 0 | 0.0% | 24 | 36.4% | |||||||
| 3 | 0 | 0.0% | 6 | 9.1% | |||||||
| p-value % | 0.000 | 0.000 | |||||||||
Furthermore, the p-values associated with the comparisons between the two groups are consistently significant across all time intervals (p < 0.001). This indicates strong statistical evidence supporting the differences observed in symptom severity and treatment response. The standard deviations of the mean values within each group suggest some variability; however, the differences between the Betahistine and Placebo groups remain statistically significant.
The severity of nausea was lower in group B than in group P at all times of the study (up to the first hour of recovery, at all time durations, p < 0.001) (Fig. 2). The term “recovery” refers to the period following the surgical procedure, during which the patient is monitored and gradually regains consciousness from the effects of anesthesia.
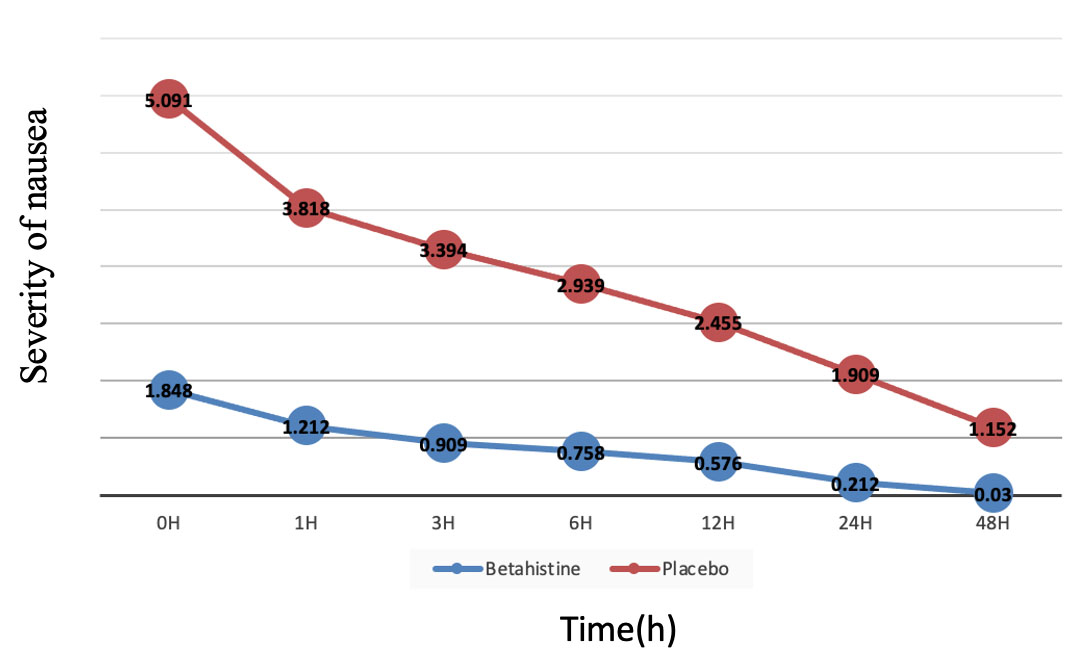
Changes in nausea score during 48 h in the two groups of Betahistine (B) and Placebo (P).
According to Table 3, a significant difference was observed between the two groups (p < 0.001) in the overall outcome of the nausea severity score during the study. Moreover, the effect of time at recovery was significant (p <0.001). The effects of time and group were also significant (p < 0.001). The model analysis indicated that the effect of the B group on the placebo was significant (p < 0.001). In addition, nausea score changes in the follow-up stages compared to the placebo at recovery were significant, p < 0.001. Furthermore, the interaction between the group and time, as well as the placebo group and the pre-test time, was significant, indicating a decrease in nausea observed in the B group over time.
| Effect Source | Parameter | B | Std. Error | 95% Wald Confidence Interval | Wald Chi-Square | df | P* | P* | |
|---|---|---|---|---|---|---|---|---|---|
| Low | Up | ||||||||
| Fix amount | 1.629 | 0.0806 | 1.471 | 1.787 | 408.592 | 1 | 0.000 | 0.022 | |
| Groupa | [group=exc] | -1.004 | 0.1501 | -1.299 | -0.710 | 44.758 | 1 | 0.000 | 0.000 |
| Timeb | [time=1h] | -0.286 | 0.0528 | -0.389 | -0.182 | 29.255 | 1 | 0.000 | 0.000 |
| [time=3h] | -0.403 | 0.0513 | -0.503 | -0.302 | 61.574 | 1 | 0.000 | ||
| [time=6h] | -0.546 | 0.0561 | -0.656 | -0.436 | 94.850 | 1 | 0.000 | ||
| [time=12h] | -0.725 | 0.0616 | -0.846 | -0.604 | 138.305 | 1 | 0.000 | ||
| [time=24h] | -0.974 | 0.0611 | -1.094 | -0.855 | 254.662 | 1 | 0.000 | ||
| [time=48h] | -1.474 | 0.0625 | -1.597 | -1.352 | 556.140 | 1 | 0.000 | ||
| Reaction Time and groupa,b |
[time=1h] * [group=ex] | -0.130 | 0.0719 | -0.271 | 0.011 | 3.269 | 1 | 0.071 | 0.000 |
| [time =3h] * [group=ex] | -0.290 | 0.1093 | -0.504 | -0.075 | 7.026 | 1 | 0.008 | ||
| [time =6h] * [group=ex] | -0.322 | 0.1246 | -0.566 | -0.078 | 6.676 | 1 | 0.010 | ||
| [time =12h] * [group=ex] | -0.403 | 0.1600 | -0.716 | -0.089 | 6.341 | 1 | 0.012 | ||
| [time =24h] * [group=ex] | -1.053 | 0.2439 | -1.531 | -0.575 | 18.630 | 1 | 0.000 | ||
| [time =48h] * [group=ex] | -1.823 | 0.3022 | -2.415 | -1.230 | 36.371 | 1 | 0.000 | ||
The number of vomiting episodes during 48 h after surgery in group B was less than that of the P group (30.3% vs. 60.6%, p= 0.013) (Table 3).
The mean additional dose of ondansetron over 48 hours after surgery in group B was less than that of the P group (2.42 mg vs. 7.27 mg, p = 0.02).
The mean dose of morphine during 48 h after surgery was similar in group B compared to the P group (2.24 vs. 1.94, p = 0.909) (Table 4).
| Treatments | Betahistin Group | Placebo Group | P-value | ||
|---|---|---|---|---|---|
| Mean | Standard Variation | Mean | Standard Variation | ||
| Ondansetron | 2.42 | 3.73 | 7.27 | 6.74 | 0.002 |
| Morphine | 2.24 | 2.49 | 1.94 | 1.69 | 0.9090 |
| Vomiting episode | 20 | 30.3% | 40 | 60.6% | 0.013 |
Side effects (blurred vision, headache, skin rash, dry mouth, itching)
The overall rate of blurred vision in the first 48 h after surgery in group B was higher than that of the P group (10.6% vs. 0.0%, p= 0.042).
Eighteen patients (27.3%) in group B and 4 patients (6.1%) in group P experienced dry mouth, and the rate of dry mouth in group B was higher than in group P (p = 0.021).
The number of patients with headaches was not significantly different between groups B and P (6.1% vs. 6.1%, p = 1.00). Furthermore, only two patients (3%) experienced a skin rash in group B. No cases of skin rash were observed in group P, and there was no difference between the two groups in terms of skin rash (p = 0.314). No patient experienced itching (Table 5).
| Side Effects | Betahistin Group | Placebo Group | P-value |
|---|---|---|---|
| Frequency | Frequency | ||
| Blurrd vision | (10.6%)7 | (0.0%)0 | 0.042 |
| Headache | (6.1%)4 | (6.1%)4 | 1.00 |
| Rush | (3.0%)2 | 0 | 0.314 |
| Dry mouth | (27.3%)18 | (6.1%)4 | 0.021 |
| Itching | 0 | 0 | - |
5. DISCUSSION
In this study, we investigated the effects of betahistine on the prevention of postoperative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy. Our findings revealed that betahistine significantly decreased nausea compared to placebo after the surgery. This highlights the potential of betahistine as an effective treatment option for PONV in high-risk patients.
Betahistine significantly reduced nausea severity compared to placebo at all time points (recovery: 1.2±1.5 vs 3.8±2.1; 6 hours: 0.8±1.2 vs 3.0±1.9; 24 hours: 0.4±0.7 vs 1.9±1.4; all p < 0.001). It lowered PONV risk by 41% (RRR=0.41, 95%CI:0.22-0.62) with an NNT of 4 (95%CI:3-7), demonstrating both statistical and clinical significance in PONV prevention.
Our results align with betahistine’s known pharmacokinetics: rapid oral absorption (Tmax: 3–4 hours) and short half-life (~3–4 hours) match the critical PONV window post-laparoscopy. Its H1/H3 modulation likely mitigates both the early (vestibular) and delayed (inflammatory) phases of nausea, explaining the sustained reduction in NRS scores up to 48 hours.
Betahistine demonstrated significant efficacy in reducing both the incidence (66.7% vs. 39.4%, p = 0.008) and severity (p < 0.001) of PONV, while also decreasing the need for rescue antiemetics (p = 0.002). Its favorable safety profile was underscored by only mild, infrequent adverse effects (e.g., dry mouth, blurred vision), with no serious complications reported. Mechanistically, betahistine’s dual action as an H1 agonist and H3 antagonist offers a distinct advantage by targeting vestibular pathways, complementing existing 5-HT3 antagonists and expanding options for high-risk patients or those requiring combination therapy.
Effective treatment of PONV is one of the most important aspects of postoperative care [18]. In some studies, patients have described PONV as even worse than postoperative pain [19]. PONV influences the patient's satisfaction and clinical prognosis, including duration of hospitalization, rate of readmission, and complications such as dehydration, aspiration, electrolyte disturbances, increased blood pressure, suture stretching, increased bleeding from the skin flaps, and secondary complications, including rupture of the esophagus and pneumothorax [20, 21]. Moreover, it occurs in about 30-80% of patients without prophylaxis [22]. Hence, a serotonin receptor antagonist (5-HT3) is recommended due to its high effectiveness and low side effects compared to standard PONV treatment [23]. Lack of side effects of sedation following the administration of PONV control drugs, such as dopamine D2 receptor antagonist, is one of the advantages of this class of drugs that has led to SWITICHING of physicians to these drugs in post-anesthesia care [24]. Betahistine is a structural analog of histamine with H1 receptor agonist and H3 receptor antagonist effects. In the present study, the 16 mg oral betahistine regimen was prescribed in contrast to other injectable antiemetic drugs, which are important for nursing staff. Betahistine is completely absorbed after oral administration, with a plasma peak reached approximately 3-4 hours after administration. It is cleared from the plasma within 24 hours, making it a suitable candidate for PONV treatment. When making drugs, Institutional Policies such as safety, effectiveness, risk factor, low cost, type of prescription, ease of purchase, and patient satisfaction are considered. As an anti-nausea drug, betahistine showed optimal effectiveness and limited side effects. In addition, as an H1 receptor agonist and H3 receptor antagonist, it showed acceptable anti-nausea effects compared to a placebo. Furthermore, HT3-5 receptor antagonist has been reported to be much more powerful and effective than droperidol and metoclopramide in pediatric meta-analyses. HT3-5 receptor antagonist does not have side effects of extrapyramidal (metoclopramide), sedation (phenothiazine, droperidol, butyrophenone), or prolongation of the QT fragment (droperidol). Therefore, if the patient is polypharmaceutical and is receiving drugs from the family of antidepressants, antipsychotics, and some antibiotics such as macrolides and quinolones, betahistine can be easily used. In addition, combination therapy is more effective than monotherapy in high-risk and susceptible individuals with PONV, and this scenario of the use of HT3-5 receptor antagonists with phenothiazines may cover the acute phase after surgery. Dexamethasone is another effective treatment option that includes ramostron and tropisterone, and conditions such as pregnancy, diabetes, patients exposure to anxiety, and gastric ulcer should be used with caution [25]. Betahistine may be more effective in clinical uses.
Sun Suncho et al.'s study demonstrated that the incidence of complete response in the betahistine group was higher than in the placebo group, and the severity of nausea was lower in the betahistine group compared to the placebo group. Moreover, the pain score was similar in both groups. It was also found that betahistine combined with ondansetron was more effective than ondansetron alone in preventing PONV and dizziness in high-risk patients undergoing laparoscopic gynecologic surgery [26]. The present study also showed that the complete response rate in the betahistine group was higher than in the placebo group, and the severity of nausea in the betahistine group was lower than in the placebo group. The incidence of postoperative vomiting in the betahistine group was lower than in the placebo group. Moreover, betahistine was more effective than placebo in preventing PONV and vomiting in high-risk patients undergoing laparoscopic cholecystectomy under general anesthesia. The present study is consistent with Cho's findings, which confirm the results obtained from our study. Mukhopadhyay's study was a randomized, double-blind clinical trial conducted on 100 patients undergoing middle ear surgery under local anesthesia. The patients were divided into two groups, each consisting of 50 individuals. Patients in group A received 16 mg of betahistine and 8 mg of ondansetron. Patients in group B received a placebo and 8 mg of ondansetron 3 h before surgery. Nausea, vomiting, and dizziness were assessed during surgery and 24 h after surgery. This study is a double-masked randomized clinical trial with 132 patients. The patients were divided into two groups. In both groups, 66 patients were participated. The first group received 16 mg of oral betahistine tablets, and the second group received one oral placebo 3 hours before surgery. The primary reason for obtaining similar results is the use of the same sample size and methodology. Patients were evaluated at intervals of 1, 3, 6, 12, 24, and 48 hours after drug administration. Our study focused exclusively on high-risk patients undergoing laparoscopic cholecystectomy under general anesthesia, whereas Mukhopadhyay’s study involved patients undergoing middle ear surgery. Additionally, Jin Sun Cho conducted a double-blind, randomized trial on 168 patients undergoing laparoscopic gynecological surgery. In that study, one group received a placebo, and the other received 18 mg of betahistine tablets 3 hours before surgery. Both groups were also administered 4 mg of ondansetron at the end of surgery and 8 mg along with fentanyl via a PCA pump.
Furthermore, Mukhopadhyay et al. evaluated the incidence of nausea, vomiting, and dizziness during surgery and 24 h after surgery. The complete response in patients in the B group was higher than in the P group. Vomiting during surgery and after surgery in the B group was lower than in the placebo group. Dizziness was the same in the B and P groups. The results of this study indicated that betahistine significantly decreased PONV, and the incidence of postoperative nausea and dizziness was lower in patients who were given betahistine [27]. The present study also showed that the complete response rate in the B group was higher than in the placebo group, and the severity of nausea in the B group was lower than in the P group. The incidence of postoperative vomiting in the B group was lower than in the P group. Moreover, betahistine was more effective than placebo in preventing PONV and vomiting in high-risk patients undergoing laparoscopic cholecystectomy under general anesthesia [28, 29]. The present study is consistent with Mukhopadhyay's findings, which confirm the results obtained from our study. Sokolova et al. compared the deficiency of beta-histin and Ginkgobiloba in patients with peripheral dizziness. The severity of vertigo was assessed, and disability caused by dizziness improved significantly in both groups treated with Ginkgobiloba betahestine. There were no significant differences between treatment groups due to treatment-related changes. This study showed that betahistine and GINK gobiloba extract had the same effect in treating dizziness [30, 31]. Moreover, the incidence of complete response was higher in the betahistine group than in the placebo group. The severity of nausea was also lower in the betahistine group than in the placebo group [32]. Incidence of postoperative vomiting was lower in the betahistine group than in the placebo group. This study revealed that betahistine was more effective in preventing PONV and vomiting in high-risk patients undergoing laparoscopic cholecystectomy with general anesthesia [33, 34].
The baseline comparability of the groups and the use of adjusted statistical models reinforce the conclusion that the reduction in PONV incidence and severity was indeed due to betahistine, rather than confounding factors.
Therefore, the present study is consistent with Sokolova's findings, which confirm the results obtained in our study.
CONCLUSION
Betahistine significantly reduced the incidence and severity of PONV while demonstrating a favorable safety profile (e.g., minimal headache and dry mouth), supporting its role as an effective antiemetic option. However, this single-center study had limitations, including a modest sample size (n = 132) and a 48-hour follow-up, which may affect generalizability and miss delayed PONV events. The lack of an active comparator (e.g., ondansetron) and exclusion of high-risk subgroups (e.g., BMI > 35) also restricts broader clinical applicability, and mild side effects (e.g., blurred vision) should be considered. Future studies with extended observation periods and direct comparative designs are needed to validate these findings further.
LIST OF ABBREVIATIONS
| PONV | = Postoperative nausea and vomiting |
| BMI | = Body mass index |
| GEE | = Generalized estimating equations |
| NRS | = Numerical Rating Scale |
| CNS | = Central Nervous System |
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: Dr. F.H.K. and Dr. A.S.: Conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised it; Dr. M.M. and Dr. M.E.: Designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript; Dr. S.A.E.: Coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content. All authors reviewed the results and approved the final version of the manuscript;.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences, Iran (IR.MAZUMS.IMAMHOSPITAL.REC.1397.058).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (both institutional and national), as well as the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written consent forms were obtained from all patients before participation. For participants between 18 and 20 years old, their parents or legal guardians provided informed consent on their behalf.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


