All published articles of this journal are available on ScienceDirect.
Common Tongue Manifestations in Patients with Diabetes and Hypertension
Abstract
Introduction
The tongue, a sensitive organ in the oral cavity, is often affected by various systemic conditions. Diabetes mellitus (DM) and hypertension (HTN) are prevalent chronic diseases potentially associated with oral manifestations. This study aimed to investigate the relationship between systemic diseases and common tongue pathologies.
Materials and Methods
This cross-sectional study examined 30 patients at the University of Kufa’s College of Dentistry in Iraq. Patients aged 20-70 years underwent oral examinations using a mirror and probe to identify various tongue pathologies, including coated tongue, hairy tongue, geographic tongue, fissured tongue, and fibroma. Data on pre-existing DM and/or HTN were collected. Statistical analysis was employed to evaluate the relationship between systemic diseases and tongue pathologies.
Results
The mean age of the participants was 43.46 years, with a male predominance of 63.3%. Moreover, 63.3% of participants had chronic medical conditions: 26.7% had both DM and HTN, 16.7% had DM only, and 20% had HTN only. Fissured tongue (53.3%) and coated tongue (20%) were the most prevalent tongue pathologies. However, no statistically significant correlation (P = 0.816) was found between systemic disease (DM and/or HTN) and the presence of tongue pathology.
Discussion
The absence of a statistically significant association in this limited sample (n=30) contrasts with some existing literature suggesting links between diabetes/hypertension and oral manifestations, possibly due to insufficient statistical power in this study. While fissured and coated tongues were common, their direct statistical link to the studied systemic diseases was not established. The findings highlight the need for cautious interpretation of results from small-scale exploratory studies and underscore the importance of larger, more investigations to clarify these complex relationships.
Conclusion
While fissured and coated tongues were common in this study population, no statistically significant association was demonstrated between diabetes mellitus or hypertension and tongue pathology in this limited sample. Despite this, the tongue's sensitivity suggests that individuals with systemic diseases, such as diabetes mellitus (DM) and/or hypertension (HTN), may be more vulnerable to tongue changes. Further research with larger samples is needed to clarify these relationships.
1. INTRODUCTION
The oral cavity, often regarded as a window to systemic health, frequently exhibits signs and symptoms that may indicate underlying medical conditions [1, 2]. Among these, the tongue, a highly mobile and sensory organ, provides valuable diagnostic information [3]. Beyond its crucial roles in speech, taste, and swallowing, the tongue's specialized mucosa and rich vascular network make it susceptible to a range of pathological changes. Clinically relevant tongue manifestations, such as coated tongue, fissured tongue, geographic tongue, hairy tongue, fibromas, and atrophic glossitis, are commonly encountered and deserve careful clinical attention [4]. These alterations are not always isolated oral issues; they can be sentinel signs, prompting an investigation into possible systemic conditions [5].
Of particular significance are the highly prevalent chronic systemic diseases, such as diabetes mellitus (DM) and hypertension. Diabetes mellitus, a global health concern, is characterized by persistent hyperglycemia resulting from defects in insulin secretion or action or both [6]. This complex metabolic disease, affecting millions worldwide, is diagnosed based on glycemia thresholds and is associated with an increased risk of cardiovascular diseases [7]. Chronic hyperglycemia in diabetes mellitus (DM) leads to long-term microvascular complications that affect the kidneys, eyes, and nerves [7-9]. Type 2 diabetes, the most prevalent form, is linked to insulin resistance, hyperglycemia, and relative insulin deficiency, influenced by genetic, behavioral, and environmental factors [10]. Type 1 diabetes, resulting from the autoimmune destruction of pancreatic islet β-cells, leads to a complete loss of insulin secretion [11]. Diabetes mellitus presents with various clinical characteristics and potential complications that can impact dental management [12].
Hypertension, also known as high blood pressure (HBP), is another widespread chronic illness defined by a persistent elevation of blood pressure in the arteries [13]. Often symptomless, hypertension is a significant risk factor for peripheral vascular disease, atrial fibrillation, stroke, heart failure, vision loss, and chronic kidney disease [14]. Primary or essential hypertension, where the cause is unknown, is the most common type, accounting for 80-95% of cases [15]. Secondary hypertension, caused by underlying conditions affecting the kidneys, the endocrine system, or other organs, represents the remaining cases [16]. Persistently high blood pressure is a critical health concern globally, contributing significantly to cardiovascular morbidity and mortality [15, 16].
Despite the clinical importance of tongue manifestations and the high prevalence of diabetes and hypertension, a comprehensive understanding of the specific connections between these systemic conditions and tongue pathologies remains incomplete. While clinical observations and some research suggest links, further systematic investigation is needed. The oral manifestations of DM and HTN are well-documented; this study provides exploratory data from a specific Iraqi population, contributing to regional knowledge. Therefore, this study addressed this knowledge gap by examining the association between systemic diseases (diabetes and hypertension) and common tongue pathologies. By investigating this relationship in a patient population at the University of Kufa, Iraq’s College of Dentistry, this research aimed to contribute to a more informed understanding of oral-systemic health interactions and potentially improve clinical practice in dentistry.
2. MATERIALS AND METHODS
2.1. Study Design and Setting
This cross-sectional observational study was conducted at the Oral Diagnosis Department of the Faculty of Dentistry, University of Kufa, Najaf, Iraq. The study was conducted over a three-month period, from November 2022 to January 2023. The Faculty of Dentistry clinic serves as a primary dental care center in the Najaf region, providing access to a diverse patient population. This timeframe was chosen to allow for sufficient patient recruitment within the available resources and study period.
2.2. Study Population and Sample
The study involved 30 adult participants (n = 30), who served as the experimental units for this investigation. Participants were selected using a convenience sampling method and recruited consecutively as they presented for dental examinations.
2.2.1. Inclusion Criteria
Participants included were adults aged 20-70 years who presented for routine or initial oral examinations at the clinic and provided informed consent to participate.
2.2.2. Exclusion Criteria
Individuals younger than 20 years or older than 70 years, as well as those who declined to participate, were excluded from the study.
2.2.3. Sample Size Rationale and Justification
The sample size of 30 participants was determined primarily based on feasibility considerations for this preliminary investigation, which included the limited three-month timeframe allocated for data collection, available resources, and typical patient flow at the single clinical site. It is important to note that a formal a priori power analysis was not conducted before the study to determine the optimal sample size needed to detect potential associations with a desired level of statistical power; this represents a significant limitation. Consequently, while deemed practical for recruitment within the study's constraints, this sample size was intended only for the exploratory nature of this initial study. The aim was to gather preliminary data to identify potential trends and generate hypotheses regarding common tongue manifestations and systemic conditions, such as diabetes and hypertension, within this specific patient population rather than to test these associations with adequate statistical power definitively. The limitations of this sample size are further discussed in the limitations section.
2.3. Data Collection Procedures
Prior to participation, informed consent was obtained from each patient after the study's purpose and procedures were explained. Available clinic records were checked during the patient encounter to confirm the self-reported DM/HTN status where possible. Researchers from the Oral Diagnosis Department calibrated for the task and performed the data collection. Each participant underwent a standardized intraoral examination using a dental mirror and probe under adequate clinical lighting. The examination focused on the dorsal surface of the tongue to identify and document the presence of tongue pathologies based on visual and tactile examination, as shown in Figs. (1 and 2). The tongue pathologies observed in this study are as follows:
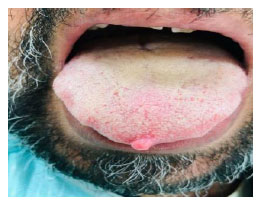
A 43-year-old male patient with fibroma of the tongue and hypertension.
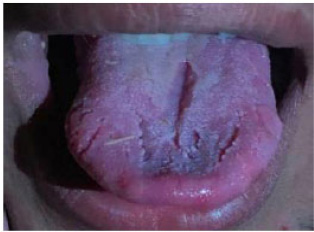
A 20-year-old patient with a fissured tongue and diabetes.
- Coated Tongue: Characterized by a whitish or yellowish layer covering the dorsal surface of the tongue.
- Hairy Tongue: Characterized by elongated filiform papillae on the dorsal surface of the tongue, which give a hair-like appearance and often exhibit pigmentation.
- Geographic Tongue (Benign Migratory Glossitis): Characterized by irregular patches of depapillation with raised, whitish borders, giving a map-like appearance, and known to change location over time.
- Fissured Tongue (Plicated Tongue, Grooved Tongue): The presence of grooves or fissures of varying depths on the dorsal surface of the tongue.
- Fibroma: A benign, smooth-surfaced, pedunculated, or sessile nodule on the tongue mucosa, typically firm to palpation.
2.4. Demographic and Systemic Condition Data
Patient demographics, including age and gender, were recorded. Information on pre-existing, diagnosed systemic conditions, specifically diabetes mellitus (DM) and hypertension (HTN), was collected via patient self-report. This information was cross-referenced and confirmed, where feasible, using available patient medical history within the dental clinic's records system. Participants were subsequently categorized into one of four groups based on their systemic health status: (1) No chronic medical condition, (2) Hypertension only, (3) Diabetes mellitus only, or (4) Both hypertension and diabetes mellitus.
2.5. Data Analysis
The collected data were entered and analyzed using the statistical software SPSS. Descriptive statistics, including frequencies and percentages, were used to summarize the prevalence of tongue pathologies and the distribution of patients across age, gender, and systemic disease categories. Chi-square tests or Fisher's exact test (where appropriate due to small sample size or zero cell counts) were employed to assess the statistical association between tongue pathologies and systemic diseases and between tongue pathologies and gender. A p-value of < 0.05 was considered statistically significant.
2.6. Ethical Considerations
This study was reviewed and approved by the Research Ethics Committee of the Faculty of Dentistry, University of Kufa (Reference number: 899; Date: October 2022). All procedures performed involving human participants were conducted according to the ethical standards of the institutional research committee and adhered to the principles outlined in the Declaration of Helsinki. The study protocol and reporting also adhered to the Sex and Gender Equity in Research (SAGER) guidelines where applicable [17]. Written informed consent was obtained from all participants included in the study before their participation, after a full explanation of the study's purpose and procedures. Patient confidentiality was maintained throughout the study.
3. RESULTS
A total of thirty participants took part in the study, all of whom were diagnosed with tongue pathology.
3.1. Participant Demographics
The age range of participants was 20–70 years (mean = 43.46 ± 15.2 years). As illustrated in Fig. (3), the majority of participants (63.3%) were aged 40 years or older. (Fig. 4) illustrates the gender distribution, which shows a higher proportion of males (63.3%) compared to females (36.7%), resulting in a male-to-female ratio of 1.72:1.
3.2. Prevalence of Chronic Medical Diseases
The distribution of reported chronic medical conditions among participants is detailed in Table 1. Overall, 63.3% (n = 19) of participants reported having at least one chronic condition. Specifically, 26.7% (n = 8) had both hypertension (HTN) and diabetes mellitus (DM), 16.7% (n = 5) had DM only, 20.0% (n = 6) had HTN only, while 36.7% (n = 11) reported no chronic conditions.
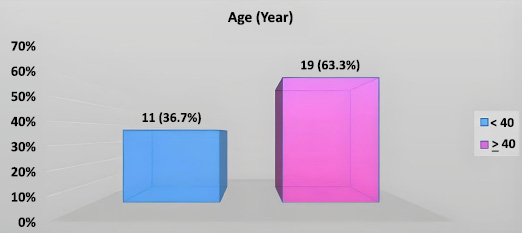
Distribution of study patients by age.
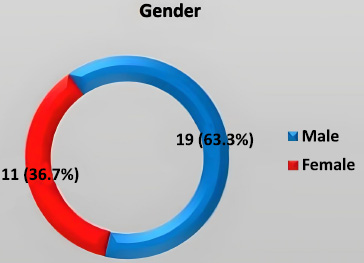
Distribution of study patients by gender.
| Chronic Medical Diseases | No. (n=30) | Percentage (%) |
|---|---|---|
| HTN+DM | 8 | 26.7 |
| DM | 5 | 16.7 |
| HTN | 6 | 20 |
| NO | 11 | 36.7 |
| Tongue Pathology | Systemic Disease | P-value | |
|---|---|---|---|
| Yes (%) n=19 | No (%) n=11 | ||
| Fissure tongue | 9 (47.4) | 7 (63.6 | 0.85 |
| Coated tongue | 4 (21.1) | 2 (18.2) | |
| Geographic tongue | 2 (10.5) | 1 (9.1) | |
| Hairy tongue | 2 (10.5) | 1 (9.1) | |
| Fibroma on tongue | 2 (10.5) | 0 (0) | |
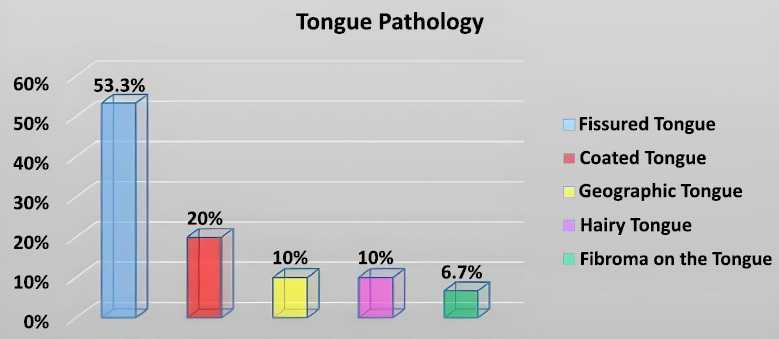
Prevalence (%) of different tongue pathologies observed in the study.
3.3. Prevalence of Tongue Pathologies
Fig. (5) displays the prevalence of the specific tongue pathologies identified. The fissured tongue was the most frequent finding, affecting 53.3% of the participants. A coated tongue was the second most common observation, noted in 20.0% of participants.
4. ASSOCIATION ANALYSES
4.1. Tongue Pathology and Systemic Disease
Fisher's exact test analysis revealed no statistically significant association between the presence of any tongue pathology and having a systemic disease (DM and/or HTN) (P = 0.850). Table 2 provides the detailed distribution of specific pathologies across participants categorized by the presence or absence of systemic disease.
4.2. Tongue Pathology and Gender
Similarly, Fisher's exact test indicated no statistically significant association between gender and the presence of any tongue pathology (P = 0.400). Table 3 shows the distribution of specific pathologies between male and female participants.
5. EXPLORATORY ANALYSIS OF INDIVIDUAL PATHOLOGIES VS. SYSTEMIC DISEASE AND GENDER
5.1. Systemic Disease Status
The results of these comparisons, using Fisher's exact test, are detailed in Table 4. No statistically significant association was found between any single specific tongue pathology (fissured tongue, coated tongue, geographic tongue, hairy tongue, or fibroma) and systemic disease (DM and/or HTN). All calculated P-values were well above the 0.05 significance threshold (P = 0.478 for fissured tongue; P = 1.000 for coated, geographic, and hairy tongue; and P = 0.530 for fibroma).
5.2. Gender
Similarly, the association between the presence of each specific tongue pathology and patient gender was examined. The results are presented in Table 5. Again, Fisher's exact test revealed no statistically significant associations. The comparison for the fissured tongue, coated tongue, geographic tongue, hairy tongue, and fibroma yielded P-values of 0.478, 0.345, 0.294, 0.255, and 1.000, respectively.
| Tongue Pathology | Systemic Disease | P-value | |
|---|---|---|---|
| Male (%) n=19 | Female (%) n=11 | ||
| Fissure tongue | 9 (47.4) | 7 (63.6) | 0.4 |
| Coated tongue | 5(26.3) | 1 (9.1) | |
| Geographic tongue | 1(5.3) | 2 (18.2) | |
| Hairy tongue | 3 (15.8) | 0 (0) | |
| Fibroma on tongue | 1 (5.3) | 1 (9.1) | |
| Tongue Pathology Comparison | Systemic Disease Yes | Systemic Disease No | Fisher's Exact P-value |
|---|---|---|---|
| Fissured Tongue | |||
| Present (n=16) | 9 | 7 | 0.478 |
| Absent (n=14) | 10 | 4 | |
| Coated Tongue | |||
| Present (n=6) | 4 | 2 | 1.000 |
| Absent (n=24) | 15 | 9 | |
| Geographic Tongue | |||
| Present (n=3) | 2 | 1 | 1.000 |
| Absent (n=27) | 17 | 10 | |
| Hairy Tongue | |||
| Present (n=3) | 2 | 1 | 1.000 |
| Absent (n=27) | 17 | 10 | |
| Fibroma on Tongue | |||
| Present (n=2) | 2 | 0 | 0.530 |
| Absent (n=28) | 17 | 11 |
| Tongue Pathology Comparison | Male (n=19) | Female (n=11) | Fisher's Exact P-value |
|---|---|---|---|
| Fissured Tongue | |||
| Present (n=16) | 9 | 7 | 0.478 |
| Absent (n=14) | 10 | 4 | |
| Coated Tongue | |||
| Present (n=6) | 5 | 1 | 0.345 |
| Absent (n=24) | 14 | 10 | |
| Geographic Tongue | |||
| Present (n=3) | 1 | 2 | 0.294 |
| Absent (n=27) | 18 | 9 | |
| Hairy Tongue | |||
| Present (n=3) | 3 | 0 | 0.255 |
| Absent (n=27) | 16 | 11 | |
| Fibroma on Tongue | |||
| Present (n=2) | 1 | 1 | 1.000 |
| Absent (n=28) | 18 | 10 |
6. DISCUSSION
This study investigated the prevalence of tongue manifestations in patients with diabetes mellitus and hypertension. Our findings indicated that fissured tongue and coated tongue were the most frequently observed tongue pathologies within our sample of 30 patients. Specifically, the fissured tongue was present in over half of the participants (53.3%), followed by coated tongue (20%). However, the lack of statistically significant associations found in our analyses (P > 0.05) must be interpreted cautiously, primarily due to the study's small sample size and resulting low statistical power.
Despite the lack of statistical significance in our sample, it is valuable to consider our findings in the context of existing literature. The high prevalence of fissured tongue in our study aligns with observations regarding hypertensive patients. Saleh et al. (2021) [18] identified hyposalivation as a clinical feature in patients with hypertension. They linked hyposalivation to both hypertension itself and the use of antihypertensive medications, particularly diuretics. As the tongue plays a significant role in breathing, speech, taste, and digestion [19], any condition affecting its moisture and health is noteworthy. Hyposalivation is a known contributing factor to the fissured tongue. It can also increase the risk of oral candidiasis [19-21], potentially explaining the prevalence of the fissured tongue observed in our study, especially given that a portion of our sample had hypertension or both hypertension and diabetes. It is also important to recall that elevated blood pressure, characteristic of hypertension, is a critical indicator of cardiovascular health, and oral manifestations may potentially reflect broader systemic vascular issues [22, 23].
Regarding coated tongue, our finding of its prevalence in 20% of participants resonates with the work of Ahrari et al. (2025) [24]. They reported a higher likelihood of coated tongue in individuals with type 2 diabetes mellitus. Diabetes mellitus, characterized by elevated blood sugar levels resulting from insufficient insulin action or production, can have a wide range of effects on the body. While our study did not demonstrate a statistically significant correlation between coated tongue and systemic disease, the presence of coated tongue in our sample, including patients with diabetes, is consistent with previous observations suggesting a link between diabetes and coated tongue. This could be related to altered oral microflora or other metabolic changes associated with diabetes [6, 25].
Furthermore, the literature cited by Rahnuma and Mainul (2021) [25] highlights various tongue manifestations in diabetic patients, including rhomboid glossitis, benign migratory glossitis (also known as geographic tongue), fissured tongue, and atrophic glossitis. While our study's statistical analysis did not confirm a direct link to systemic disease in our limited sample, we did observe cases of geographic tongue and fissured tongue, consistent with these reported associations in diabetic populations. Rahnuma and Mainul [25] also attribute Candida infection as a factor in rhomboid glossitis in diabetic individuals, which is relevant since diabetes is known to increase susceptibility to candidiasis, potentially contributing to various tongue changes [26, 27].
Clinically, this suggests that oral health professionals should be particularly attentive to tongue changes during examinations of patients with diabetes and hypertension.
Future research with larger, more diverse patient populations and longitudinal study designs is warranted to further investigate the relationship between systemic diseases and tongue manifestations. Exploring the specific mechanisms through which diabetes and hypertension might influence tongue health and examining the impact of disease severity, oral hygiene, and medication types would also be valuable directions for future studies.
CONCLUSION
This study aimed to investigate the prevalence of common tongue manifestations in a sample of patients attending a dental clinic in Iraq and explore potential associations with diabetes mellitus (DM) and hypertension (HTN). However, statistical analyses, employing Fisher's exact test due to the small sample size, did not reveal any significant association between the presence of these common tongue pathologies (either individually or overall) and the presence of DM and/or HTN (P > 0.05), nor between tongue pathologies and gender (P > 0.05).
It is imperative to interpret these non-significant findings with considerable caution. The study suffers from significant limitations, most notably the very small sample size obtained through convenience sampling at a single center. This resulted in low statistical power, meaning the study likely lacked the ability to detect true associations if they existed (a high risk of Type II error). Therefore, the absence of statistically significant findings should not be interpreted as definitive evidence of no association; rather, it reflects the constraints of this preliminary investigation.
Despite these limitations and the absence of statistical significance in this specific study, our findings and existing literature support the conclusion that individuals with systemic diseases, such as diabetes and hypertension, may be more susceptible to developing tongue manifestations than healthy individuals. As a sensitive indicator of overall health, the tongue may reflect systemic imbalances associated with these conditions. Therefore, while fissured and coated tongues were prevalent in the study population, these findings do not provide statistical confirmation of a link to these specific systemic diseases in this cohort due to the high risk of findings being influenced by chance, indicating the critical need for further research. Future investigations should employ substantially larger, more diverse samples recruited through probability sampling methods across multiple centers to enhance generalizability. Prospective study designs are needed to better establish temporal relationships. Critically, future studies must perform a priori power analyses to ensure adequate sample sizes for detecting clinically meaningful effects and should control for potential confounding factors, such as disease duration, severity, medication use, and oral hygiene status. Until such larger, methodologically robust studies are conducted, definitive conclusions regarding the relationship between common tongue manifestations and DM/HTN remain elusive. This study serves as an exploratory step, highlighting prevalent conditions but underscoring the need for more extensive validation.
LIMITATIONS OF THE STUDY
It is crucial to acknowledge the limitations of our study, primarily the small sample size (n = 30) recruited via convenience sampling at a single site. This limited sample size significantly reduces the statistical power to detect subtle but potentially real associations between systemic diseases and tongue pathologies. Therefore, our findings' lack of statistical significance should be interpreted cautiously. Findings, particularly non-significant ones, might be attributable to chance, given the limitations. Additionally, the convenience sampling method and the cross-sectional design may limit the generalizability of our findings. Although corroborated with medical records where possible, reliance on patient self-report for systemic disease status also introduces a potential for information bias.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: S.H.A.A.H. Study conception and design; T.S.A.: Validation; S.M.I.: Visualization; N.B.A.: Resources; A.M.K.: Draft manuscript;. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DM | = Diabetes Mellitus |
| HTN | = Hypertension |
| HBP | = High Blood Pressure |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was reviewed and approved by the Research Ethics Committee of the Faculty of Dentistry, University of Kufa, Iraq (Reference number: 899; Date: October 2022).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS:
The data supporting the findings of this study are available from the corresponding author, [S.M.I.] upon reasonable request.
ACKNOWLEDGEMENTS
The authors would like to appreciate the assistance and resources provided by the Department of Oral Diagnosis at the Al Kufa College of Dentistry.


