All published articles of this journal are available on ScienceDirect.
Prioritizing Strategic Fields for International Collaborative Research In Healthcare: A Focus on Korea-UK Partnership
Abstract
Introduction
Global collaboration in healthcare research has become increasingly essential for addressing complex health challenges. While Korea and the UK have established cooperative efforts, a structured methodology for selecting priority areas for joint research remains underdeveloped.
Methods
This study employed a three-dimensional framework to prioritize collaborative fields between Korea and the UK, integrating technological competitiveness, the need for international collaboration, and policy alignment. Thirty-five healthcare technologies were assessed using data from the 2022 Healthcare Industry Technology Level Evaluation by KHIDI. Principal Component Analysis (PCA) was used to evaluate Korea’s technology levels, and survey responses identified collaboration needs. Policy alignment was assessed via qualitative analysis of investment priorities from the MRC and NIHR. Each technology was scored from 0 to 6 based on the three evaluation dimensions, and those scoring 5 or above were selected as priority areas.
Results
Among 35 evaluated technologies, xenotransplantation and medical data standardization were identified as top priorities. This was based on comparatively low competitiveness in Korea, need of high international collaboration, and strong alignment with UK policy directions.
Discussion
These two areas address global health challenges and offer mutual benefits: xenotransplantation can alleviate organ shortages, while standardizing medical data is crucial for AI-driven diagnostics and the interoperability of healthcare systems. . The findings emphasize the strategic necessity of bilateral coordination.
Conclusion
This study presents a replicable, evidence-based framework for identifying strategic international healthcare R&D partnerships. The approach may inform future policy decisions and facilitate practical bilateral cooperation between Korea and other global partners.
1. INTRODUCTION
With the intensification of global technological competition, strategic international collaboration in key technologies has become essential for enhancing national security and technological capabilities. Major countries now recognize science and technology as core components of economic, security, and diplomatic power, driving efforts to secure technological superiority and foster strategic partnerships [1]. Recent evidence suggests that the national impact of research is driven more by international collaboration than by improvements in domestic performances [2].
In the healthcare sector, the COVID-19 pandemic highlighted the limitations of individual national efforts in addressing global health challenges, underscoring the critical importance of international cooperation. To efficiently address complex global health problems, prioritizing research areas and allocating limited resources to high-impact fields is crucial [3, 4]. Setting research priorities in international collaboration enables the pooling of expertise and resources from multiple countries, facilitating the resolution of shared health issues [5].
Despite the significant advantages of international collaborative research, challenges remain, including difficulties in selecting optimal partners [6], the potential for knowledge leakage [7], and issues related to intellectual property rights. Structured consensus-building tools such as workshops, stakeholder engagement, and the Delphi method have been proposed as effective means to address these challenges and establish research priorities [8].
While international collaboration in healthcare R&D has been discussed broadly, this study focuses specifically on the bilateral dynamics between Korea and the UK. The Korea–UK partnership is unique in that both countries have recently expanded their health R&D funding and policy priorities, particularly in areas such as digital health and dementia research [9, 10]. Korea's strong push for internationalization in R&D aligns with the UK’s strategic investment plans under the MRC and NIHR. However, gaps still remain in policy synchronization, institutional coordination, and thematic focus areas between the two nations. This study aims to identify and address these specific gaps through a structured priority-setting framework.
In 2024, Korea's health R&D budget is projected to reach 1.6 billion USD, accounting for 10.1% of the total national R&D budget of 15.9 billion USD. Among these, the Ministry of Health and Welfare’s R&D budget amounts to 572 million USD, representing 35.7% of the total health R&D expenditure. Furthermore, the ministry’s global R&D budget is set at 98 million USD, comprising 17% of its total R&D budget. This figure represents a substantial increase of 448% compared to the 17 million USD allocated for global R&D in 2023, reflecting the growing importance of international collaboration in healthcare R&D [9].
In this context, the objective of this study is to identify priority areas for healthcare collaboration with the United Kingdom. The UK has been a key partner for Korea since 2010, with ongoing joint initiatives such as Alzheimer’s research partnership awards, and dementia collaboration projects [10]. This study utilizes objective data from the healthcare industry technology level evaluation report published by public institutions in Korea. The report includes a comparative analysis of technological levels and gaps across major countries and assesses the need for international collaboration based on expert opinions, ensuring the reliability of the findings. Additionally, to evaluate policy alignment, which is a critical factor for successful international collaboration, the study has incorporated an analysis of investment direction reports of the UK’s major funding agencies, namely the Medical Research Council (MRC) and the National Institute for Health Research. (NIHR).
The structure of this study is as follows: Section 2 reviews the theoretical background and previous studies on international collaborative research. Section 3 presents the research methodology. Section 4 applies the methodology to healthcare technologies to identify priority areas for collaboration with the UK. Finally, Section 5 summarizes the results and discusses the implications and limitations of the study.
2. THEORETICAL BACKGROUND
2.1. Definition and Scope of International Collaborative Research
The scope and nature of international collaborative research vary significantly depending on the specific objectives, project types, and characteristics of each initiative. Generally, international collaborative research involves joint efforts by governments, institutions, organizations, or individuals from different countries who contribute scientific and technological resources, such as R&D funding, research facilities, and researchers, to achieve shared research goals.
Several previous studies have explored methodologies for identifying priority areas and selecting partners for international collaborative R&D. Oxley and Sampson (2004) demonstrated that concerns about potential knowledge leakage could limit the scope of collaborative research [11]. Fatehi and Choi (2009) identified critical factors for international R&D collaboration, including technology acquisition, global business expansion, and cost reduction [12]. Lee (2022) proposed a methodology for deriving priority fields in international collaborative R&D by analyzing technological levels, gaps, and policy alignment [13]. Additionally, Kim (2023) emphasized the importance of international collaboration in the defense sector and proposed ten key technologies, including artificial intelligence, quantum technology, and space exploration [14].
In another study, Jeon (2017) proposed a framework for identifying priority areas by considering national policies, technological levels, and R&D expenditure as a percentage of GDP [15]. Samuel (2024) argued that international collaborative research should focus on addressing global issues and fostering knowledge transfer, social equity, and sustainable development through policy-driven collaboration [16].
Recent studies have highlighted the significant role of international collaboration in enhancing research quality and impact. Okamura (2023) analyzed the evolution of global scientific collaboration over the past five decades, noting the formation of strong bilateral partnerships [17]. Similarly, Thelwall et al. (2024) found that international co-authorship, particularly between countries like Korea and the UK, is associated with higher quality research outputs [18].As Korea’s national strategy prioritizes technological innovation and seeks alignment with international investment priorities in digital health, this study focuses on technological competitiveness to ensure contextual relevance to Korea-UK collaboration [9]. In summary, the above can be represented as shown in Table 1.
| Category | Criteria | Related Studies |
|---|---|---|
| Technology | Technological level, technological gap, and complementarity | Oxley & Sampson (2004), Fatehi & Choi (2009), Lee (2022), Kim (2023) |
| Policy | Policy priorities | Jeon (2017), Lee (2022), Samuel (2024) |
| Market | Securing overseas markets and achieving economies of scale | Fatehi & Choi (2009), Lee (2015), Maria et al. (2024) |
| Infrastructure | R&D investment capacity | Yoo et al. (2019), Fatehi & Choi (2009), Maria et al. (2024), Kokhan (2023) |
2.2. Evaluation of Healthcare Industry Technological Levels
This study utilizes data from the “2022 Healthcare Industry Technology Level Evaluation” [19] conducted by the Korea Health Industry Development Institute (KHIDI). The evaluation aims to diagnose Korea’s technological standing in healthcare by comparing it with other major countries and identifying potential areas for improvement. The most recent evaluation conducted in 2022 is summarized in Table 2.
The evaluation standardized the technological levels and gaps by setting the highest technological level at 100% and the smallest gap at 0 years. The range of responses for technological levels was set between 0% and 100%, while the technological gap ranged from 0 to 50 years. The study covered 42 disease-related technologies and 35 industrial technologies. The evaluation also included a survey to identify key factors necessary for enhancing Korea’s technological levels, allowing respondents to rank the top three factors.
2.3. Korea-UK International Healthcare Collaboration
Korea and the United Kingdom have maintained a long-standing partnership in the healthcare sector since the signing of the Korea-UK Joint Committee on Science and Technology Cooperation in 2010. Following this agreement, collaborative research on Alzheimer’s disease was initiated in 2011, focusing on researcher exchange and joint studies. In 2014, a memorandum of understanding (MOU) was signed between the Korea Health Industry Development Institute (KHIDI) and the UK’s Medical Research Council (MRC), strengthening bilateral healthcare cooperation [10].
| Category | Details |
|---|---|
| Survey Target | Experts in healthcare, including academia, industry, and research institutions |
| Target Fields | 42 disease-related technologies and 35 industrial technologies in the healthcare sector |
| Target Countries | United States, Europe, Japan, Korea, China |
| Key Survey Contents | - Technological level and gap compared to the leading country - Strategies for technological advancement |
| Method/Period/Participants | - Delphi survey with experts - July 8, 2022 – November 30, 2022 - 563 participants |
Since 2016, the KHIDI-MRC Partnering Award program has been supporting joint research projects and facilitating networking between researchers from both countries. This initiative paved the way for further collaboration, including the Korea-UK Joint Dementia Research Project launched in 2019, which aimed to support early diagnosis and treatment of dementia [9].
In addition to MRC, KHIDI has been collaborating with the UK’s National Institute for Health Research (NIHR) since 2023. As part of this collaboration, a Korea-UK Smart Clinical Trials Joint Symposium was held in 2024, and a joint research initiative on smart clinical trials is scheduled to begin in 2025. This initiative will adopt a matching fund model, with both countries contributing equally to support research projects, thereby promoting advanced clinical trials using innovative technologies [20].
Given the strong history of collaboration and the planned expansion of joint initiatives, further research is needed to enhance the effectiveness of Korea-UK healthcare partnerships.
3. MATERIALS AND METHODS
This study aims to identify priority areas for collaboration in healthcare research between Korea and the United Kingdom. Based on a review of previous studies (Table 1), we derived the following research framework. The 35 target technologies were selected and assessedbased on Healthcare Industry Technology Level Evaluation conducted by the Korea Health Industry Development Institute (KHIDI) in “2022 ” [19]. To determine Korea-UK priority collaboration areas, the study conducted three levels of analysis.
First, technological competitiveness was analyzed. While international collaborative research is conducted for various purposes, this study focuses on identifying collaboration areas that can enhance Korea's technological competitiveness. As such, the analysis targets areas where Korea's technological level is relatively low compared to other countries.
Second, the need for international collaboration was assessed. Even if Korea's technological level is relatively low, international collaboration may not be fruitful if such collaboration cannot improve the said technology significantly. Therefore, survey results regarding the necessity of international collaboration for each field were incorporated into the analysis.
The KHIDI survey applied a modified Delphi method across two rounds, with a third round conducted for technologies with low consensus. A total of 929 experts participated in the first round and 563 in the second round, contributing responses across 77 healthcare technologies [19]. Experts were drawn from academia (54.7%), industry (21.7%), and research institutions (17.1%), with over 50% having more than 15 years of R&D experience [19]. Technological level and gap assessments were standardized, and consensus reliability was assessed by KHIDI using interquartile range (IQR) analysis to determine agreement levels among experts [19].
Finally, policy alignment with the UK was evaluated. This study analyzed the alignment of the technologies with the UK’s policies by utilizing investment direction reports from two major UK funding agencies, the Medical Research Council (MRC) and the National Institute for Health and Care Research (NIHR). To evaluate policy alignment with the United Kingdom, this study analyzed the strategic priorities outlined in the Strategic Delivery Plan 2022 to 2025 of the Medical Research Council (MRC) [21] and the Annual Report 2023/24 of the National Institute for Health and Care Research (NIHR) [22]. Policy alignment was assessed by an expert panel based on a structured review of UK strategy documents; however, inter-rater reliability could not be calculated due to the anonymity of responses.
Equal weighting was applied to the three evaluation dimensions—technological competitiveness, collaboration need, and policy alignment—based on the assumption that each dimension independently contributes to the strategic value of international collaboration. This design choice was made to ensure methodological consistency and transparency in the absence of established weighting guidelines. The study framework is illustrated in Fig. (1).
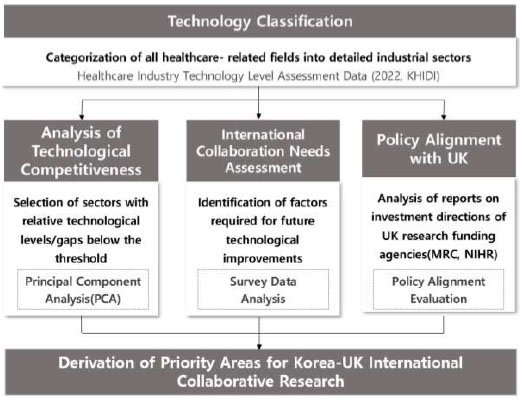
Study framework.
4. RESULTS AND DISCUSSION
This section applies the previously outlined methodology to identify priority areas for international collaborative research and development (R&D) in the healthcare sector. Given the increasing emphasis on global collaboration in healthcare following the COVID-19 pandemic, this study focuses on analyzing healthcare technologies that necessitate international efforts to tackle complex global health challenges. Moreover, effective international research collaboration requires not only researcher exchange but also a structured framework for organizational capacity building and strategic management, as emphasized in recent literature.
4.1. Classification of Healthcare Technologies
The classification of healthcare technologies used in this study is based on the “2022 Healthcare Industry Technology Level Evaluation” conducted by the Korea Health Industry Development Institute (KHIDI) [19]. The study categorizes technologies into eight categories, encompassing 35 specific technologies within the healthcare and biomedical sectors. These classifications serve as the foundation for identifying priority areas for international collaboration. (Table 3).
4.2. Technological Competitiveness Analysis
This study utilized data from the “2022 Healthcare Industry Technology Level Evaluation” to extract standardized scores for relative technological levels and technological gaps across various fields. The results, normalized by mean and standard deviation, are presented in Table 4. Standardization allows for an objective comparison of whether a specific technology field's level or gap is above or below the overall average (0) [19].
| Category | Technology | Definition |
| Drug Development | Synthetic Drugs(A1) | Drugs developed and produced through the synthesis of chemical compounds |
| Protein Therapeutics(A2) | Drugs formulated with peptides or proteins as key active ingredients | |
| Antibody Therapeutics(A3) | Drugs targeting specific antigens, leveraging antibodies to enhance immune response or inhibit specific pathways | |
| Microbiome(A4) | Therapeutic agents utilizing microorganisms within specific environments to regulate disease-related imbalances or improve health | |
| Vaccines(A5) | Biological preparations aimed at preventing or controlling infectious diseases by inducing immunity | |
| Drug Delivery Systems(A6) | Technologies or methods designed to effectively deliver drugs to target sites within the body | |
| Traditional Korean Medicine Therapies | Herbal Medicine(B1) | Medicines derived from natural plants, providing therapeutic effects for a variety of health conditions |
| Traditional Medicine Devices(B2) | Devices specifically designed for traditional medicine practices to enhance diagnosis and treatment outcomes | |
| Traditional Medicine Services(B3) | Comprehensive services that integrate traditional medical practices for diagnosis, treatment, and care | |
| Biomarker Discovery | Biomarker Discovery(C1) | Identification of DNA, RNA, proteins, or metabolites to predict, diagnose, or monitor disease progression |
| Regenerative Medicine | Stem Cells (Cell Reprogramming) (D1) | The process of reprogramming somatic cells into induced pluripotent stem cells |
| Xenotransplantation(D2) | Use of organs, tissues, or cells derived from other species for transplantation | |
| Tissue Engineering Products/Composite Products(D3) | Tissue regeneration products that function by transplanting cultured tissues developed in vitro | |
| Cell Therapy Products(D4) | Medicinal products manufactured using living cells that are cultured, expanded, or selected ex vivo through physical, chemical, or biological methods | |
| Gene Therapy Products(D5) | Medicinal products that include genetic material designed to influence gene expression or modified cells containing altered or introduced genetic material | |
| Medical Device | Diagnostic Imaging Devices(E1) | Systems that visualize internal tissues or organs, either invasively or non-invasively, to extract and process clinical information for diagnosis or treatment |
| Bio-Signal Monitoring Devices(E2) | Devices that measure weak bio-signals, such as potential differences, magnetic fields, pressure changes, and flow variations, to analyze and diagnose various diseases | |
| In Vitro Diagnostic Devices(E3) | Devices that perform diagnostic testing outside the human body using biological samples collected from patients | |
| High-Energy Delivery Therapeutic Devices(E4) | Devices that deliver high-energy (e.g., radiofrequency, low-frequency, radiation) into the body for diagnostic or therapeutic purposes | |
| Automated and Interventional Surgical Devices(E5) | Systems and devices that integrate automation technologies to provide safer and more convenient medical services | |
| Rehabilitation Devices(E6) | Devices designed to assist individuals in performing tasks they cannot manage independently or to help individuals with physical disabilities achieve superior mobility | |
| Dental Materials and Devices(E7) | Devices and materials used for dental treatments, including the prevention, diagnosis, and treatment of diseases or abnormal conditions affecting teeth and surrounding tissues | |
| Digital Health | Telemedicine(F1) | Medical activities conducted remotely between physicians and patients using communication technologies to assess patient conditions and provide appropriate care |
| Digital Therapeutics(F2) | Software-based medical devices intended to prevent, manage, or treat diseases beyond traditional diagnostic and monitoring capabilities | |
| Virtual & Mixed Reality Technologies in Digital Health(F3) | Technologies such as AR, VR, and MR integrated with big data and ICT to provide advanced healthcare services | |
| Mobile Health (mHealth) and Digital Health Systems(F4) | Mobile Health (mHealth): Services using mobile devices like smartphones to manage health without visiting healthcare facilities Digital Health: Systems utilizing discrete variables to process and analyze health-related information |
|
| Healthcare Analytics(F5) | A field of study that focuses on digitizing health and medical-related information to provide stable and efficient healthcare services | |
| Medical Information | Medical Data Generation and Collection(G1) | Technologies for collecting and storing various medical-related data, such as health and clinical information |
| Standardization of Medical Data(G2) | Establishing principles for naming, defining, formatting, and applying information elements related to health and clinical data across systems | |
| Medical Data Security and Protection(G3) | Technologies designed to safeguard various medical-related data, such as health and clinical information, from accidents, intentional modifications, damage, or data breaches | |
| Medical Information Systems(G4) | Systems that utilize computers to collect, manage, and enable the retrieval of medical-related information as needed | |
| Medical Artificial Intelligence | AI-Based Disease Diagnosis and Treatment Systems(H1) | Systems that analyze medical big data, such as clinical records, biometrics from medical devices, medical imaging, and genetic information, using techniques like CNN, RNN, reinforcement learning, deep learning, and GAN to diagnose or treat diseases |
| AI-Based Disease Prevention and Prediction Systems(H2) | Systems that leverage medical big data, including clinical records, biometrics from medical devices, medical imaging, and genetic information, trained with CNN, RNN, reinforcement learning, deep learning, and GAN to predict or prevent diseases | |
| AI-Based Drug Development Algorithms(H3) | Algorithms that utilize medical big data, such as clinical records, biometrics, medical imaging, and genetic information, trained through CNN, RNN, reinforcement learning, deep learning, and GAN to facilitate drug discovery | |
| AI-Based Healthcare Resource Optimization Systems(H4) | Systems that employ AI technologies to optimize the allocation of healthcare resources, enhancing the quality and efficiency of medical services |
| Technology | Standardized Relative Technological Level | Standardized Relative Technological Gap | First Principal Component Value | Technological Competitiveness Lower Sector (Method 1) | Technological Competitiveness Lower Sector (Method 2) |
| Synthetic Drugs(A1) | -0.42 | 0.52 | -0.67 | 1 | 1 |
| Protein Therapeutics(A2) | -0.42 | -0.22 | -0.14 | 0 | 1 |
| Antibody Therapeutics(A3) | -0.06 | 1.37 | -1.01 | 1 | 1 |
| Microbiome(A4) | 0.47 | 0.62 | -0.1 | 0 | 1 |
| Vaccines(A5) | -1.32 | 0 | -0.93 | 0 | 1 |
| Drug Delivery Systems(A6) | 1.19 | 0 | 0.85 | 0 | 0 |
| Herbal Medicine(B1) | 0.47 | -1.07 | 1.09 | 0 | 0 |
| Traditional Medicine Devices(B2) | 1.37 | -1.07 | 1.73 | 0 | 0 |
| Traditional Medicine Services(B3) | 2.27 | -2.13 | 3.12 | 0 | 0 |
| Biomarker Discovery(C1) | 0.47 | 0 | 0.34 | 0 | 0 |
| Stem Cells (Cell Reprogramming) (D1) | 0.92 | 0 | 0.66 | 0 | 0 |
| Xenotransplantation(D2) | -2.22 | 2.11 | -3.07 | 1 | 1 |
| Tissue Engineering Products/Composite Products(D3) | 1.23 | -1.07 | 1.62 | 0 | 0 |
| Cell Therapy Products(D4) | -1.32 | 0.52 | -1.3 | 1 | 1 |
| Gene Therapy Products(D5) | -0.96 | -0.54 | -0.3 | 0 | 1 |
| Diagnostic Imaging Devices(E1) | 1.19 | 0.3 | 0.62 | 0 | 0 |
| Bio-Signal Monitoring Devices(E2) | 0.47 | 0 | 0.34 | 0 | 0 |
| In Vitro Diagnostic Devices(E3) | 1.01 | -1.07 | 1.47 | 0 | 0 |
| High-Energy Delivery Therapeutic Devices(E4) | -1.32 | 2.64 | -2.81 | 1 | 1 |
| Automated and Interventional Surgical Devices(E5) | 0.11 | -0.11 | 0.16 | 0 | 0 |
| Rehabilitation Devices(E6) | -0.42 | 1.05 | -1.04 | 1 | 1 |
| Dental Materials and Devices(E7) | 0.47 | -1.07 | 1.09 | 0 | 0 |
| Telemedicine(F1) | -0.42 | -1.07 | 0.45 | 0 | 0 |
| Digital Therapeutics(F2) | -1.32 | 1.05 | -1.68 | 1 | 1 |
| Virtual & Mixed Reality Technologies in Digital Health(F3) | -0.42 | 0 | -0.29 | 0 | 1 |
| Mobile Health (mHealth) and Digital Health Systems(F4) | 0.47 | -0.54 | 0.71 | 0 | 0 |
| Healthcare Analytics(F5) | 0.47 | 1.05 | -0.4 | 0 | 1 |
| Medical Data Generation and Collection(G1) | 0.02 | 0 | 0.02 | 0 | 0 |
| Standardization of Medical Data(G2) | -2.22 | 1.58 | -2.69 | 1 | 1 |
| Medical Data Security and Protection(G3) | 0.47 | -0.54 | 0.71 | 0 | 0 |
| Medical Information Systems(G4) | 0.47 | -1.07 | 1.09 | 0 | 0 |
| AI-Based Disease Diagnosis and Treatment Systems(H1) | -0.42 | -0.11 | -0.21 | 0 | 1 |
| AI-Based Disease Prevention and Prediction Systems(H2) | -0.15 | 0 | -0.1 | 0 | 1 |
| AI-Based Drug Development Algorithms(H3) | -0.6 | 0 | -0.42 | 0 | 1 |
| AI-Based Healthcare Resource Optimization Systems(H4) | 0.47 | -1.07 | 1.09 | 0 | 0 |
Fig. (2) visualizes the relative technological levels and gaps for each technology field. Two approaches were used to identify fields with lower technological competitiveness:
Method 1: Technologies with a standardized technological level below 0 or a technological gap above 0 were classified as having lower competitiveness.
Method 2: Recognizing the high correlation between technological levels and gaps (Pearson correlation coefficient: -0.676), principal component analysis (PCA) was employed to reduce the two-dimensional data into a single dimension (PC1). PC1 accounted for 83.1% of the total variance, and was most strongly loaded on relative technical level (+0.707) and relative technical gap (-0.708). PC1 represents a composite indicator of technological competitiveness derived from technological levels and gaps. Technologies with PC1 values below 0 were classified as lower competitiveness under Method 2.
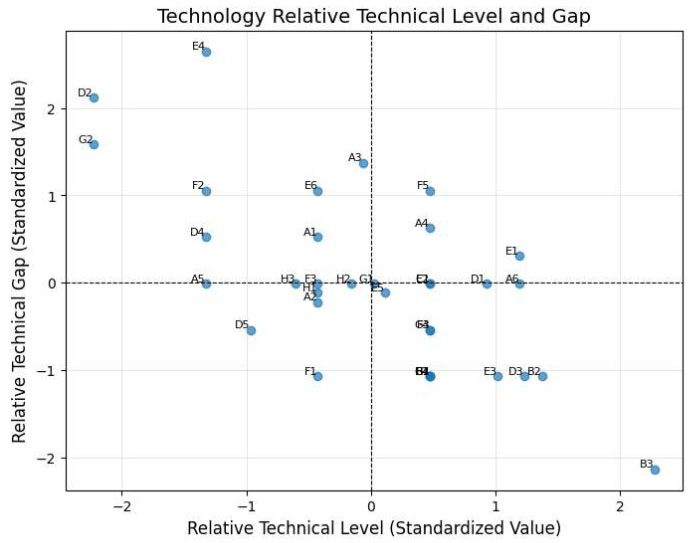
Relative technological levels and gaps across technology fields.
| Technology | International Collaboration Need | Top 25% | Above Mean +Std |
| Synthetic Drugs(A1) | 9.1 | 0 | 0 |
| Protein Therapeutics(A2) | 40 | 1 | 1 |
| Antibody Therapeutics(A3) | 10 | 0 | 0 |
| Microbiome(A4) | 0 | 0 | 0 |
| Vaccines(A5) | 0 | 0 | 0 |
| Drug Delivery Systems(A6) | 25 | 1 | 1 |
| Herbal Medicine(B1) | 4.8 | 0 | 0 |
| Traditional Medicine Devices(B2) | 9.1 | 0 | 0 |
| Traditional Medicine Services(B3) | 15.8 | 1 | 0 |
| Biomarker Discovery(C1) | 11.1 | 0 | 0 |
| Stem Cells (Cell Reprogramming) (D1) | 7.1 | 0 | 0 |
| Xenotransplantation(D2) | 28.6 | 1 | 1 |
| Tissue Engineering Products/Composite Products(D3) | 0 | 0 | 0 |
| Cell Therapy Products(D4) | 5.3 | 0 | 0 |
| Gene Therapy Products(D5) | 20 | 1 | 0 |
| Diagnostic Imaging Devices(E1) | 7.1 | 0 | 0 |
| Bio-Signal Monitoring Devices(E2) | 0 | 0 | 0 |
| In Vitro Diagnostic Devices(E3) | 9.1 | 0 | 0 |
| High-Energy Delivery Therapeutic Devices(E4) | 22.2 | 1 | 1 |
| Automated and Interventional Surgical Devices(E5) | 10 | 0 | 0 |
| Rehabilitation Devices(E6) | 13.3 | 0 | 0 |
| Dental Materials and Devices(E7) | 0 | 0 | 0 |
| Telemedicine(F1) | 0 | 0 | 0 |
| Digital Therapeutics(F2) | 9.1 | 0 | 0 |
| Virtual & Mixed Reality Technologies in Digital Health(F3) | 11.1 | 0 | 0 |
| Mobile Health (mHealth) and Digital Health Systems(F4) | 23.1 | 1 | 1 |
| Healthcare Analytics(F5) | 0 | 0 | 0 |
| Medical Data Generation and Collection(G1) | 12.5 | 0 | 0 |
| Standardization of Medical Data(G2) | 18.2 | 1 | 0 |
| Medical Data Security and Protection(G3) | 11.1 | 0 | 0 |
| Medical Information Systems(G4) | 11.1 | 0 | 0 |
| AI-Based Disease Diagnosis and Treatment Systems(H1) | 10 | 0 | 0 |
| AI-Based Disease Prevention and Prediction Systems(H2) | 8.3 | 0 | 0 |
| AI-Based Drug Development Algorithms(H3) | 14.3 | 1 | 0 |
| AI-Based Healthcare Resource Optimization Systems(H4) | 9.1 | 0 | 0 |
Fig. (2) visualizes the standardized scores of Relative Technical Gap (x-axis) and Relative Technical Level (y-axis) across 35 healthcare technologies. Technologies in the upper right quadrant exhibit both lower technical performance and larger development gaps compared to global benchmarks, indicating priority areas where international collaboration is critically needed to strengthen Korea’s healthcare R&D competitiveness.
4.3. International Collaboration Need Analysis
The need for international collaboration was assessed based on survey data from the “2022 Healthcare Industry Technology Level Evaluation. [19]” Respondents ranked the factors necessary for improving Korea’s technological levels, including government research funding, expert training and recruitment, and international collaboration. Among the 12 factors surveyed, the study focused on the responses identifying “international collaboration” as a critical factor. The analysis used two approaches: 1. Technologies within the top 25% of responses were classified as requiring international collaboration. 2. Technologies with response rates above one standard deviation from the mean were also classified as requiring collaboration. The results are summarized in Table 5.
4.4. Analysis of Policy Alignment with the UK
Policy alignment with the UK was assessed by analyzing the strategic investment priorities outlined in reports from the Medical Research Council (MRC) and the National Institute for Health and Care Research (NIHR). Using expert evaluations, the study determined whether the 35 technologies aligned with the policies of these institutions. The alignment results are presented in Table 6.
The Medical Research Council (MRC), as outlined in its Strategic Delivery Plan 2022 to 2025 [21], accelerates human health and economic prosperity through biomedical research, aligning with the UK government’s Life Sciences Vision. The MRC prioritizes investment in cutting-edge technologies and innovations, particularly in stem cell research, gene therapy, regenerative medicine, digital health, and artificial intelligence (AI)-driven diagnostic and therapeutic technologies. By translating research outcomes into clinical and policy applications, the MRC fosters real-world impact. Moreover, the organization emphasizes data-intensive research and interdisciplinary approaches, strengthening global collaborations to address critical challenges such as infectious diseases and environmental change. These strategic directions underscore the alignment of technologies like stem cell and gene therapy, as well as digital health, with the UK’s national policy priorities.
The National Institute for Health and care Research (NIHR), as detailed in its Annual Report 2023/24 [22], focuses on advancing health and social care through innovative research aligned with the UK’s Health Mission. The NIHR emphasizes developing cutting-edge solutions such as AI-powered diagnostic tools, digital health innovations, and precision medicine. By fostering collaboration with the NHS, researchers, and public health organizations, NIHR ensures the integration of research outcomes into practical healthcare improvements. Furthermore, the institute prioritizes health equity, supporting studies that address disparities in care delivery and outcomes across diverse populations. This strategic alignment highlights the relevance of technologies such as digital therapeutics, biomarker discovery, and AI-based systems to the UK’s policy goals for improving healthcare accessibility and efficiency. Although this study analyzed UK policy documents in depth, direct validation from UK-based stakeholders or domain experts was not conducted. This limitation is acknowledged, and future studies will incorporate cross-verification through bilateral consultation to enhance reliability and contextual relevance.
| Technology | International Collaboration Need | Top 25% | Above Mean +Std |
| Synthetic Drugs(A1) | 9.1 | 0 | 0 |
| Protein Therapeutics(A2) | 40 | 1 | 1 |
| Antibody Therapeutics(A3) | 10 | 0 | 0 |
| Microbiome(A4) | 0 | 0 | 0 |
| Vaccines(A5) | 0 | 0 | 0 |
| Drug Delivery Systems(A6) | 25 | 1 | 1 |
| Herbal Medicine(B1) | 4.8 | 0 | 0 |
| Traditional Medicine Devices(B2) | 9.1 | 0 | 0 |
| Traditional Medicine Services(B3) | 15.8 | 1 | 0 |
| Biomarker Discovery(C1) | 11.1 | 0 | 0 |
| Stem Cells (Cell Reprogramming) (D1) | 7.1 | 0 | 0 |
| Xenotransplantation(D2) | 28.6 | 1 | 1 |
| Tissue Engineering Products/Composite Products(D3) | 0 | 0 | 0 |
| Cell Therapy Products(D4) | 5.3 | 0 | 0 |
| Gene Therapy Products(D5) | 20 | 1 | 0 |
| Diagnostic Imaging Devices(E1) | 7.1 | 0 | 0 |
| Bio-Signal Monitoring Devices(E2) | 0 | 0 | 0 |
| In Vitro Diagnostic Devices(E3) | 9.1 | 0 | 0 |
| High-Energy Delivery Therapeutic Devices(E4) | 22.2 | 1 | 1 |
| Automated and Interventional Surgical Devices(E5) | 10 | 0 | 0 |
| Rehabilitation Devices(E6) | 13.3 | 0 | 0 |
| Dental Materials and Devices(E7) | 0 | 0 | 0 |
| Telemedicine(F1) | 0 | 0 | 0 |
| Digital Therapeutics(F2) | 9.1 | 0 | 0 |
| Virtual & Mixed Reality Technologies in Digital Health(F3) | 11.1 | 0 | 0 |
| Mobile Health (mHealth) and Digital Health Systems(F4) | 23.1 | 1 | 1 |
| Healthcare Analytics(F5) | 0 | 0 | 0 |
| Medical Data Generation and Collection(G1) | 12.5 | 0 | 0 |
| Standardization of Medical Data(G2) | 18.2 | 1 | 0 |
| Medical Data Security and Protection(G3) | 11.1 | 0 | 0 |
| Medical Information Systems(G4) | 11.1 | 0 | 0 |
| AI-Based Disease Diagnosis and Treatment Systems(H1) | 10 | 0 | 0 |
| AI-Based Disease Prevention and Prediction Systems(H2) | 8.3 | 0 | 0 |
| AI-Based Drug Development Algorithms(H3) | 14.3 | 1 | 0 |
| AI-Based Healthcare Resource Optimization Systems(H4) | 9.1 | 0 | 0 |
4.5. Comprehensive Analysis Results
By integrating the three dimensions—technological competitiveness, international collaboration needs, and policy alignment—priority areas for Korea-UK healthcare R&D collaboration were identified. Each dimension was evaluated using the methods outlined above, and the total score for each technology was calculated. Technologies scoring 5 or above were classified as priority areas. The results identified two key technologies for priority collaboration Table 7, as visually represented in Fig. (3).
Out of 35 technologies evaluated, two fields-xenotransplantation and standardization of medical data - scored ≥5 across all three criteria. While this may seem limited, Table 7 also lists technologies that ranked highly in one or two dimensions but did not meet the composite threshold. This scoring system ensures that selected technologies reflect the most strategic convergence of Korea’s technological needs and the UK’s policy agenda.
Xenotransplantation (D2): With a total score of 5, this field demonstrated high competitiveness (2 points), significant collaboration need (2 points), and strong policy alignment with MRC (1 point). Xenotransplantation addresses critical organ shortages and represents a transformative innovation in regenerative medicine.
Standardization of Medical Data (G2): Also scoring 5, this field is essential for improving interoperability in medical data systems, enabling advancements in digital health and AI-driven diagnostics. The technology aligns strongly with the policies of both MRC and NIHR.
The analysis identified two technologies— xenotransplantation and standardization of medical data—as the highest priority areas for Korea-UK healthcare collaboration. These fields align with Korea’s needs for technological enhancement, the UK’s policy priorities, and the overarching goal of addressing global health challenges through collaborative efforts.
| Technology | Technological Competitiveness | Collaboration Need | Policy Alignment | Sum | |||
| Method 1 | Method 2 | Top 25% | Above Mean +Std | MRC | NIHR | ||
| Synthetic Drugs(A1) | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Protein Therapeutics(A2) | 0 | 1 | 1 | 1 | 1 | 0 | 4 |
| Antibody Therapeutics(A3) | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Microbiome(A4) | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Vaccines(A5) | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| Drug Delivery Systems(A6) | 0 | 0 | 1 | 1 | 1 | 0 | 3 |
| Herbal Medicine(B1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Traditional Medicine Devices(B2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Traditional Medicine Services(B3) | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Biomarker Discovery(C1) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Stem Cells (Cell Reprogramming) (D1) | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Xenotransplantation(D2) | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| Tissue Engineering Products/Composite Products(D3) | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Cell Therapy Products(D4) | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| Gene Therapy Products(D5) | 0 | 1 | 1 | 0 | 1 | 1 | 4 |
| Diagnostic Imaging Devices(E1) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Bio-Signal Monitoring Devices(E2) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| In Vitro Diagnostic Devices(E3) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| High-Energy Delivery Therapeutic Devices(E4) | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| Automated and Interventional Surgical Devices(E5) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Rehabilitation Devices(E6) | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Dental Materials and Devices(E7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Telemedicine(F1) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Digital Therapeutics(F2) | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Virtual & Mixed Reality Technologies in Digital Health(F3) | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| Mobile Health (mHealth) and Digital Health Systems(F4) | 0 | 0 | 1 | 1 | 1 | 0 | 3 |
| Healthcare Analytics(F5) | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| Medical Data Generation and Collection(G1) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Standardization of Medical Data(G2) | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| Medical Data Security and Protection(G3) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Medical Information Systems(G4) | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| AI-Based Disease Diagnosis and Treatment Systems(H1) | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| AI-Based Disease Prevention and Prediction Systems(H2) | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| AI-Based Drug Development Algorithms(H3) | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| AI-Based Healthcare Resource Optimization Systems(H4) | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
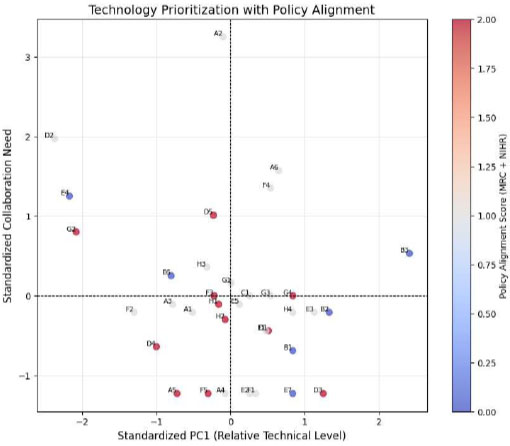
Technological competitiveness and international collaboration need with policy alignment.
Fig. (3) visualizes the final prioritization of healthcare technologies based on three evaluation dimensions: technological competitiveness (PC1, x-axis), international collaboration need (y-axis), and policy alignment (color coding). Red markers indicate technologies with strong alignment to the UK strategy. Technologies in the upper-left quadrant with red and grey coloring represent the most strategic candidates for bilateral research, demonstrating combined weaknesses in competitiveness and high alignment with collaborative and policy goals.
CONCLUSION
This study proposed a strategic framework to identify priority areas for international healthcare research collaboration between Korea and the UK. By integrating technological competitiveness, collaboration needs, and policy alignment, two key fields—xenotransplantation and medical data standardization—were identified as high-potential areas for bilateral cooperation.
These findings offer practical insights for national policymakers and funding agencies, particularly as Korea and the UK plan to initiate a joint matching fund program in 2025. Rather than claiming universal replicability, the methodology presented in this study is intended as an adaptable model that can be customized to fit other international contexts. Its transparency in scoring and evaluation logic may inform similar efforts elsewhere, but local validation and stakeholder engagement are essential for its contextual application. Moreover, effective international research collaboration requires not only researcher exchange but also a structured framework for organizational capacity building and strategic management, as emphasized in recent literature [16].
However, this study has several limitations. First, the technological levels of the UK were not assessed, which may have limited the balance of the bilateral evaluation and restricted full comparability across countries. Second, equal weighting was applied across all analytical dimensions, although in practical contexts, some criteria—such as policy alignment—may warrant greater emphasis. Third, expert judgment was employed to evaluate the policy alignment with the UK, which may have introduced subjective bias. Future studies could mitigate subjectivity by incorporating structured coding protocols, employing multiple independent raters, and applying inter-rater reliability checks. Fourth, this study did not address regulatory and geopolitical barriers that often complicate international research collaboration. For example, cross-border data sharing is subject to data protection regulations such as the General Data Protection Regulation (GDPR) in the UK and Europe. Ethical review processes and institutional requirements also vary by country, which can delay or constrain research design, especially in clinical contexts. These constraints should be systematically considered in future bilateral or multilateral research planning. Lastly, this study was limited to the Korea–UK context; extending the framework to include additional countries would improve the generalizability and strategic applicability of the findings.
Despite these limitations, this research provides a structured and data-driven approach to enhance the effectiveness of international R&D partnerships. Future studies should explicitly incorporate bilateral benchmarking of technological capabilities to ensure a more symmetrical evaluation, and explore the inclusion of diverse partner countries to further validate the framework’s applicability and robustness.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: A.R.K.: Analysis and interpretation of results; J.Y.: Draft manuscript; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| AI | = Artificial Intelligence |
| GDP | = Gross Domestic Product |
| GDPR | = General Data Protection Regulation |
| IQR | = Interquartile Range |
| KHIDI | = Korea Health Industry Development Institute |
| MOU | = Memorandum of Understanding |
| MRC | = Medical Research Council |
| NIHR | = National Institute for Health and Care Research |
| NHS | = National Health Service |
| PCA | = Principal Component Analysis |
| R&D | = Research and Development |
| UK | = United Kingdom |
| US | = United States |
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of the article is available in the Korea Health Industry Development Institute (KHIDI) repository at https://www.khidi.or.kr/ board/view?linkId=48891835&menuId=MENU01435, reference number [19].
ACKNOWLEDGEMENTS
The authors would like to thank all those who contributed to the completion of this research.


