All published articles of this journal are available on ScienceDirect.
Applying Formal Consensus Methods To Enhance The Credibility of Public Health Guideline Development – A Case Study
Abstract
Introduction
This article examines the use of formal consensus methods employed in the adaptation of the United Kingdom's National measles guidelines for the public health management of measles in Ireland. The authors explore the efficacy of a modified e-Delphi approach and Nominal Group Technique (NGT) adopted for these purposes. In articulating the findings, the authors will adhere to the ACCURATE Consensus Reporting Document (ACCORD) guidelines.
Methods
This study was undertaken in accordance with a Consensus-Based Recommendations (CBR) protocol. The study phases comprised modified e-Delphi rounds and two NGT meetings with a consensus panel. The intention was to gather panellist input and agree on a conclusive list of guideline recommendations.
Results
Following a review of evidence about international jurisdictions, and contextualisation of evidence to an Irish frame of reference, all draft recommendations received consensus (100%) among all panellists following requisite modifications to the text.
Discussion
This is the first study to establish a public health guideline in Ireland through the explicit use of formal consensus methods and to report on this process in compliance with the ACCORD guideline. Globally, formal consensus methods are not yet routinely used for developing public health guidelines. This underlines the significance of this study for enhancing international understanding of the fundamentals of applying formal consensus methods.
Conclusion
This case study has demonstrated that, when used in tandem with the “GRADE-ADOLOPMENT” approach, formal consensus methods are effective in fusing the requisite evidence sources to ensure a rigorous, comprehensive, and equitable evidence base for public health practice.
1. INTRODUCTION
Developing evidence-informed guidance is a core function of the National Health Protection Office (NHPO) within Ireland’s Health Service Executive. The purpose is to enhance health outcomes for patients, diminish variation in practice, and support and improve the quality of clinical decision-making. This is delivered through the work of the NHPO’s Research and Guideline Development Unit (RGDU), which leads the development of evidence-informed health protection guidance. Recently, the RGDU has developed a range of resources of methodological interest to ensure the quality of guidance produced to support health protection practice in Ireland. These include a protocol that focuses specifically on advancing consensus-based recommendations in the context of public health guideline development.
Conceptually, this is a complex evolution, designed to facilitate comprehensive, systematic, transparent, and authentic processes, thereby catalyzing expert opinion in circumstances where contemporaneous evidence is ambiguous, limited, unavailable, or still evolving [1]. Formal methods have previously been established for the purposes of healthcare guideline development (Delphi Method [2] and Nominal Group Technique [3]) that enhance the consensus process by engaging appropriate subject matter expertise, facilitating objective input, and diminishing the potential for bias in group decision making. Despite this recognition, except for the World Health Organisation (WHO), the use of formal consensus methods has not yet become a routine approach for public health guideline development [4].
The aim of this article was to examine the use of formal consensus methods employed in the adaptation of the United Kingdom Health Security Agency (UKHSA) ‘National measles guidelines’ [5] for the public health management of measles in Ireland. The authors will explore the efficacy of a modified e-Delphi approach and Nominal Group Technique adopted for these purposes. In addition, the future significance of this methodology is considered within the context of adapting and contextualizing public health guidelines.
In articulating the findings from this consensus study, the authors will adhere to the ACCURATE Consensus Reporting Document (ACCORD) guidelines (S1) [6]. This is designed to ensure rigour, accuracy, and transparency when reporting on consensus processes and subsequent outcomes.
The explicit processes associated with the adaptation, adoption, and de novo development of guideline recommendations have been previously outlined using the “GRADE-ADOLOPMENT” (Grading of Recommendations Assessment, Development, and Evaluation) approach [7]. While the core principles of evidence-based guideline development apply to both clinical and public health guidelines, public health guidelines often require a more nuanced and contextualised approach to address the complexities of population-level interventions and policies. As a consequence, in view of the hierarchy of evidence applied to clinical guidelines, this is frequently more challenging in the context of public health practice [8, 9]. With relevance to this study, the contextualisation process facilitates an examination of established guidelines and supportive evidence with a potential positive impact upon resources typically associated with this endeavour. This case study is shaped around the Guideline International Network (GIN) McMaster Guidelines Development Checklist (GDC) (S2), which reinforces the guideline development process and underpins the development and implementation of authentic public health guidelines for Ireland.
Historically, within the healthcare context, consensus methods have been applied as a means of enhancing clinical decision-making and advancing health policy [10]. Regardless of previous recommendations from organisations including National Institute for Health and Clinical Excellence (NICE) [11] and Public Health Scotland (PHS) [12], evidence suggests that there are potentially significant shortcomings with informal consensus methods, applied to decision-making within guideline development, and most notably these concern: power imbalance of individuals within the consensus panel resulting in dominance over discourse and subsequent recommendations; a lack of transparency on complex issues as a result of unstructured processes [13]. In addition, it is considered that informal consensus methods are more likely to be founded upon both insufficient criteria and arrangements for explicit consensus. As a consequence, guidelines developed using this approach are frequently subjective and inadequately defined [3].
Recognizing the limitations of informal consensus methods, formal consensus methods have been promoted to provide systematic and transparent support for group decision-making, reduce potential biases, and ensure equal opportunities for engagement among all members [14]. Formal consensus methods are considered a suitable approach to address the aforementioned complexities, and to support the adaptation and adoption of public health guidelines in Ireland when situated within a structured and transparent framework [15]. Although it is theoretically understood that consensus models are typically iterative, it is strongly recommended that this approach be situated within an evidence-based framework [16]. The RGDU has adopted the “GRADE-ADOLOPMENT” methodology for this purpose, and this framework is endorsed by organisations including the WHO, ECDC (European Centre for Disease Prevention and Control), and NICE.
Through the methods discussed below, formal consensus approaches can facilitate meaningful engagement among subject matter experts relevant to the topic under examination. As a consequence, this synergy facilitates valuable consultation among expert panelists and, correspondingly, limits the impact of narrow, influential behavior, where there may be insufficient supportive evidence, thereby mitigating negative consequences for guideline developers [17]. In tandem with the best available scientific evidence, formal consensus methods incorporate rigorous and explicit processes that fuse the knowledge, experiences, and perspectives of stakeholders representing multiple clinical specialties, patient and public forums, and additional subject matter experts. This facilitates the production of recommendations and guidelines that are more credible and considered.
In previous studies [14, 19], researchers have employed a combination of formal consensus methods, which typically include both the Delphi Method and the Nominal Group Technique. This should enable researchers to exploit the perceived benefits of both mechanisms. If applied properly, this combination has been demonstrated to maximize judgment among group members, resulting in optimal reliability of outcomes [20].
The underlying premise for utilizing these methods is that group decision-making among subject matter experts offers considerable benefits, such as consolidating expert knowledge, perspectives, and experiences on a wide range of topic areas. If applied methodically, these methods should also enhance participant anonymity, group participation, controlled feedback, and statistical group consensus [21].
The Delphi Method has been widely applied by health scientists, and more pertinently, to elicit consensus on guideline recommendations. It can provide guideline developers with a structured method of soliciting opinions from a panel of experts by virtue of a questionnaire designed specifically for this purpose. Expression of opinion is facilitated anonymously through the questionnaire, enabling guideline developers to collate the information and engage in an iterative process via multiple rounds of evaluating and refining submissions. In support of this approach, Rowe and Wright (1999) [22] suggest that the four main tenets of the Delphi method are focused on anonymity, iteration, controlled feedback, and statistical aggregation of group responses. The final purpose was to achieve expert consensus and advance the formulation of recommendations [18]. It has been previously suggested that many Delphi studies do not adequately define criteria for achieving consensus, and that even when consensus has been defined, it is not always clear whether the prespecified criteria for consensus have been a factor in deciding when to stop the Delphi process [23].
The Delphi Method is frequently combined with the Nominal Group Technique (NGT) in a hybrid formal consensus approach towards guideline development [20]. The NGT is a facilitated group interaction that empowers group members to have their voices heard and opinions considered by fellow members [24]. Originally designed by Delbecq and Van de Ven [25] it comprises four key stages, although these are open to adaptation when employed alongside additional consensus methods. When used in conjunction with the Delphi Method, NGT meetings generally follow the initial Delphi round(s) and are guided by an experienced facilitator. It is crucial that the criteria for selecting panel experts, the group size, the procedure, and the principles for reaching consensus are transparent, and the entire process is recorded, including how and when consensus was reached [18]. The NGT approach is conducive to extracting relevant and trustworthy qualitative information from a panel of experts. It is suggested that the attributes of the NGT, specifically the focus on collaboration, enhance ownership of the items under discussion and thus increase the potential for positive outcomes [26].
2. METHODS
This study was undertaken in accordance with a consensus-based recommendations (CBR) protocol. This protocol is available on the RGDU website and is part of a suite of documents supporting the development of public health guidelines in Ireland.
2.1. Consensus Methods Literature Review: Search Strategy
The original search was undertaken during June 2024 and repeated in December 2024 (S3). The project team identified a total of 791 potentially relevant records from a systematic search of Medline, Embase, and CINAHL, of which 409 duplicates were removed at this point. A total of 382 records were then screened in Rayyan [27] by title and abstract, with a further 241 excluded on the basis of incorrect outcome, population, study design, foreign language, or duplication. The remaining 141 records were subjected to full-text screening by two authors using Rayyan in blinded mode. A thematic analysis of full-text records was applied based on additional inclusion criteria that considered consensus articles reporting on the risk of bias in group decision-making, ensuring transparency in the process, systematic methods adopted, and a comprehensive perspective in decision-making [28]. Of these, a further 70 records were excluded, leaving 71 remaining. A hand search also yielded a further 15 records, therefore, a final total of 86 records were included in the review. (Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram PRISMA Fig. (1).
The search strategy was reviewed by the lead author and the Knowledge Management Officer (RGDU). Any differences of opinion between reviewing authors were resolved by consensus discussion.
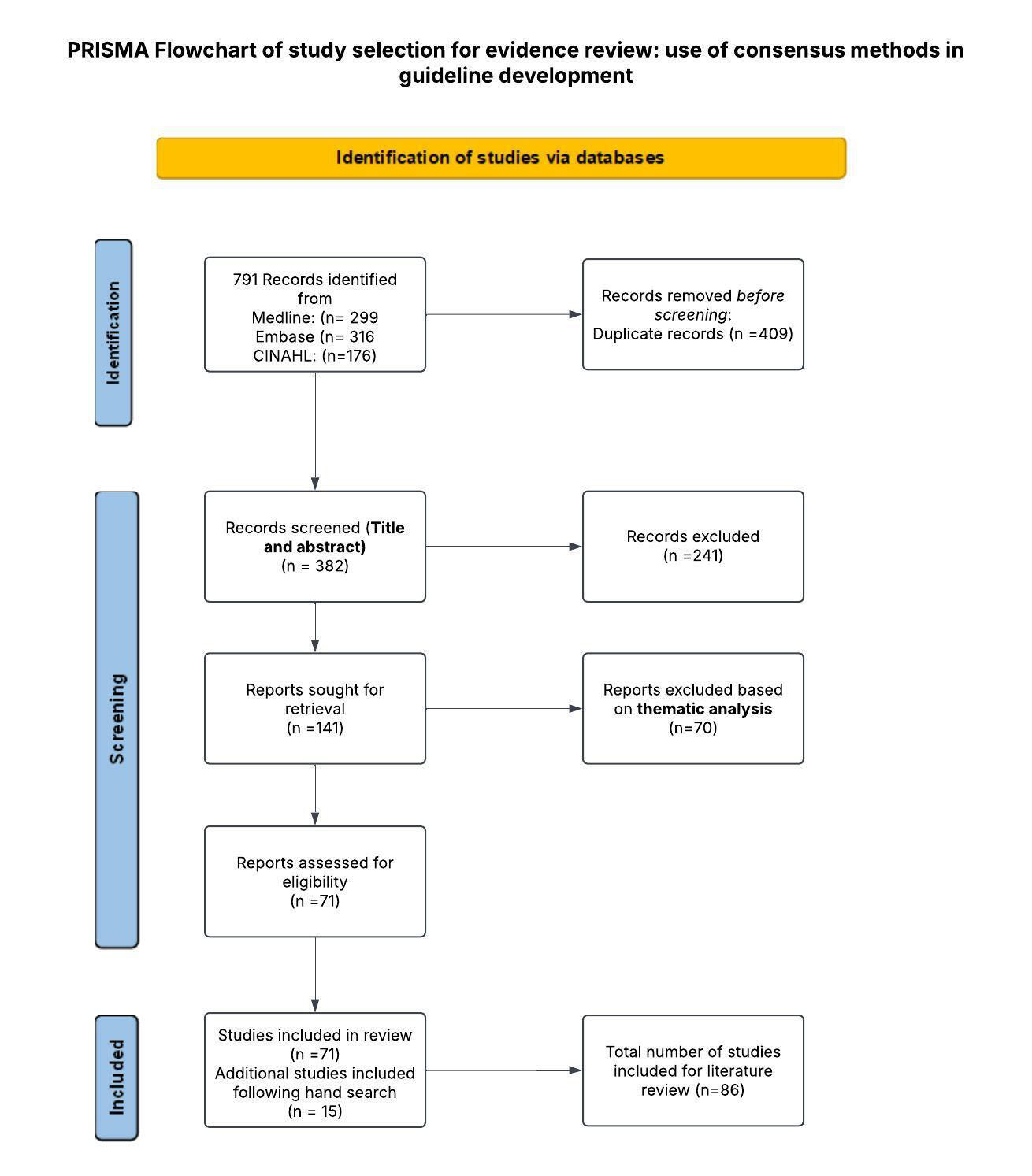
PRISMA flowchart.
2.2. Defining Consensus
It has been previously espoused that there is no requirement to characterise consensus as unanimity among all guideline development group (GDG) members [17]. To that end, many studies have purposely formulated, a priori, definitive rules that aggregate results, thus indicating the strength of agreement. For this case study, the research team adopted the meaning of consensus purported by the World Health Organisation (2014) [4] “In guideline development groups, consensus decision-making is a process whereby the consent of all committee members is pursued. When consensus has been reached, it generally means that every committee member finds the proposed resolution acceptable – or at least lends it support, even if less than wholeheartedly”.
This understanding of consensus is further underlined by Siwiec and colleagues (2019) [18], who emphasize the role of negotiation as an instrument for achieving formal consensus. Rather than pursuing concessions from panel members, it is argued that the purpose of consensus is recognising solutions that are acceptable to all involved. [18] Similarly, Nair et al. (2011) [17] have affirmed that perfect consensus is seldom realized; therefore, the objective of consensus methods is to establish a central tendency within the group and grade the resultant level of agreement.
2.3. Membership of Guideline Development Panels
Participants in this project included those who were members of the GDG and subsequently formed subject matter expert topic groups (SME-TGs). Membership was established on the basis of several key principles, supported by the World Health Organization, to ensure that the panels provide credible, balanced, and relevant public health guidance [29]. These included relevant subject matter clinical expertise and experience, relevant academic expertise, diversity of knowledge and experience, commitment and participation, and conflict of interest disclosure. Although participation bias is to some extent inevitable in populating public health expert panels, this was mitigated by recruiting panel members from a broad range of backgrounds, specialties, and experiences to ensure no single group dominated the discussions [30].
The Terms of Reference were ratified for the GDG, delineating the role of the Chair, the responsibilities of group members, and the responsibilities of the RGDU. This document also outlined principles for working together, which included openness, respect, transparency, and confidentiality (S4). The GDG was chaired by a subject matter expert/Consultant in Public Health Medicine, with methodological expertise provided by the RGDU. The GDG comprised twenty-seven multidisciplinary members. The records from GDG meetings reflect a consistent picture among group members throughout the project's lifetime. This was important for ensuring continuity, cohesion, and high-quality decision-making, particularly with reference to consensus panels [31]. The rationale for the number of panel members was to ensure an optimal group size to achieve a topic-appropriate balance of expertise and adequate representation on the guideline panel and SME-TGs. An iterative process was initiated with membership reviewed at all meetings to ensure requisite experience and content expertise throughout the guideline development process.
Membership of the GDG reflected stakeholders from across the entire spectrum of national public health, including infectious diseases (adult and paediatric), microbiology, Irish College of General Practitioners (ICGP), Antimicrobial Resistance and Infection Control (AMRIC) 1, National Social Inclusion Office (NSIO), Irish Prison Services (IPS), National Virus Reference Laboratory (NVRL), National Immunisation Office (NIO), Pharmacy and Occupational Health.
Members were recruited to the GDG via email by the National Measles Incident Management Team (N-IMT), a collaborative national structure convened by the Director of National Health Protection in response to the measles threat in early 2024. Members had experience, knowledge, and skills in evidence-based medicine and guideline development. The national RGDU provided methodological oversight and project management support throughout the process. The consensus panel, derived from GDG membership, played a significant role in developing the guideline through agreement on recommendations based on summaries of evidence.
The SME-TG comprised evidence review experts, all of whom had training in data analysis and evidence-based medicine. They performed systematic searches, appraisal, and synthesis of evidence for specific content. An SME-TG lead supervised group members who developed evidence summaries and recommendations and presented these to the Consensus Panel. Roles and functions are outlined in Table 1.
No patients were involved in any significant role in the development of this guideline. However, in advance of the publication of the Guidelines for the Public Health Management of Measles in Ireland (2025), the GDG engaged with the National Patient and Service User Forum, and a final draft copy was made available for feedback. This was completed in February 2025.
2.4. Conflict of Interest
To adequately moderate potential conflicts of interest and uphold standards of integrity, conduct, and concern for the public interest, all GDG members were required to complete a conflict-of-interest declaration form as a prerequisite for effective participation. The Chair and the RGDU screened and reviewed submissions by panel nominees for potential conflicts of interest. A policy for managing conflicts of interest was implemented in accordance with the HSE Code of Governance 2021 [32].
2.5. Sources of Evidence/Preparatory Research
The RGDU developed a detailed search strategy document (S5), and a systematic search was undertaken across Medline, CINAHL, PubMed, and the TRIP (Turning Research into Practice) database to identify potentially relevant resources. A hand search of Google and Google Scholar was also undertaken. As a consequence, four international guidelines were identified that met the inclusion criteria that had been set a priori and these included National measles guidelines (UKHSA); Measles for Healthcare Providers (Centers for Disease Control and Prevention); Health New Zealand (Ch 12 Measles) Communicable Disease Control Manual; Measles - Communicable Diseases Network Australia (CDNA) National Guidelines for Public Health Units.
1AMRIC is a national team, established by the HSE, to address the challenges facing the Irish health service in dealing with healthcare-associated infection and antimicrobial resistance.
| Group and number recruited | Composition | Role description |
|---|---|---|
|
Guideline Development Group (GDG) 27 members |
Multidisciplinary stakeholders with diverse experience and expertise | • Define the scope and purpose of the guideline. • Constitute SME-TGs and Consensus Panel • Reviewing the evidence and developing recommendations for practice. • Stakeholder consultation (both internal and external). • Ongoing evaluation and review of the guideline • Liaise with the Health Protection Advisory Committee for Infectious Diseases (HPAC-ID) regarding publication and dissemination of the guideline. |
|
Subject Matter Expert Topic Groups (SME-TGs) 8-10 members |
Experts with previous knowledge of evidence‐based medicine and evidence synthesis |
• Synthesise evidence and expert opinions to formulate recommendations for public health practice. • Identify variations in practice upon analysis of the evidence summaries and recommendations from the source guideline. • Developed and presented the recommendations and evidence to the consensus panel. |
|
Consensus panel (CP) 23 members |
Multidisciplinary stakeholders with diverse experience and expertise | • Participate in iterative rounds of voting to reach consensus on recommendations. • Address areas of practice where variation may exist, and rigorous evidence may be inadequate. • Appraise draft guidelines to ensure they are comprehensive, clear, and applicable to public health practice. |
These guidelines were independently assessed for methodological rigour and transparency using the Appraisal of Guidelines for Research and Evaluation (AGREE II) Instrument. Each guideline was appraised by two reviewers selected from a review group that comprised methodological expertise (RGDU) and subject matter expertise (GDG) [33]. High-quality guidelines were defined as those where the AGREE II score >70%.
A novel Stakeholder Engagement Form (S6) was employed to summarize the content agreed upon from the source guideline, and this was circulated to all members of the GDG to ensure that all relevant topics for the guideline had been addressed. Topics were identified as follows: Background, Public Health Management, and Specific Settings and Situations. Accordingly, the GDG decided to adopt and adapt the National measles guidelines (UKHSA).
In July 2024, upon the development of the three SME-TGs, it was evident that detailed training and education were required to enable coherence across each group to effectively implement the formal consensus methodology for the purposes of synthesizing evidence and expert opinions and formulating recommendations. The project team delivered bespoke training sessions based on a consensus-based recommendations protocol. This approach took account of the diverse previous experience of group members in both guideline development and the application of consensus methods.
2.6. Assessing Consensus
The formal consensus measures employed in this study involved multiple phases, including a modified e-Delphi round and two NGT meetings with the consensus panel. Adopting a modified e-Delphi approach meant that the project team established the relevant questions at the outset before submitting these to the consensus panel [34]. Primarily, the process involved extracting evidence summaries and recommendations from the source guideline and preparing decision logs for consideration by the consensus panel Fig. (2). The intention was to gather panellist input and agree on a conclusive list of recommendations for inclusion in a guideline for the public health management of measles in Ireland.
2.7. e-Delphi
Evidence summaries and recommendations were extracted from the source guideline for the agreed-upon topics. SME-TGs identified variations in practice upon analysis of the evidence summaries and recommendations from the source guidelines. The SME-TGs formulated a total of 36 statements around the topics included in the scope of the guideline. Consensus decision logs were prepared as MS Forms by each SME-TG for their area of expertise based on these statements. The aim at this juncture was to achieve consensus from all panel members on the statements presented. Statements were presented as either “updated content” or “new content,” with the rationale and evidence source for the modification stated. Panel members were presented with three response options to each statement: “Proposed content acceptable”, “Proposed content acceptable (with modifications)”, “Proposed content unacceptable”. Panellists were required to explain their responses if they found any of the statements to be either “acceptable with modifications” or “unacceptable”. The questionnaires with the list of statements are available as supplementary material (S7a, S7b, S7c).
Results from the e-Delphi round were returned individually by panel members. For ease of use, results were collated by the RGDU using MS Forms and Excel. A subsequent meeting of each SME-TG was held to formulate another iteration of statements based on the results collected from the panel. These statements were then presented to the consensus panel at the NGT meeting in October 2024.
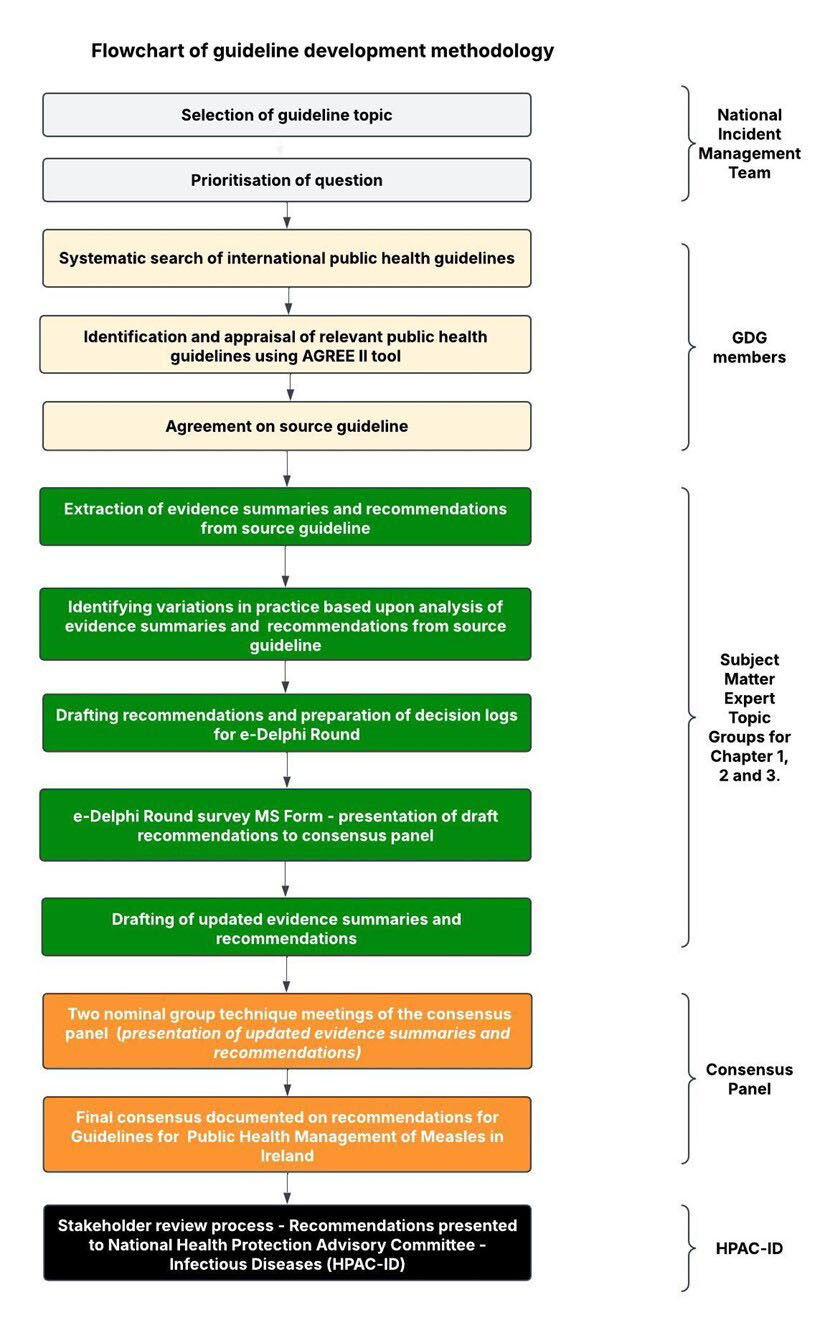
Flowchart of guideline development methodology.
2.7.1. Nominal Group Technique Meetings
Within this study, the purpose of the NGT meetings was to consider the updated statements that the participants had reviewed and commented on during the e-Delphi Round. The first meeting focused primarily on the statements that were the source of disagreement from Round 1, i.e., those that received responses of “Proposed content acceptable (with modifications)” or “Proposed content unacceptable.” Statements that had reached consensus “Proposed content acceptable” in the previous round were not included in subsequent rounds. The nominal group meeting was facilitated by the Chair of the GDG, an experienced NGT facilitator, which helped ensure that the process ran smoothly and that high-quality decisions were made. When all members had been given the opportunity to respond, there was a discussion to clarify, defend, or challenge the issues. All members of the group were given the opportunity to discuss all the issues they wished to. There was then an opportunity for each participant to re-rate the revised statements. Consensus was achieved when all panellists found the revised content “Acceptable,” and for the purpose of this study, it meant that every committee member found the proposed resolution acceptable, or at least lent it support, even if less than wholeheartedly. The final consensus was documented for all statements in the Decision Log.
2.8. Ethical Considerations
This study involves ‘normal and statutory public health work’ aimed at reviewing evidence and developing recommendations with members of the GDG. The Reference Research Ethics Committee Midlands and Corporate Services (HSE Dublin & Midlands) [35] confirmed that, as such, ethical approval was not required for this project.
3. RESULTS
3.1. Formal Consensus Phase 1: e-Delphi Round – Chapter 1 (Background)
This e-Delphi round was scheduled to close on 3rd October 2024, and in advance of this, the SME-TG for this specific chapter populated a decision log with six draft recommendations and supportive evidence summaries. Four of these draft recommendations had been adapted from the source guideline, and two represented de novo content. As stated previously, an MS form was established containing all relevant information and presenting panellists with three response options (S7a, S7b, S7c). A copy of the draft guideline content was also circulated to panellists as an aide-memoire.
A response rate of 87% (n = 20) of panellists was recorded for Chapter 1. Regarding the draft content, one of the draft recommendations was accepted by all (100%) panellists without modification; three draft recommendations were accepted without modification by nineteen (95%) panellists, with one panellist accepting these recommendations with modifications to the text. Regarding the remaining draft recommendations, one was accepted without modifications by eighteen (90%) panellists, with two panellists accepting the recommendations with modifications to the text. The final draft recommendation was again accepted without modifications by nineteen (95%) panellists, with one panellist finding the proposed content unacceptable.
3.2. Formal Consensus Phase 1: e-Delphi Round – Chapter 2 (Public Health)
This e-Delphi round was coordinated to close on 11th October 2024 and followed an identical arrangement to that outlined above. Within this chapter, the SME-TG identified 19 draft recommendations, considering content adapted from the source guideline (11) and de novo content (8).
Once again, a response rate of 87% (n = 20) of panellists was recorded for Chapter 2. Three of the nineteen draft recommendations were accepted without modifications by all (100%) of the panellists who responded. Of the remaining sixteen draft recommendations, there were no instances where panellists indicated that proposed content was unacceptable. However, there were varying numbers (1-6) among panellists who indicated that the proposed content would be acceptable following modifications to the text. This was particularly applicable to the adapted draft recommendation on ‘Defining the infectious period’; and de novo content on ‘Defining exposure risk to vulnerable immunocompetent individuals’ e.g., infants and pregnant women.
3.3. Formal Consensus Phase 1: e-Delphi Round – Chapter 3 (Specific Settings)
The final e-Delphi round was coordinated to close on October 22, 2024, and, once again, followed the same approach. Within this chapter, the SME-TG identified 11 draft recommendations, considering content adapted from the source guideline (7) and de novo content (4).
A response rate of 70% (n=16) was recorded for chapter 3. Two of the eleven draft recommendations were accepted without modification, and of the nine remaining recommendations, there were no instances where panelists indicated that the proposed content was unacceptable. Once again, there were varying numbers (1-4) among panellists who indicated that the proposed content would be acceptable following modifications to the text. This outcome was more focused on draft recommendations related to ‘Measles in settings for underserved populations’ (de novo content); and amended content referring to ‘Considerations for healthcare staff’; ‘Educational and Childcare settings’; ‘Air Travel’; and ‘Protecting hospitalised children from measles’. Consensus levels related to all draft recommendations are presented in Table 2, while a summary of the results is outlined in Table 3.
Table 2.
| Formal Consensus Phase | Statement Number | Consensus Decision | Panel Attendance % |
|---|---|---|---|
| Phase 1 - Delphi Round 1 | 1 | Proposed content acceptable - 95% Proposed content unacceptable - 5% | 87% |
| Phase 1 - Delphi Round 1 | 2 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 1 | 3 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 1 | 4 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 1 | 5 | Proposed content acceptable - 100% | 87% |
| Phase 1 - Delphi Round 1 | 6 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 1 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 2 | 2 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 3 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 4 | Proposed content acceptable - 85% Proposed content acceptable (with modifications) – 15% | 87% |
| Phase 1 - Delphi Round 2 | 5 | Proposed content acceptable - 70% Proposed content acceptable (with modifications) – 30% | 87% |
| Phase 1 - Delphi Round 2 | 6 | Proposed content acceptable - 75% Proposed content acceptable (with modifications) – 25% | 87% |
| Phase 1 - Delphi Round 2 | 7 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 2 | 8 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 2 | 9 | Proposed content acceptable - 100% | 87% |
| Phase 1 - Delphi Round 2 | 10 | Proposed content acceptable - 100% | 87% |
| Phase 1 - Delphi Round 2 | 11 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 12 | Proposed content acceptable - 85% Proposed content acceptable (with modifications) – 15% | 87% |
| Phase 1 - Delphi Round 2 | 13 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 2 | 14 | Proposed content acceptable - 85% Proposed content acceptable (with modifications) – 15% | 87% |
| Phase 1 - Delphi Round 2 | 15 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 16 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 2 | 17 | Proposed content acceptable - 95% Proposed content acceptable (with modifications) – 5% | 87% |
| Phase 1 - Delphi Round 2 | 18 | Proposed content acceptable - 100% | 87% |
| Phase 1 - Delphi Round 2 | 19 | Proposed content acceptable - 90% Proposed content acceptable (with modifications) – 10% | 87% |
| Phase 1 - Delphi Round 3 | 1 | Proposed content acceptable - 75% Proposed content acceptable (with modifications) – 25% | 70% |
| Phase 1 - Delphi Round 3 | 2 | Proposed content acceptable - 81% Proposed content acceptable (with modifications) – 19% | 70% |
| Phase 1 - Delphi Round 3 | 3 | Proposed content acceptable - 94% Proposed content acceptable (with modifications) – 6% | 70% |
| Phase 1 - Delphi Round 3 | 4 | Proposed content acceptable - 100% | 70% |
| Phase 1 - Delphi Round 3 | 5 | Proposed content acceptable - 94% Proposed content acceptable (with modifications) – 6% | 70% |
| Phase 1 - Delphi Round 3 | 6 | Proposed content acceptable - 75% Proposed content acceptable (with modifications) – 25% | 70% |
| Phase 1 - Delphi Round 3 | 7 | Proposed content acceptable - 81% Proposed content acceptable (with modifications) – 19% | 70% |
| Phase 1 - Delphi Round 3 | 8 | Proposed content acceptable - 75% Proposed content acceptable (with modifications) – 25% | 70% |
| Phase 1 - Delphi Round 3 | 9 | Proposed content acceptable - 94% Proposed content acceptable (with modifications) – 6% | 70% |
| Phase 1 - Delphi Round 3 | 10 | Proposed content acceptable - 100% | 70% |
| Phase 1 - Delphi Round 3 | 11 | Proposed content acceptable - 100% | 70% |
| Phase 2 - NGT meeting | Requisite 100% consensus achieved on all items with exception of the following: ‘Immunocompetent vulnerable contacts: pregnant women’ (Chapter 2); ‘Considerations for healthcare staff’ (Chapter 3); ‘Air travel’ (chapter 3); ‘Measles in prisons and places of detention’ (chapter 3); ‘Protecting hospitalised children from measles’ (Chapter 3). | 100% | |
| Phase 3 - NGT meeting | All outstanding items received consensus among all panellists (100%) following requisite modifications to text. | 100% |
| Formal Consensus Phase | Context | Outcomes |
|---|---|---|
|
Phase 1 - Delphi Round 1 Chapter 1 |
Six draft recommendations were presented (4 adapted from existing guidelines, 2 de novo), with supportive evidence summaries. | • 1 recommendation accepted by all (100%) without modification. • 3 recommendations accepted without modification by 19 (95%); 1 panellist accepted with modifications. • 1 recommendation accepted without modification by 18 (90%); 2 panellists accepted with modifications. • 1 recommendation accepted without modification by 19 (95%); 1 panellist found it unacceptable. |
|
Phase 1 - Delphi Round 1 Chapter 2 |
19 draft recommendations presented (11 adapted, 8 de novo), with supportive evidence summaries. | • 3 recommendations accepted by all (100%) without modification. • For the remaining 16, no panellist found the content unacceptable, but 1–6 panellists per item requested modifications. This was especially relevant for recommendations on ‘Defining the infectious period’ and ‘Defining exposure risk to vulnerable immunocompetent individuals’ (e.g., infants, pregnant women). |
|
Phase 1 - Delphi Round 1 Chapter 3 |
11 draft recommendations presented (7 adapted, 4 de novo) with supportive evidence summaries. | • 2 recommendations accepted without modifications. • For the remaining 9, no content was found unacceptable, but 1–4 panellists per item requested modifications. This feedback focused on recommendations for ‘Measles in settings for underserved populations’, ‘Considerations for healthcare staff’, ‘Educational and Childcare settings’, ‘Air Travel’, and ‘Protecting hospitalised children from measles’. |
| Phase 2 - NGT meeting | Decision logs for all chapters were reviewed, reflecting modifications based on e-Delphi feedback. Panel discussions focused on recommendations that had previously been in disagreement. Voting was conducted for unresolved items. |
• Chapter 1: Consensus reached on all except ‘Transmission of primary measles’ (referred to NIAC for further review). • Chapters 2 & 3: Consensus (100%) achieved for most recommendations, with exceptions: • ‘Immunocompetent vulnerable contacts: pregnant women’ (Ch. 2) • ‘Considerations for healthcare staff’ (Ch. 3) • ‘Air travel’ (Ch. 3) • ‘Measles in prisons and places of detention’ (Ch. 3) • ‘Protecting hospitalised children from measles’ (Ch. 3) These exceptions required further evidence review or modifications. |
| Phase 3 - NGT meeting | NIAC provided clarification on ‘Transmission of primary measles’. Outstanding recommendations were reviewed, with evidence contextualised for Ireland. |
• Consensus (100%) was achieved for all previously unresolved recommendations after text modifications. • Agreement to submit the complete guideline document for approval by the National Health Protection Advisory Committee for Infectious Diseases in February 2025. |
3.4. Formal Consensus Phase 2: Nominal Group Technique Meeting
The preliminary NGT meeting was convened on the 5th of November 2024. All members of the consensus panel were present, representing both regional and national stakeholders and expertise. The meeting was chaired by a public health consultant with previous experience in facilitating these groups. As was the case with the e-Delphi exercise, the processes underpinning the NGT meeting had been previously clarified for panel members by the RGDU. Nevertheless, the project team accepted the opportunity to summarise the approach once again, ensuring that all participants were familiar with the functions and purpose of the meeting.
Panel members were provided with copies of the decision logs for chapters one, two, and three, which had been amended following the conclusion of the e-Delphi phase. In the intervening period, the SME-TGs responsible for each individual chapter had reassembled to address the feedback received from the e-Delphi exercise. Consequently, evidence summaries were reviewed and actions focused on modifying proposed recommendations where necessary. These modified recommendations were now presented to the panel for review, discussion, and consensus.
Similar to Singh and colleagues (2013) [19], the benefit, in this case, of commencing a Delphi process in advance of the NGT meeting is that it created space for domains of consensus and non-consensus to be revealed. The impact of this was that the NGT meeting was able to effectively concentrate on the domains where there were differences of opinion and thus lacked overall consensus.
Panellists reviewed the proposed recommendations, with a particular focus on areas where previous variations in practice and divergence had been identified. The Chair moderated the ensuing discussion, ensuring that all panellists had an equal opportunity to submit an informed opinion. Following this, voting was facilitated on all draft recommendations where consensus remained outstanding following the e-Delphi phase. Consensus was established among all panellists for all draft recommendations within chapter one, except for that relating to ‘Transmission of primary measles’. In this case, it was agreed that the National Immunisation Advisory Committee (NIAC) should be requested to undertake a further review of evidence on this matter.
The process for advancing consensus was facilitated in an iterative manner for chapters 2 and 3 as well Fig. (3). As regards these chapters, the NGT process was followed for all draft recommendations and the requisite level for consensus (100%) was attained with the exceptions of items relating to ‘Immunocompetent vulnerable contacts: pregnant women’ (Chapter 2); ‘Considerations for healthcare staff’ (Chapter 3); ‘Air travel’ (chapter 3); ‘Measles in prisons and places of detention’ (chapter 3); ‘Protecting hospitalised children from measles’ (Chapter 3). The expert consensus panel determined that additional time was needed to review current evidence in respect of proposed recommendations impacting these settings and, if necessary, request further evidence synthesis and/or modifications.
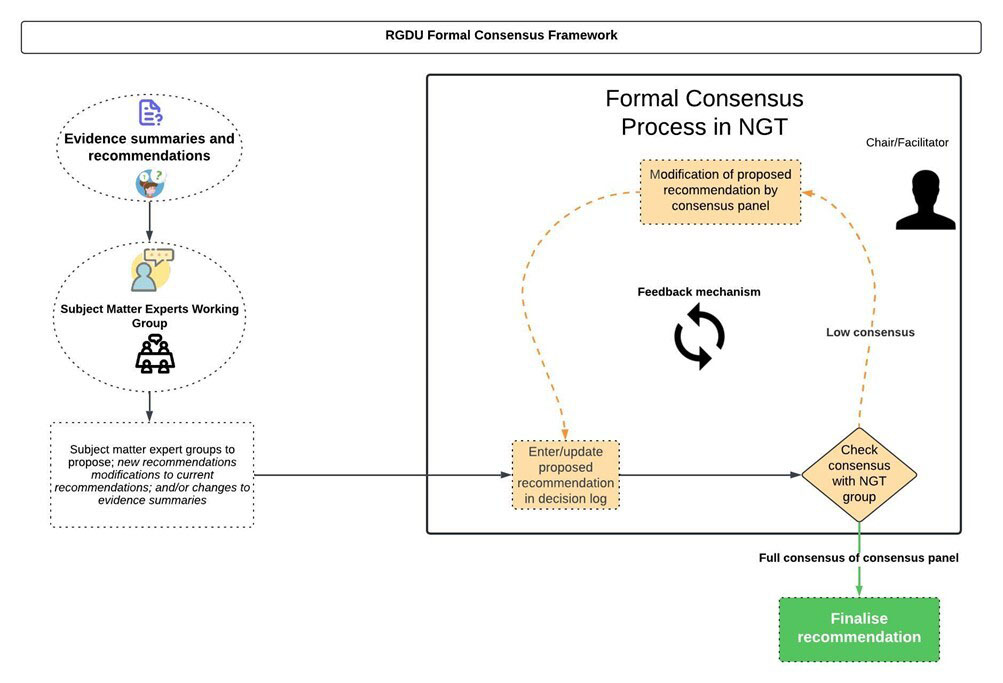
RGDU formal consensus framework.
3.5. Formal Consensus Phase 3: Nominal Group Technique Meeting
A second Nominal Group meeting was convened on 19th December 2024. Once again, all consensus panellists were present, and the meeting was facilitated by the Public Health Consultant who had presided over phase 2. In the interim period, clarification had been received from NIAC on evidence impacting ‘Transmission of primary measles’. In addition, based on the feedback from the consensus panel during Phase 2, a review of evidence pertaining to other jurisdictions, and contextualization of the evidence within an Irish frame of reference, all outstanding draft recommendations received consensus among all panellists (100%) following the requisite modifications to the text. It was further agreed that the complete guideline document should be considered for approval by the national Health Protection Advisory Committee for Infectious Diseases in February 2025.
4. DISCUSSION
To the best of our knowledge, this is the first study to establish a public health guideline in Ireland through the explicit use of formal consensus methods and to report on this process in compliance with the ACCORD guideline. Moreover, on a global scale, formal consensus methods are not yet universally or routinely used for developing public health guidelines [14]. This underlines the significance of this particular study, and the inherent methodological examination for enhancing international understanding of the fundamentals of applying formal consensus methods for this purpose.
The methodological strengths of this study are reflected across a number of key criteria, although specific challenges endure that are not unique to this particular project. Rating the certainty of evidence and moving from evidence to decision is a constant challenge in the context of public health guideline development [36]. This is partly due to the lack of public health-related randomised studies. Having acknowledged this, it is also important to accept that the legitimacy of evidence is not solely predicated on the fact that it is derived from a randomised controlled trial, but rather that it reflects the most relevant source for the matter under examination [3]. To moderate these requirements, previously published guidelines were assessed for quality using the AGREE II instrument, and recommendations were adopted, adapted, or contextualized to the Irish context. Where indicated, further evidence review was also undertaken as outlined during ‘formal consensus phase two’. Aligned with the aspirations of the GIN adaptation working group, the intention was to enhance the proficiency of the guideline development process whilst upholding requirements for rigour and transparency [37].
The transparency of the methods employed was of particular importance in developing the recommendations, and the appropriate application of AGREE II ensured that the methodological quality of the source guideline was a key consideration. Furthermore, detailed information relevant to each recommendation was recorded in the consensus decision log, providing a transparent association across panel members, supporting evidence, and final recommendations. Where areas of contention had arisen, these were also clearly documented, including the resolution achieved through formal consensus methods.
A number of additional core characteristics also underpinned the application of the study methodology. A cardinal attribute among these involved the adoption of systematic methods – a multi-phase consensus approach was followed, which included both a modified e-Delphi technique and NGT meetings. The advantages of this ‘hybrid’ model have been previously recognised [24] and this has enabled the project team to balance flexibility with a rigorous and systematic approach. It has also been recognised that within many contemporary studies that report the use of consensus methods, the implementation phases are poorly illustrated and are systematically inconsistent. [21] The research team was cognizant of the need to explicitly highlight the methods applied, which are emphasized in Figs. (1 and 2). Similar to Wang and colleagues (2018) [38], the authors would also contend that these frameworks, adopted for guideline adaptation and the application of formal consensus methods, facilitated systematic measures for guideline adaptation that heightened methodological rigour and, ultimately, the quality of the adapted guideline. As a consequence, the recommendations produced reflect the Irish context whilst remaining aligned with the definitive sources of evidence.
A further pivotal aspect was the heterogeneous composition of the GDG and consensus panel. The impact of this was particularly apparent during phases two and three, helping to ensure that diverse perspectives were considered and that the recommendations were grounded in both scientific data and practical expertise. Similar to Hennessy et al. (2022) [39], the authors recognize the rich diversity across the GDG and consensus panels and the opportunities this provides to learn from each other, while being granted ‘space’ for reflection and discussion.
Overall, the methodology applied in developing this guideline helped to diminish the potential for bias to impact panel decision-making. During the e-Delphi rounds, all panel members had the opportunity to anonymously record their opinions on the evidence incorporated and the wording of the recommendations. This helped to counter the potential for dominance by any single voice and ensured that all viewpoints were represented. Similarly, the structured facilitation of NGT meetings provided additional safeguards, thus limiting any inherent risk of bias.
5. STRENGTHS AND LIMITATIONS
The methodological strengths of this study lie in the adherence to a cohesive framework that incorporates the “GRADE-ADOLOPMENT” approach with formal consensus methods. This aligns with the ACCORD reporting guideline, which enables transparency and a systematic structure to the decision-making process. The consensus panel composition was also a significant factor in enabling more robust and innovative outcomes. Within panel discussions, this was evident in the breadth of perspectives presented, which impacted the applicability of the recommendations.
Upon reflection, the number of consensus rounds (n=5) applied in this study may present the potential for participant burnout and a lack of enthusiasm to engage in later rounds. It is evident that, during e-Delphi round three, the attendance level among panellists dropped to 70%. However, it is also apparent that, during phases two and three, there was 100% attendance among panel members. An overall attendance percentage of 89% indicates a high level of active engagement and provides a solid foundation for more balanced decision-making during the consensus process.
The authors recognize that the inclusion of a patient representative may have enabled opportunities to develop more patient-centered content within the guideline. At the outset, the GDG discussed the complexities of patient involvement in the guideline process, which highlighted several potential barriers. These included issues related to technical language associated with a public health guideline, the potential for a limited scope of patient involvement, and difficulty in integrating patient experience in a meaningful way within this guideline. Nonetheless, it is also conceivable that a more limited perspective can emerge during consensus discussions when patient representation is absent. The authors acknowledge that issues of transparency and trust can be effectively addressed through the presence of meaningful patient engagement.
CONCLUSION
This case study has demonstrated that, when used in tandem with the “GRADE-ADOLOPMENT” approach, formal consensus methods are effective in fusing the requisite evidence sources to ensure a rigorous, comprehensive, and equitable evidence base for public health practice. The transparency created through the structured processes, which were invoked to capture expert opinion, ensured clarity, reproducibility, and mitigation of arbitrary decisions, thus increasing stakeholder confidence in the guideline content. In applying this approach, the GDG was able to adopt, adapt, and contextualize a range of evidence-informed recommendations that will enhance the public health management of measles in Ireland.
This paper consolidates the work of several writers [14, 15, 28, 39, 40] who have previously articulated the practical experience of adopting formal consensus methods for guideline development. The authors will continue to learn and evolve our processes based upon these experiences. In addition, through a range of measures, including the reduction of bias, inclusion of diverse expertise, systematic and transparent processes, and evidence-informed decision-making, this work may enhance the future development and credibility of public health guidelines internationally.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ACCORD | = Accurate Consensus Reporting Document |
| AGREE II | = Appraisal of Guidelines for Research and Evaluation Instrument |
| AMRIC | = Antimicrobial Resistance and Infection Control |
| CBR | = Consensus-Based Recommendations |
| CDNA | = Communicable Diseases Network Australia |
| ECDC | = European Centre for Disease Prevention and Control |
| GDC | = Guidelines Development Checklist |
| GDG | = Guideline Development Group |
| GIN | = Guideline International Network |
| GRADE | = Grading of Recommendations Assessment, Development and Evaluation |
| ICGP | = Irish College of General Practitioners |
| IPS | = Irish Prison Services |
| NGT | = Nominal Group Technique |
| NHPO | = National Health Protection Office |
| NIAC | = National Immunisation Advisory Committee |
| NICE | = National Institute for Health and Clinical Excellence |
| N-IMT | = National Measles Incident Management Team |
| NIO | = National Immunisation Office |
| NSIO | = National Social Inclusion Office |
| NVRL | = National Virus Reference Laboratory |
| PHS | = Public Health Scotland |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RGDU | = Research and Guideline Development Unit |
| SME-TGs | = Subject Matter Expert Topic Groups |
| UKHSA | = United Kingdom Health Security Agency |
| WHO | = World Health Organisation |
ETHICAL STATEMENT
The Reference Research Ethics Committee Midlands and Corporate Services (HSE Dublin & Midlands) confirmed that, as such, ethical approval was not required for this project.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
We would like to express our thanks and appreciation to the study participants and to the Health Service Executive for their support in conducting this study.


