All published articles of this journal are available on ScienceDirect.
Evaluating the Feasibility and Usability of Blood-based Abbott CheckNOW™ HIV SELF TEST among Men Who Have Sex with Men and Transgender Women in KwaZulu-Natal, South Africa: A Cross-Sectional Study
Abstract
Introduction
Key populations, particularly men who have sex with men and transgender women, face significant challenges in accessing HIV testing services in public health facilities, despite their availability. Lack or avoidance of HIV testing among these populations results in delayed diagnosis and increased HIV transmission risk. HIVST has emerged as a potential approach to increasing HIV testing uptake. This study evaluated the usability, feasibility, and acceptability of the CheckNOW™ HIV SELF TEST kit to inform public health strategies and policies aimed at enhancing HIV testing and linkage to care among these vulnerable populations.
Methods
A cross-sectional study using convenience sampling was conducted between February and April 2024 in two Kwa-Zulu Natal districts in South Africa. A sample of 250 men who have sex with men and transgender women aged 18 years of age and above was included in the study. Data were collected through a paper-based questionnaire exploring the feasibility and usability of the blood-based CheckNOW HIV Self-Test. Completed questionnaires were digitised into Epicollect. Data were analyzed using SPSS Version 29, employing descriptive statistics to assess overall usability and feasibility, and Cohen’s coefficient of agreement to evaluate concordance between staff and participant test interpretations. Error occurrences were categorized as critical or non-critical to assess operational challenges.
Results
Of the 245 participants who completed the test, 89.3% (CI: 85.2-92.6%) found it easy to carry out the whole test, and 91.7% (CI: 87.7-94.7%) rated the test as acceptable. Additionally, 40.8% (CI: 34.8-47.1%) of participants were able to draw blood from the basin without generating bubbles, while 76.6% (CI: 71.7-82.6%) of participants found it easy to collect enough blood.
Discussion
The study reported high usability and feasibility scores for the test; however, this evaluation revealed broader perspectives that offer room for improvement. This was achieved through the survey design, which investigated and identified points of confusion or hesitation, as well as any critical and non-critical errors made during the self-testing procedure.
Conclusion
The study findings support the notion that HIVST is highly usable, feasible, and acceptable, therefore informing its scale-up to help address a critical gap in the HIV prevention and treatment efforts among these key populations.
1. INTRODUCTION
Sub-Saharan Africa (SSA) has the highest HIV infection rate, particularly among key populations, including MSM and transgender women, making the use of provider-initiated testing widely recommended [1, 2]. This recommendation, however, does not adequately account for the barriers these populations face to access HIV testing services, including HIV-related stigma, discrimination, privacy and confidentiality concerns, and logistical challenges [2-6]. Consequently, these populations avoid testing, leading to delayed diagnosis and increased transmission risk.
HIV Self-Testing (HIVST) has emerged as an effective approach to provide HIV testing services to unreached populations, therefore, overcoming these barriers while improving the testing uptake. This approach is recommended by the World Health Organization (WHO) as an additional effective testing method to use in public health initiatives to access unreachable populations for HIV testing services [7]. HIVST incorporates self-sampling of the individual’s blood specimen to perform the HIV Rapid Diagnostic Test (RDT), followed by self-interpretation of the results [8]. HIVST benefits include allowing people to test themselves in the privacy of their own homes, providing immediate and confidential results without worrying about issues such as stigma, potential breach of confidentiality, distance, and time required to travel to testing sites [9-12].
There has been a widespread adoption of HIVST kits in South Africa. One test is the Abbott CheckNOW™ HIV SELF TEST (“the Test”) that has been introduced among the several test kits that have been evaluated for use in the country. The test is a single-use, in vitro, visually read, qualitative immunoassay for the detection of antibodies to HIV in human whole blood. The Test met WHO Prequalification status on 6 April 2022 - Product code: 29012-W01, Application Number: PQDx 0481-032-00. The test is also currently Conformité Européenne (CE) marked and has obtained Expert Review Panel for Diagnostics (ERPD) approval. The specificity (99.9%) and sensitivity (100%) of the test have been identified through clinical evaluations conducted in South Africa, Congo, Vietnam, and Spain. Abbott’s CheckNOW™ HIV SELF TEST kit is to be used as an aid in the diagnosis of HIV infection. The self-test is intended for use by untrained individuals, utilizing a blood sample collected via a finger stick puncture. The test has been designed for ease of use and requires only two additional components, in addition to the self-collected fingerstick blood sample: the test device and a buffer solution.
There is a growing body of supporting evidence showing the acceptability and usability of HIVST in various key populations and groups, with further plans for evaluations on device performance. Public pilot HIVST programs in sub-Saharan Africa show high acceptability, feasibility, and correct usage of HIVST kits [11, 13]. However, there is a limited body of research on assessing the usability and feasibility of the CheckNOW™ HIVST, and existing studies have not thoroughly explored whether these attributes extend to diverse contexts, such as the eThekwini and uMgungundlovu districts in South Africa, particularly among high-risk populations such as men who have sex with men (MSM) and transgender women (TGW). This study aimed to evaluate the CheckNOW™ HIV SELF TEST devices specifically in these regions among MSM and TGW to understand their usability, feasibility, and acceptability. This study provides a comprehensive insight into the user experience, including label comprehension and result interpretation, while enhancing efforts to reach the UNAIDS 95-95-95 targets.
2. METHODS
2.1. Study Design
This was a cross-sectional study using convenience sampling, where eligible participants who came to Aurum Institute POP INN facilities for routine medical care or were invited to the study were approached for prospective enrollment. The POP INN clinics are specialised facilities that provide healthcare services for MSM and TGW. Recruitment continued until a desired sample size was reached. The targeted outcomes of interest were the usability, feasibility, and acceptance of blood-based HIVST kits in the hands of untrained lay users. Usability was defined as the count and proportion of participants who found it easy to complete all testing steps correctly without assistance and accurately interpreted the results. Feasibility included assessing lay users’ ability to correctly utilize the self-test, successfully obtain an interpretable result, and correctly interpret the results. Acceptability focused on the lay user’s acceptance of HIVST, willingness to recommend the test, trust in the accuracy of the test results, and comfort level in using it at home.
2.2. Study Site
The study was conducted in KwaZulu-Natal in the EThekwini and Umgungundlovu districts due to their high HIV prevalence rates of 17.6% and 19.5%, respectively [14]. Moreover, these districts represent diverse settings, with eThekwini being predominantly urban and uMgungundlovu characterized by peri-urban and rural communities, facilitating the evaluation of HIV self-testing across different population contexts.
2.3. Study Population
The study population consisted of key population clients, particularly MSM and TGW, seeking HIV testing and prevention services at the Aurum Institute POP INN facilities/clinics. The eligibility criteria included individuals aged 18 years of age and above. Participants who were known to be HIV-positive, known to be HIV-negative, or had unknown HIV status and had no prior experience with self-testing for HIV were included in the study. Clients were excluded if they had any prior experience with HIVST, were healthcare professionals or lay providers, and had experience with HIV rapid testing.
2.4. Sample Size
Using the standard formulas for cross-sectional studies (n=Z ^2 *p * (1−p) / e^2), the minimum sample size for this cross-sectional study was calculated based on an expected HIV self-testing usability rate of 85%, a 95% confidence interval, and a 5% margin of error, resulting in an estimate sample of 196 participants. The final sample size included 250 untrained individuals, with 40 participants being known HIV-positive, 82 participants being known HIV-negative participants and 128 participants not knowing their HIV status, based on results obtained from their previous testing at the POP INN clinic. The increase in sample size beyond the estimated value was to account for potential data loss and to increase statistical power. Post-hoc power analysis indicated that this sample size provided approximately 80–85% power to detect a minimum 10% difference in usability outcomes between demographic groups.
The recruitment of clients with various HIV statuses was to ensure that bias was not given to any particular group.
2.5. Data Collection
A paper-based questionnaire (Appendix A) was used to collect the data, which was then digitized into Epicollect upon completion. A unique participant ID was assigned to each participant, matched to the test conducted. Data collection was conducted by 14 HIV testing services (HTS) counsellors from 22 February - 11 April 2024. The healthcare providers invited participants to participate in the study through a study enrollment screening process that ensured the inclusion of the correct number of participants with the required HIV status and that the participants had sufficient information about the study and its requirements. Participants who met the eligibility criteria and agreed to participate in the study were asked to sign the consent form.
The enrolled participant then conducted the CheckNOW™ HIV SELF TEST based on the product instructions for use (IFU). Participants were given one HIVST kit with no further information about the device or test procedure and asked to perform the test in front of a health staff observer. A trained study staff member supervised the testing and result interpretation but did not assist with the IFU understanding, testing, or result interpretation. The staff did not interact with the participant unless intervention was required for a study participant’s safety, well-being, or privacy. During the self-testing procedure, the study staff used product-specific, semi-structured questionnaires with an observation checklist of the HIVST process, as well as a sheet for recording test results. After testing, the study staff member confirmed the untrained user’s result, and each test result was photographed and labelled with the participant’s ID number (ID).
2.6. Data Analysis
The data from the surveys was coded and imputed into Microsoft Excel for data cleaning and coding. All data with more than 50% missing information and duplicates were excluded, which led to a total sample size of 245. Cleaned data were analyzed on SPSS Version 29. Descriptive statistics were calculated for the overall usability of the self-test to give an overview of the attitudes that are held by the clients. The survey completed by the staff member for each participant was analysed for concordance between the staff and the participant regarding: correct interpretation, correct test use, and reading of instructions. This was evaluated using Cohen’s coefficient of agreement.
Errors were further analyzed and categorized as critical or non-critical. Critical errors occurred when participants made operational mistakes during the assay that could potentially invalidate the results, such as spilling the developer buffer or prematurely terminating the process. Non-critical errors occurred when participants made mistakes that deviated from the IFU but were not considered to lead to false or invalid results, such as holding the buffer vial rather than placing it on the table. The proportion of participants committing critical errors was calculated.
3. RESULTS
3.1. Demographic Characteristics of the Participants
The study sample consisted of 245 adult MSM and TGW participants aged 18–56 years, with a median age of 38 years (IQR: 56-18). Most participants (30.2%) were between the ages of 18-24 years, with 67.3% having completed high school or obtained a diploma equivalent. Over 71.0% of the participants were from urban areas. Regarding monthly income, 22% of participants reportedly earned R0-R1000 per month, while 54.8% reported an income range of R1001-R50000 per month (Table 1).
3.2. Usability of CheckNOW™ HIV SELF TEST
The average usability index of the Abbott CheckNOW™ test was 84.6% (Table 2). Among the 245 eligible participants, 89.3% (CI: 85.2-92.6%) found it easy to carry out the whole test, whereas 10.7% experienced challenges. Of the 245 participants, 95.5% (CI: 92.1-97.5%) found it easy to remove the cap from the lancet, and 95.1% (CI: 89.6-96.2%) were able to easily apply the buffer to the test. Participants struggled with preventing bubbles from forming when transferring blood from the basin to the test device. Only 40.8% (CI: 34.8-47.1%) of the participants were able to correctly transfer blood from the basin to the test device with no bubbles generated (Fig. 1).
3.3. Feasibility of the CheckNOW™ HIV SELF TEST
Among the 245 untrained lay users, the majority (93%) were able to use the self-test successfully and correctly (Table 3). A specific challenge involved generating bubbles, where only 40.8% (34.8-47.1%) of participants were able to draw blood from the basin without generating bubbles (Table 2). Of the participants, 76.6% (CI: 71.7-82.6%) faced challenges in drawing enough blood to conduct the test.
Table 1.
| - | Category | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Age | 18 - 24 years | 74 | 30.2 |
| 25 - 29 years | 46 | 18.8 | |
| 30 - 34 years | 53 | 21.6 | |
| >35 years | 56 | 22.9 | |
| Education level | Never attended school | 2 | 0.8 |
| Primary School | 10 | 4.1 | |
| High school or diploma equivalent | 165 | 67.3 | |
| Some college or technical degree | 30 | 12.2 | |
| College or postgraduate degree | 28 | 11.4 | |
| Prefer not to answer | 10 | 4.1 | |
| Monthly income | R0 - R1000 per month | 54 | 22.0 |
| R1 001 - R2 500 per month | 35 | 14.3 | |
| R2 501 - R5 000 per month | 33 | 13.5 | |
| R5 0001 - R10 000 per month | 35 | 14.3 | |
| R10 001 - R50 000 per month | 31 | 12.7 | |
| >R50 000 per month | 3 | 1.2 | |
| Prefer not to answer | 54 | 22.0 | |
| - | In a rural area | 55 | 22.4 |
| Geographical location | In an urban area | 175 | 71.4 |
| Not listed, please specify below: | 15 | 6.1 |
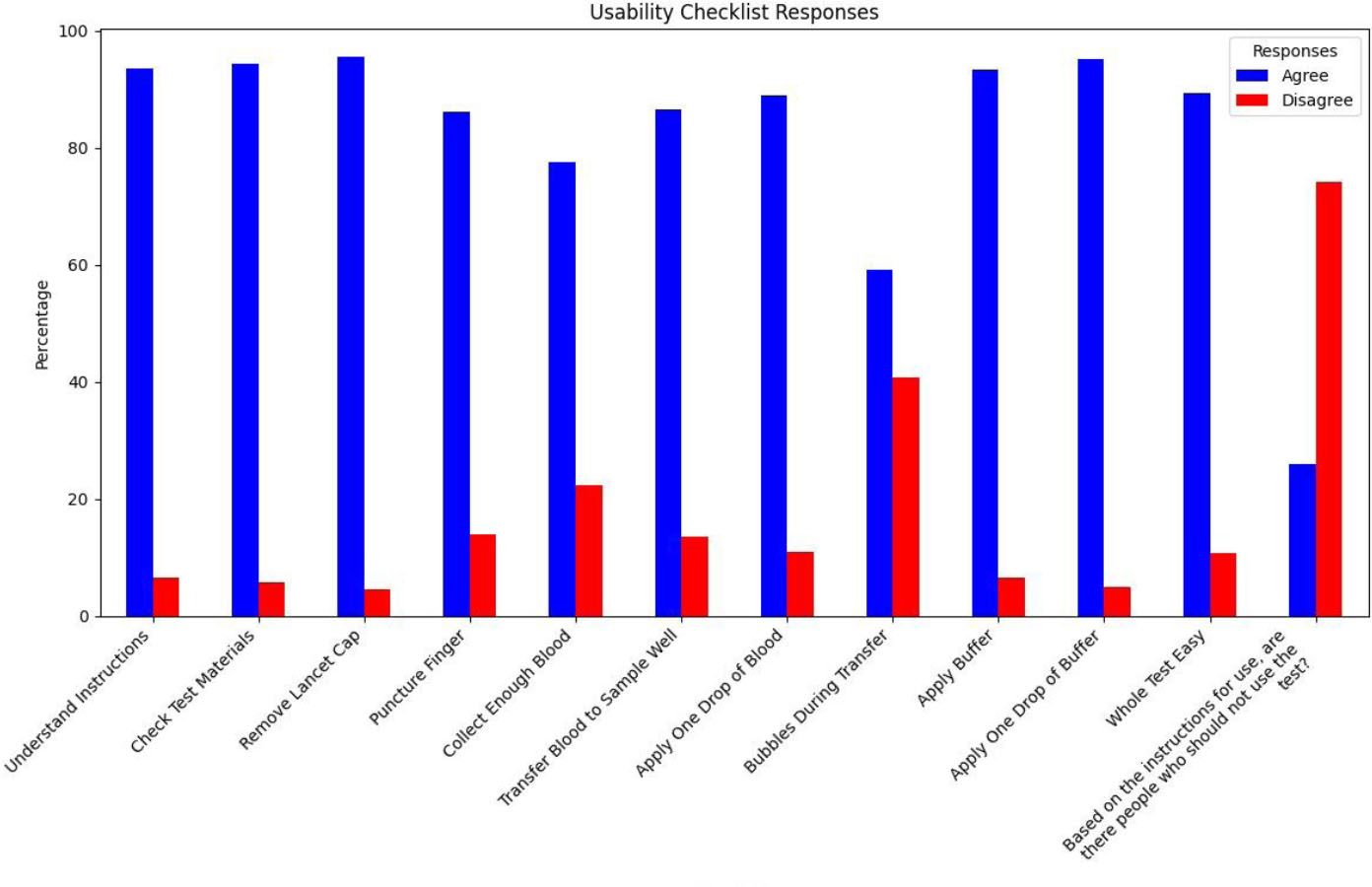
Participants' usability checklist responses.
| Usability Checklist | Yes n (%) | No n (%) | Usability Index (%) | 95% CI |
|---|---|---|---|---|
| How Easy was it to understand the instructions for use | 229 (93.5) | 16 (6.5) | 93.5 | 89.6-96.2% |
| How Easy was it to identify and check that all of the test materials are present before starting the procedure as described in the Instructions for Use? | 231 (94.3) | 14 (5.7) | 94.3 | 90.6-96.7% |
| How Easy was it to remove the cap of the lancet? | 234 (95.5) | 11 (4.5) | 95.5 | 92.1-97.5% |
| How Easy was it to puncture your finger with the lancet? | 211 (86.1) | 34 (13.9) | 86.1 | 81.1-90.1% |
| How Easy was it to collect enough blood? | 190 (77.6) | 55 (22.4) | 77.6 | 71.7-82.6% |
| How Easy was it to transfer the blood to the Sample Well using the Specimen Dropper? | 212 (86.5) | 33 (13.5) | 86.5 | 81.6-90.4% |
| Did you apply ONLY one drop of blood to the test device? | 218 (89.0) | 27 (11.0) | 89.0 | 84.4-92.3% |
| Were bubbles generated during the transfer of blood from the bowl/basin to the test device? | 145 (59.2) | 100 (40.8) | 40.8 | 34.8-47.1% |
| How Easy was it to apply the buffer to the test? | 228 (93.4) | 16 (6.6) | 93.4 | 89.6-96.2% |
| Did you apply only ONE drop of the buffer? | 233 (95.1) | 12 (4.9) | 95.1 | 91.6-97.2% |
| How Easy was the whole test to carry out? | 218 (89.3) | 26 (10.7) | 89.3 | 85.2-92.6% |
| Based on the instructions for use, are there people who should not use the test? | 63 (25.9) | 180 (74.1) | 74.1 | 20.8-31.8% |
| - | - | Number of Correct Responses | Total Number of Responses | Proportion | 95% CI | |
|---|---|---|---|---|---|---|
| Feasibility | The participant used the test correctly | 227 | 244 | 93.0 | 89.2 - 95.6% | |
| Acceptability | How likely would you trust the test if you performed it in your home? | 216 | 244 | 90.0 | - | |
| - | How likely would you recommend this product to your family or friends who might be interested in testing themselves, based on your own experience? | 216 | 244 | 90.0 | - | |
| - | Based on the instructions for use information, would the test have been appropriate for you to use at home? | 228 | 240 | 95.0 | - | |
3.4. Acceptability of CheckNOW™ HIV SELF TEST
Of the 245 eligible clients, 91.7% (CI: 87.7-94.7%) found the test acceptable. 95% (CI: 91.5-97.1%) of the participants indicated a high level of comfort with conducting the HIV self-test in their homes, while a significant proportion 90% (85.8-93.3%) expressed trust in the accuracy of the test results and 90% (85.8-93.3%) reported willingness to recommend the test to friends and family (Table 3).
3.5. Concordance between Counsellors and Users
The average scores from the staff (HTS counsellors and nurses) and participants on the test procedures, results, and interpretation were compared to determine concordance. Overall, the staff rated the participants higher than the participants rated themselves. The mean score given by the staff was 3.80, compared to 2.83 for the participants (Table 4). Notably, both groups rated their understanding of the test steps equally. However, the most significant difference was observed in the correct use of the test, where the mean difference and Cohen’s D were most pronounced. The effect size between the two scores (0.93 and 0.06) was large at 2.067, indicating a substantial disparity in perceptions of test usage accuracy between staff and participants.
4. DISCUSSION
The study aimed to evaluate whether CheckNOW™, a blood-based HIVST kit, was usable, feasible, and acceptable in the hands of untrained lay users, particularly MSM and TGW in the KwaZulu-Natal province. Results from this study were consistent with those from recent studies conducted in Vietnam, the Democratic Republic of the Congo, Kenya, and Brazil, where participants considered the test easy to use, easy to interpret, and acceptable, such that they were willing to recommend it to their friends and family [11, 15-17].
The study reported high usability and feasibility scores for the test; however, this evaluation revealed broader perspectives that offer room for improvement. This was achieved through the survey design, which investigated and identified points of confusion or hesitation, as well as any critical and non-critical errors made during the self-testing procedure. In the CheckNOW™ test, participants encountered a few difficulties with instructions, test procedures, and kit components. This included participants self-reporting feeling too scared to prick themselves, as well as others feeling pain from the lancet [18, 19]. They also reported having issues with transferring blood using the specimen dropper without generating bubbles, and some clients reported not having enough blood when they pricked themselves.
| - | - | Mean | Std Deviation |
Significance (p-value) |
Cohen’s D |
|---|---|---|---|---|---|
| Pair 1 | SE- The participant was able to correctly interpret their result | 0.98 | 0.127 | 0.004 | 0.188 |
| PE- How Easy was the test to read and interpret? | 0.94 | 0.233 | 0.187 | ||
| Pair 2 | SE- The participant appeared comfortable using the test | 0.95 | 0.225 | 0.001 | 0.220 |
| PE- How Easy was the whole test to carry out? | 0.89 | 0.309 | 0.219 | ||
| Pair 3 | SE- The participant used the test correctly | 0.93 | 0.255 | 0.001 | 2,074 |
| PE- Did you understand the steps of the test enough to be confident you were performing it correctly? | 0.06 | 0.233 | 2,067 | ||
| Pair 4 | SE- The participant appeared to understand the instructions | 0.93 | 0.248 | 1,000 | 0.000 |
| PE- How Easy was it to understand the instructions for use | 0.93 | 0.248 | 0.000 | ||
| Pair 5 | SE (Total) | 3.80 | 0.719 | 0.001 | 1,756 |
| PE (Total) | 2.83 | 0.516 | 1,750 |
Addressing these difficulties is important for the improvement and increase of ease-of-use and reduction of misuse of the self-test. This contributes to the low self-rating of correctly using the test by participants in comparison to the staff’s evaluation rating. The HTS counsellors mainly reported test procedure challenges related to the participants not understanding how to use the lancet, how to draw blood, and how to transfer the blood to the basin [20, 21]. This is similar to what was reported in a previous study conducted in rural and peri-urban KwaZulu-Natal, South Africa. Other notable comments were related to the client’s requiring reassurance that the test is accurate. Similar results were reported in a study on Cape Tonian adolescents [22].
Focusing on key populations allowed for the assessment of the usability of HIVST in groups that often face more substantial barriers to access healthcare than the general population. The high accessibility, user satisfaction, and effectiveness in key populations add substantial value to the generalizability of the test, supporting its broader adoption in South Africa and beyond. While the sample size was constrained by resources, the post-hoc power analysis suggests adequate power for primary usability outcomes within the MSM and TGW populations. However, the limited sample size and recruitment setting may have reduced the generalizability of results to the wider MSM and TGW communities, as well as to other key populations. Future studies with larger, more diverse samples of MSM and TGW across different social contexts are needed to validate these findings and inform targeted interventions.
5. LIMITATIONS
This study presented several limitations. The convenience sampling method may have introduced a selection bias due to the characteristics of individuals who seek POP INN services (i.e., those who are cognizant and up to date with their medical care). Some responses may have been influenced by participants’ prior HIV testing experience and post-test counselling prior to this study, which is high in the sampling area, leading to experience bias. The testing process was observed, which could have led to a Hawthorne effect where participants altered their behaviour due to awareness of being studied. Furthermore, the cross-sectional design and absence of longitudinal follow-up restrict the assessment of the long-term usability of HIV self-testing and subsequent linkage to care.
CONCLUSION
The findings of this evaluation indicate that the CheckNOW™ HIV SELF TEST is extensively usable (ease-of-use) and acceptable in the hands of untrained lay users. This evaluation was crucial as it addresses a critical gap in HIV testing coverage and early diagnosis in South Africa, particularly in KZN, while supporting widespread adoption of the test in the country. By evaluating the potential use of HIVST devices like CheckNOW™ HIV SELF TEST, these findings can inform public health strategies and policies aimed at enhancing HIV testing and linkage to care. Additionally, the findings of this study contribute to the growing body of evidence supporting the use of HIVST devices like CheckNOW as a complementary strategy to the provider-initiated testing approach, ultimately aiding in the control of the HIV epidemic in South Africa and beyond by expanding access to HIV testing and improving early diagnosis rates.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: V.N. and J.P.: Study conception and design; G.M.: Data collection; M.M., G.M., V.N., L.M., and J.P.: Analysis and interpretation of results; M.M.: Draft manuscript;. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SSA | = Sub-Saharan Africa |
| HIVST | = HIV Self-Testing |
| WHO | = World Health Organization |
| CE | = Conformité Européenne |
| RDT | = Rapid Diagnostic Test |
| HTS | = HIV Testing Services |
| ERPD | = Expert Review Panel for Diagnostics |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the University of the Witwatersrand. Joburg, South Africa Human Research Ethics Committee (Medical) FWA Registered No IRB 00001223, Ethics reference no: 230606.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data of current study are available from corresponding author, [V.N.], on a reasonable request.
ACKNOWLEDGEMENTS
We thank all the participants for their time and willingness to contribute to the study. We also acknowledge the support of the POP INN healthcare staff who facilitated recruitment and data collection. Special thanks to Abbott Laboratories for funding support.
Evaluation questionnaire for the study staff
1. The participant was able to correctly interpret their result
| □ | □ |
| Yes | No |
2. The participant appeared comfortable using the test
| □ | □ |
| Yes | No |
3. The participant used the test correctly
| □ | □ |
| Yes | No |
4. The participant appeared to understand the instructions
| □ | □ |
| Yes | No |
5. Did the participant have any questions about the test
| □ | □ |
| Yes | No |
If yes, please explain
________________________________________________________
APPENDIX B
Participant Questionnaire
Participant Number (to be filled in by the data collector):
________________________________________________________
Date: __________________________________
Demographic survey
Age: …………………….
Highest level of education:
◻ Never attended school
◻ Primary school
◻ High school or diploma equivalent
◻ Some college or technical degree
◻ College or postgraduate degree
◻ Prefer not to answer
Average Monthly Income
◻R0-R1000 per month
◻R1 001-R2 500 per month
◻R2 501 -R5 000 per month
◻R5 001-R10 000 per month
◻R10 001-R50 000 per month
◻>R50 000 per month
◻ Prefer not to answer
Where do you live?
◻ In a rural area
◻ In an urban area
◻ Not listed, please specify below:
__________________________________________________________________________________
Client evaluation survey
1. How Easy was it to understand the Instructions for Use?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
2. Did you read the Instructions for Use from beginning to end before starting the procedure as described in the Instructions for Use?
| □ | □ |
| Yes | No |
3. How Easy was it to identify and check that all of the test materials are present before starting the procedure as described in the Instructions for Use?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
4. How Easy was it to remove the cap of the lancet?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
5. How Easy was it to puncture your finger with the lancet?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
6. How Easy was it to collect enough blood?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
7. How Easy was it to transfer the blood to the Sample Well using the Specimen Dropper?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
___________________________________________________________________________________________________________________
8. Did you apply ONLY one drop of blood to the test device?
| □ | □ |
| Yes | No |
If you answered No, please comment:
____________________________________________________________________________________________________________________
9. Were bubbles generated during the transfer of blood from the bowl/basin to the test device?
| □ | □ |
| Yes | No |
10. How Easy was it to apply the buffer to the test?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
11. Did you apply ONLY one drop of buffer?
| □ | □ |
| Yes | No |
If you answered No, please comment:
____________________________________________________________________________________________________________________
12. What did you use to measure the time for reading the result?
| Watch | Timer | Other | None |
| □ | □ | □ | □ |
If you answered Other, please specify:
____________________________________________________________________________________________________________________
13. Do you know how many minutes after sample application you read the result in the testing that you just did?
| □ | □ |
| Yes | No |
14. Was the control line visible at the time you read the test result?
| □ | □ |
| Yes | No |
15. How Easy was it to see the control line?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
16. How Confident were you that you saw the control line correctly?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Confident | Fairly Confident | Neither Confident nor Unsure | Fairly Unsure | Very Unsure |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
17. If an invalid result was obtained (i.e. the test did not work), would you know what to do next?
| □ | □ |
| Yes | No |
18. How can you be sure that the test has worked?
19. How Easy was the whole test to carry out?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
20. How Easy was the test to read and interpret?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Very Easy | Fairly Easy | Neither Easy nor Difficult | Fairly Difficult | Very Difficult |
If you answered 4 or 5, please comment:
____________________________________________________________________________________________________________________
21. Did you understand the steps of the test enough to be confident you were performing it correctly?
| □ | □ |
| Yes | No |
22. How likely would you be to trust the test if you performed it in your home?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Extremely likely | Somewhat likely | Neither likely nor unlikely | Somewhat unlikely | Extremely unlikely |
23. How likely would you recommend this product to your family or friends who might be interested in testing themselves, based on your own experience?
| 1 | 2 | 3 | 4 | 5 |
| □ | □ | □ | □ | □ |
| Extremely likely | Somewhat likely | Neither likely nor unlikely | Somewhat unlikely | Extremely unlikely |
24. Based on the instructions for use information, would the test have been appropriate for you to use at home?
| □ | □ |
| Yes | No |
25. Based on the instructions for use, are there people who should not use the test?
| 1 | 2 |
| □ | □ |
| Yes | No |
If yes, please explain
____________________________________________________________________________________________________________________
26. Can you use one test several times?
| □ | □ |
| Yes | No |
27. According to the product instructions for use, you can make independent medical decisions yourself based on the test result, without discussing with your doctor.
| □ | □ |
| True | False |
28. If the result is non-reactive, you can be completely certain that you do not have HIV infection.
| □ | □ |
| Yes | No |


