All published articles of this journal are available on ScienceDirect.
Evaluation of the Quality of Medico-technical Equipment Sterilization in National University Hospital of Cotonou in Benin in 2013
Abstract
Backgrounds:
In low income countries, hospital-acquired infections continue to develop in hospitalized patients, and may also affect medical staff. Medico-technical equipment sterilization is critical for prevention and safety care of nosocomial infections.
Objective:
To assess the quality of medico-technical equipment sterilization at the National University Hospital of Cotonou in 2013.
Method:
This cross-sectional and evaluative study was conducted at the National University Hospital of Cotonou from 10th June to 04th July 2013. A sample of 51 health workers involved in the of medico-technical equipment sterilization system, two (02) administrative authorities, the responsible of National Committee for the Fight against nosocomial infections in the hospital, 41 sterilized instruments and compresses were assessed in the study. Health workers were observed in their work environment before undergoing an individual interview as well as the administrative authorities and the Responsible of the National Committee for the Fight against nosocomial infections. Sterilized instruments are analyzed in microbiology laboratory.
Results:
More than half of the participants were male (52.9%). The average age of respondents was 41 ± 7.5 years. The sterilization unit of the hospital was managed by common surgical department of the hospital and its mission was to provide sterile medico-technical equipment. The sterilization unit did not meet the standard architecture of sterilization environment. Equipment sterilization procedure did not meet standards of quality assurance. There was no preventive maintenance procedure for autoclave and poupinel that were used for sterilization of instruments. No indoor cleaning and air sterilization of the service of sterilization were planned. However, equipment sterilization supplies were available, and 13.72% of workers surveyed were well-skilled. Microbiological tests showed that 48.8% of sterilized medical equipment was contaminated by Staphylococcus aureus, Pseudomonas aeruginosa and Enterobacter cloacae.
Conclusion:
The quality of instrument sterilization system in the HKM National University hospital of Cotonou was poor. Sterilized equipment was contaminated by pathogens. Medical equipment sterilization process needs improvement to prevent hospital-acquired infections.
INTRODUCTION
Hospital acquired infections remain an important public health concern. Preventing Nosocomial Infections (NI) is then a major objective for hospitals that aimed at providing safe health care to patients. The use of disposable equipment is the best way to ensure patient comfort and security. However, low income country health system could not acquire disposable format for certain devices due to the cost. Then some instruments need to be sterilized for re-use [1]. Sterilization is a set of methods that aims at eliminating all living organisms of any kind or form whatsoever carried on a perfectly cleaned object [2]. Sterilization helps reducing the number of germs (egg: 1,000,000 to 1) by six logarithms fold, while disinfection aims at reducing germs by 5 logarithms fold [3]. Hence, the importance of cleaning and disinfection before sterilization is critical as it is essential for ensuring that medical and surgical instruments do not transmit infectious pathogens to patients. It is recognized that any equipment could be well sterilized only if it is well cleaned [4, 5]. According to the manual on the Individualized Nursing (SFIS), sterilization is an activity related to health care, which can also be seen as an indirect care [6]. Sterilization helps preventing health care related infections and its quality reflects dysfunctions in hospital institutions.
Sterilization also aims at protecting everyone in the hospital: patients, medical staff and other hospital users. It is therefore essential for proper sterilization to establish a sterilization system based on standard references. According to the guidelines of Good Pharmacy Practices Hospital (BPPH) in France, proper treatment of medico-technical equipments requires human resources, adequate material and financial resources, trained personnel and a “quality” approach in order to prevent and reduce any risk for patients, professionals and the environment [7]. There are relatively few published assessments of equipment sterilization procedures from low income countries in general and Benin in particular. In Benin, a study reported an overall prevalence of nosocomial infections in 19.1% in hospitalized patients [8]. Despite this high frequency of nosocomial infection, little is known about the quality of instrument sterilization system. In Benin, there are no regulations along the health care quality in hospitals including instrument sterilization, despite guidelines for disinfection and sterilization in healthcare facilities being available since 2008 [9]. The objective of the study was to assess the quality of the sterilization system of medical equipment in the National University Hospital of Cotonou in Benin in 2013.
MATERIALS AND METHODOLOGY
Settings
The study was carried out at the National University hospital called CNHU-HKM of Cotonou.
The CNHU-HKM is a hospital that covers a land of 10 hectares with a capacity of 617 beds. In the last five years, the average number of patients seen in this hospital per year is 192600 [10]. Various missions are assigned to this National Hospital, including mainly:
- Investigate and provide appropriate care for complex medical cases;
- Train medical and related sector students; and
- Conduct scientific research in conjunction with schools and health training institutes.
Main sterilization methods used in the hospital are “moist heat method” and “dry heat method”. The first uses autoclaves which are machines for sterilizing things such as surgical instruments and hospital equipments. They are a bit like giant pressure cookers that use the steam under pressure to kill off bacteria, spores and germs resistant to boiling water and powerful detergents. Because water is heated in a closed container, temperatures above 100°C can be reached. The last method uses “poupinel” for sterilization by hot air (dry heat) at elevated temperature. This method is convenient for metal, heat resistant glass, and vaseline, but is not convenient for linen or gauze swabs [2].
Study Design
This was a cross-sectional and evaluative study conducted to assess the quality of the sterilization system of medical equipments in the National University Hospital of Cotonou in Benin in 2013.
Study Population
The study population consisted of primary and secondary targets. The primary targets included sterilized medico-technical equipments (instruments and compresses) and workers in CNHU-HKM. The secondary targets included the responsible of the committee of fight against nosocomial infections (CLIN) and administrative authorities of the hospital (the Director and manager of human resources). Eligible participants who had not given their consent were excluded from the study.
Sampling
The sampling method used was non probabilistic for the workers who were selected by convenience, while administrative authorities were identified using rational choice.
The sampling method was probabilistic for sterilized medico-technical equipment (instrument and compresses) using the simple random technique.
The sample of materials study included:
- Fifty-one (51) health workers involved in the sterilization process, which were selected using convenience technique;
- Two (02) administrative authorities and the president of CLIN 01, which were selected according to the technique of rational choice; and
- Forty-one (41) medical and technical equipments and swabs, which were collected using simple random selection.
Study Variables
The main variable of the study is the quality of sterilization that has two modalities:
- First modality: Good (no germ found),
- Second Modality: Poor (presence of germs),
The main variable is the quality of the sterilization system of medico-technical equipment.
The components that influence the main variable are “the inputs”, “the process” and “the result” according to the model “quality improvement” of Donabédian [11].
The component “inputs” included:
- The infrastructure’s environment, the existence of the dirty areas, the appearance of walls, ceilings and surfaces, the frequency of equipment cleaning, and the existence of instruction that prohibited entrance to anyone outside the service;
- The material resources concerning their availability, quality, functionality and effective use;
- Equipment (containers, trolleys, metal cabinets);
- Consumables: detergents, disinfectants, brushes, gloves, filters, corner strips, paper, canvas bags or packaging;
- Guideline documents for sterilization process including material traceability records, filing procedures, maintenance contracts, supplier catalogs, administrative records and databases; and
- Human resources namely health workers involved in the process of medico-technical equipment sterilization and the activities/actions relative to compliance with hospital hygiene standard precautions (hand hygiene, personal hygiene, wearing of protective barriers, immunization and prevention of accidental exposure to blood).
The component “Process” referred to the application of technical and administrative procedures in the sterilization unit.
-
Administrative procedures included:
- the existence and implementation of a work plan;
- the existence of a flowchart;
- the existence of a job description;
- the existence of a workers’ training plan;
- the existence of a monitoring - assessment plan of service activities;
- the supervision of staff; and
- the staff motivation mechanism.
- Technical Procedures included:
- written and validated sterilization procedures in force in the unit;
- existence and implementation of a policy of procurement and maintenance of autoclave and poupinel used for sterilization;
- promotion of hospital hygiene;
- controls the water and air into the sterilization department;
- achievement of physical and chemical controls;
- carrying out bacteriological controls; and
- achievement of visual ant tactile controls.
The component “result” referred to the quality of the medico-technical instrument sterilization that was assessed by the presence or absence of germs on the sterilized material.
Microbiological Analysis
The bacteriological analysis of medico-technical instruments and compresses was performed in surgery services and other medico-technical services of CNHU-HKM just after the opening of the instrument or compresses containers. Sterilized equipments were randomly selected. Sterile swabs were used to pass and/or scouring the equipments just after opening the container in the surgical operating unit or in wound repair service. The collection was introduced in incubated broth (37°C) oven for 24 hours. After 24 hours, the broth was seeded in required solid area (Agar fresh Blood, Chapman, Mueller Hinton, Eosin Methylene Blue) for 24 hours at 37°C. Positive cultures were subjected to additional tests (Gram test and other) and then the API-20E gallery for Entero-bacteria and others (Gram negative bacteria), the Coagulasse, Dnase and grouping tests for Cocci Gram-positive were carried out (Staphylococcus and Streptococcus).
Definition and Modalities of Variables
Operational definition and modalities of variables are described in Table 1.
| Variables | Definition | Modalities |
|---|---|---|
| Qualification of workers | Worker who have received basic training in a medical or paramedical training school | Yes/No |
| Staff trained in sterilization procedures | Existence of a sterilization training certificate or a diploma after trainings | Yes/No |
| Availability of workers | Three (03) or more workers are present every day for sterilisation activities | Yes/No |
| Knowledge of sterilization procedures | Worker knows in the required order of the steps of sterilization | Yes/No |
| Observance of steps of sterilization | In practice, the agent follows the normal order of the sterilization process | Yes/No |
| Performance | The worker knows and respects the different steps of sterilization | Yes/No |
| Choice of sterilization method | The agent knows how to choose the method of sterilization depending on the nature of the material, packaging, destination and level of treatment required | Yes/No |
| Choice of sterilization cycle | The worker knows how to choose sterilization cycle based on the level of treatment required | Yes/No |
| Physical sterilization control | The responsible of the unit controls the time, temperature and pressure according to the sterilizing cycle | Yes/No |
| Physico-chemical sterilization control | The responsible of the unit checks the strips bend at the end of the sterilization process | Yes/No |
| Bacteriological control of sterilization |
Presence or absence of germ on the sterilized equipment | Yes/No |
| Water control for sterilization | Existence of report on the quality of water used during sterilization process | Yes/No |
| Air control for sterilization | Existence of the air monitoring report during sterilization process | Yes/No |
| Access to sterilization unit | Access to service is limited | Yes/No |
Measurement of Study Variables
The variables were operationalized as follows. A major criterion for assessing the quality of material was chosen: the presence of germs on the medical-technical equipment after sterilization.
Each practice was assessed as follows: when the practice meets the standards, it was marked “YES”, and when the practice does not meet the standards it was marked “NO” (YES = 1 and NO = 0).
Each component was assessed “Good” if all the items that go into its composition were “YES” and there were no germs on the sterilized equipment.
Even if only one practice was listed “NO”, the component was rated “Bad.”
The quality of sterilization was “Good” if all the components were “Good”, otherwise it was rated “Bad.”
Even if all the components were rated “Good” and a germ was found on sterilized medical and technical equipment, the quality of material was assessed “Bad.”
The quality of the sterilization was “good” if all the components or the quality of medico-technical sterilization system were assessed “Good” and no germ was found on the sterilized medico-technical equipment.
A major criterion for assessing the quality of material was chosen: the presence of germs on the medical-technical equipment after sterilization.
For each component, it was assessed as follows: when the practice meets the standards required (YES), score 1 was assigned and when the practice does not meet the standards (NO), the score 0 was assigned. Each component was appreciated “Good” if all the elements that compose it met the respective required standards. Otherwise the component was appreciated “Bad”.
The quality of the sterilization was “good” if all the components or the quality of medico-technical sterilization system were appreciated “Good” and no germ was found on sterilized medico-technical equipment as shown in Table 2.
| Components | Number of variables accessed | Score | Component Result |
Quality of sterilisation system |
|---|---|---|---|---|
| Environmental factors | 6 | Yes = 1 No = 0 |
R1 | Good or Bad |
| Human and material resources | 4 | Yes = 1 No = 0 |
R2 | |
| Technical and administrative procedures | 5 | Yes = 1 No = 0 |
R3 | |
| Organizational factors | 12 | Yes = 1 No = 0 |
R4 |
Data Collection Procedures
Data were collected using various techniques:
- Direct observation was used for the appreciation of the unit of sterilization and the performance of workers regarding compliance with the steps of sterilization;
- The document review was done to check traceability of the sterilized equipment and procedures used;
- Structured individual interviews were used to collect information about administrative authorities of the hospital and the responsible of CLIN;
- Laboratory analyses were performed seeking for germs on sterilized equipment.
Ethical Considerations
The authorization to conduct the study was obtained from the Director of HKM CNHU. The objectives of the study were explained to the participants. Voluntary informed consent was obtained from each participating worker before starting the interview. This consent claims that the participants were not at risk by refusing to participate in the survey or stopping their collaboration during the study any way prior to commencing the interview. The confidentiality and anonymity of the information collected were respected.
Data Analysis
Data were analyzed using Epi Info version 3.5.3. Quantitative variables were expressed as mean and standard deviation, and categorical variables were expressed as percentages.
RESULTS
Socio-demographic Characteristics, Knowledge and Observance of Steps of Sterilization in Participants
Socio-demographic characteristics, knowledge and observance of steps of sterilization in participants are described in Table 3 as follows:
| Variables | Frequencies (n=51) | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 27 | 52.9 |
| Female | 24 | 47.1 |
| Occupation | ||
| Nurse/Mid wife | 26 | 51 |
| Nurse assistant | 15 | 29.4 |
| Laboratory skilled agents | 06 | 11.8 |
| Trainees | 04 | 7.8 |
| Training in sterilization | ||
| Yes | 28 | 54.9 |
| No | 23 | 45.1 |
| Knowledge of steps sterilization | ||
| Yes | 12 | 23.5 |
| No | 39 | 76.5 |
| Observance of steps of sterilization | ||
| Yes | 13 | 25.5 |
| No | 38 | 74.5 |
Distribution of Workers According to Their Performance Regarding Medico-technical Equipment Sterilization at HKM CNHU in Benin in 2013
Fig. (1) below summarizes proportion of workers with adequate or inadequate performance regarding medico-technical equipment sterilization at HKM CNHU in Benin in 2013.
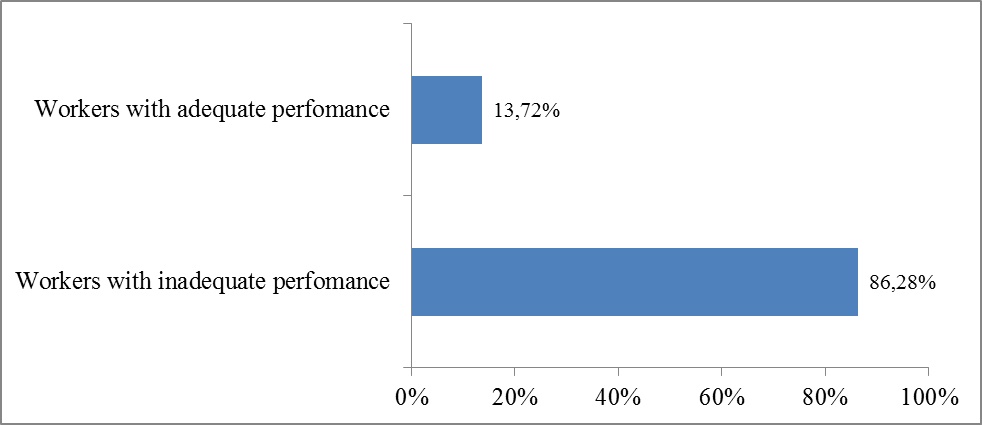
Fig. (1) shows that 86.3% of workers involved in sterilization had inadequate (low) performance, while 13.7% exhibited adequate (high) performance.
Structural and Organizational Environment of Medico-technical Equipment Sterilization at HKM CNHU
Structural and organizational environment of medico-technical equipment sterilization at HKM CNHU is described in Table 4.
| Variables | Compliance with standards | |
|---|---|---|
| Yes | No | |
| Structural aspects | ||
| Walls | X | |
| Ceilings | X | |
| Surfaces /tables | X | |
| Local air conditioner | X | |
| Cleaning according to the procedures | X | |
| Limited access to sterilization unit | X | |
| Human and material Resources | ||
| Autoclaves | X | |
| Consumables | X | |
| Individual protection | X | |
| Availability of human resources | X | |
| Control of quality | ||
| Existence of water quality monitoring report | X | |
| Existence of the air quality monitoring report | X | |
| Physical controls (pressure, temperature, duration) | X | |
| Chemical controls (Bowie and Dick test, shift indicator of passage, integrators) | X | |
| Bacteriological controls | X | |
| Organizational concerns | ||
| Material traceability document | X | |
| Recording of sterilization cycles | X | |
| Equipment maintenance contract | X | |
| Existence sterilization procedures | X | |
| Standardisation of sterilization procedures | X | |
| Centralization of the sterilization process | X | |
| Existence of local cleaning procedures | X | |
| Existence of organizational chart | X | |
| Existence of a work plan | X | |
| Existence of a training course plan | X | |
| Existence of a monitoring and evaluation plan | X | |
| Existence of supervision reports | X | |
Among the items studied, few elements complied with European standards. Among the six items regarding respect to the structural aspect, only 1 (17%) conformed to European standards. Among the 12 items considered for organizational aspect, 2 (17%) complied with European standards. According to the human and material resources, 3 out of 4 (75%) items complied with European standards. Overall, 6 (22.2%) items out of 27 conformed to European standards.
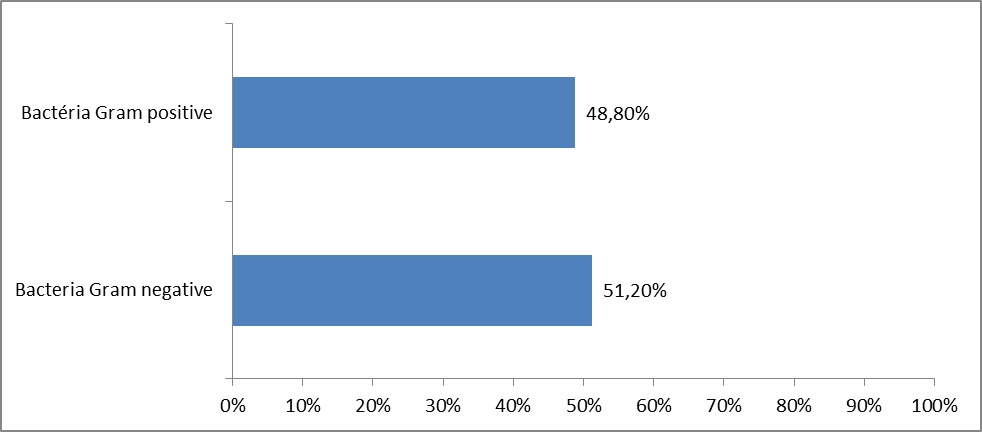
Classification of Germs Isolated from Sterilized Medico-technical Equipment
The classification of germs isolated from sterilized medico-technical equipment in CNHU-HKM in 2013 is shown in Fig. (2), Tables 5 and 6.
| Surgery department* | Sterilized materiel | Mode of sterilization | Bacteria found | Bacteria counting/ Number of colonies counted |
|---|---|---|---|---|
| Emergencies service | Instrument | Poupinel | Bacteria Gram(+) | ≥ 500 |
| Compresses | Autoclave | Coagulase-negative Staphylococcus | ≥ 500 | |
| Clinic of gynecology and obstetrics | Instrument | Poupinel | None | 0 |
| Compresses | Poupinel | Pathogen Gram(+) | 200-500 | |
| Ophthalmology | Instrument | Autoclave | None | 0 |
| Compresses | Autoclave | Coagulase-negative Staphylococcus | 200-500 | |
| Urology room 1 | Instrument | Autoclave | None | 0 |
| Compresses | Autoclave | None | 0 | |
| Visceral surgery room 2 | Instrument | Autoclave |
Staphylococcus Saprophyticus |
≥ 500 |
| Instrument | Autoclave | Pathogens Gram(+) | ≥ 500 | |
| Pediatric surgery room 3 | Instrument | Autoclave | None | 0 |
| Instrument | Autoclave | None | 0 | |
| Traumatology room 4 | Instrument | Autoclave | None | 0 |
| Instrument | Autoclave | None | 0 | |
| Surgery Room 7 | Instrument | Autoclave | Coagulase-negative Staphylococcus | ≥ 500 |
| Compresses | Autoclave | None | 0 |
| Wound care Service* | Material analyzed | Sterilization mode | Bacteria identified | Bacteria counting/ Number of colonies counted |
|---|---|---|---|---|
| Pediatric surgeon | Instrument | Poupinel | None | 0 |
| Compresses | Poupinel | None | 0 | |
| Stomatology | Instrument | Poupinel | None | 0 |
| Compresses | Poupinel | None | 0 | |
| Traumatology | Instrument | Poupinel | None | 0 |
| Compresses | Poupinel | Gram negative Cocco bacillus | ≥ 500 | |
| Clinic of gynecology and obstetrics | Instrument | Poupinel | None | 0 |
| Compresses | Poupinel | Gram positive bacteria | 200-500 | |
| Neonatology | Compresses | Poupinel | Enterobacter cloacae | 200-500 |
| External surgeon | Instrument | Poupinel | Staphylococcus aureus | ≥ 500 |
| Compresses | Autoclave | None | 0 | |
| Visceral surgeon | Instrument | Poupinel | Not identified Gram positive bacteria | ≥ 500 |
| Compresses | Poupinel | Not identified Gram positive bacteria | ≥ 500 | |
| Urology | Instrument | Poupinel | Not identified Gram positive bacteria | ≥ 500 |
| Compresses | Poupinel | Not identified Gram positive bacteria | ≥ 500 | |
| Large burned room | Instrument | Poupinel | Not identified Gram negative bacteria | 200-500 |
| Compresses | Autoclave | None | 0 |
Isolated bacteria Gram (-) were 51.2%, while isolated bacteria gram (+) were 48.8%.
Bacteriological culture of the 41 samples (medical-technical equipment and compresses) made in the various surgical blocks and other medical-technical services CNHU-HKM, just after the opening of the container showed that 20 (48.8%) of 41 materials were contaminated by bacteria. Among the identified bacteria, 50% Gram (-) bacteria included Enterobacter cloacae, Pseudomonas aeruginosa and other bacteria Gram (-) bacteria were not identified; whereas 50% Gram (+) bacteria included mostly Staphylococcus aureus, Staphylococcus saprophyticus and other bacillus.
The medico -technical equipment and compresses, sterilized in CNHU-HKM and used in operating theaters are mostly contaminated by coagulase-negative staphylococci.
The presence of pathogens, such as Staphylococcus aureus and Enterobacter cloacae, on instruments and compresses discredits the quality of the sterilization system of medical-technical equipment in various medical-technical services in the hospital.
Antibiotic Resistance Profile of Bacteria Isolated from Sterilized Medico-technical Material
Antibiotic resistance profile of bacteria isolated from sterilized medico-technical material is summarized in Table 7 below.
| Services | Bacteria identified |
Number of Antibiotics tested (N) |
Efficacy Antibiotic (%) |
Mid-efficacy antibiotics (%) |
Non-efficacy antibiotics (%) |
|---|---|---|---|---|---|
| Emergency service | Coagulase negative Staphylococcus | 30 | 83.3 | 0 | 16.6 |
| Neonatology | Enterobacter cloacae | 25 | 24 | 8 | 68 |
| External surgeon | Staphylococcus aureus | 30 | 86.6 | 0 | 13.3 |
| Ophthalmology. | Coagulase negative Staphylococcus | 30 | 66.6 | 3.3 | 30 |
| Cardiology | Pseudomonas Aeruginosa | 23 | 21.7 | 13 | 65.2 |
| Large burned service | Not identified Gram negative bacteria | 22 | 86.3 | 0 | 36.4 |
| Surgeon room 7 | Coagulase negative Staphylococcus | 26 | 34.6 | 3.8 | 61.5 |
| Visceral surgeon room 2 | Staphylococcus saprophyticus | 28 | 82.1 | 3.6 | 14.3 |
Most germs isolated on sterilized medico-technical material in CNHU HKM-2013 showed a drug resistance for tested antibiotics.
DISCUSSION
The study explored the quality of medico-technical material sterilization in National University Hospital of Cotonou through the description of the structural and organizational environment of the sterilization unit and bacteriological analysis of sterilized equipment. We found that there was no-one responsible for the quality of medical equipment sterilization process at HKM-CNHU in contrast to the situation reported by Dubaele in France in 2000. According to Dubaele, the pharmacist, with the support of the management staff of the institution, implements the “quality system” for all sterilization operations. The pharmacist is the quality manager sterilization in the hospital [12, 13]. According to standards, sterilization should be centralized with objective to implement and respect the sterilization procedures for reusable medical devices under the responsibility of the head of sterilization. This was not the case in our study. At HKM-CNHU, workers in sterilization unit have never been supervised, evaluated or motivated. None of them had the opportunity to undergo training in medico-technical sterilization process despite the progress in science and technology. The material available (autoclave) was used without preventive maintenance. Subsequently, only one choice of sterilization cycle was possible since the autoclave was blocked due to lack of maintenance. This situation did not allow assessment of compliance with the steps of sterilization. This study reported a low performance rate of 13.723% in workers, which is far from 60% reported by Viayinon in 2003 [14].
In HKM-CNHU of Cotonou, nurses or midwives who were trained in preparing equipments for surgical care were responsible for the preparation of boxes (packaging), loading and unloading of the autoclave or poupinel. The step of packaging the instruments is obscured by lack of means to accomplish it. Workers without basic paramedic training or briefing of hospital hygiene were sometimes recruited for a short duration for cleaning, rinsing and drying of soiled material. The need of training of workers at unit of sterilization is critical, as well as the need of a new modern autoclave and better organization of work in the unit.
The compliance level of structural and organizational environment of the sterilization unit in HKM-CNHU compared to the European standards was low (22.2%). Indeed, sterilization by dry heat was still the most frequently used, while Poupinel method of sterilization is prohibited in Europe [15]. The circular 2001/138 of 14 March 2001 stipulated that this process is inefficient regarding Transmissible Unconventional Agents (NCTA) called “prions” or abnormal infectious protein, a general term given to agents that cause neurodegenerative disorders.
The profession of sterilization was not recognized in Benin. It was the same in France, but the first official recognition of the sterilizing agent profession was effective in February 2011. The certification of specialization of sterilization in hospital is issued by the Academic Centre for Continuing Education (CAFOC) of Toulouse [16].
Biological controls on sterilization units could be done in two ways using small tubes containing growing environment and bacterial spores in one hand and strips impregnated with bacterial spores for evaluation after autoclaving in the other hand.
Biological tests are not performed at CNHU-HKM due to lack of adequate equipments and their scarcity in the market.
It explained why we adopted the method which consisted of just swab sterile equipments at the opening of the container in the surgical operating room or in the dressing room with sterile swabs. The absence of sterilization standardized procedures leads to the fact that, in the hospital, each service disinfects, cleans and sterilizes the material in its own way.
For example, in stomatology department, packs of instruments are sterilized in an autoclave and benchtop instruments in poupinel for a period of two hours. This protocol established by the supervisor of the service was strictly respected by the workers. During checking, no germs were identified on compresses and sterilized instruments in the service.
Staphylococcus aureus were found on instruments sterilized by poupinel in the main unit of the hospital sterilization and on those being used in the surgery department [17]. The study allowed us to identify Pseudomonas aeruginosa on the heat-sensitive equipment disinfected and unsterilized in the cardiology department. A similar situation was reported by other studies [18, 19]. It also raises the issue of sterilization of heat-sensitive equipment in HKM- CNHU. Nowadays, recommended sterilization methods are gas or ionizing radiation. The method we used for biological control allowed identification of only germs, while the biological tests recommended but not available in Benin would allow assessing the effectiveness of the sterilization process. This method was used in the high school province in Namur in Belgium by Michau in the context of surgery specialization in pediatrics [20].
The Bowie and Dick test allows us to appreciate the smooth operation of the autoclave. It should be conducted every morning, while stopped for at least four hours or after any repair work on the autoclave. This was not implemented in CNHU-HKM in 2013.
Furthermore, it has been observed that the physical, chemical and biological tests have never been done in the sterilization unit of HKM-CNHU, despite knowing that this practice is an ongoing process to provide a guarantee of optimal quality care to patients [21].
CONCLUSION
This study highlighted the inadequacy of the architectural design of the sterilization unit of CNHU-HKM. The level of compliance of structural and organizational environment of the sterilization unit in HMK-CNHU was low as compared to European standards. The level of performance of the workers involved in the sterilization process was inadequate. Germs were identified on equipment marked as “sterile”. These findings demonstrated the poor quality of the sterilization system of medical-technical equipments at HKM CNHU of Cotonou in 2013.
Improvement of the quality of the sterilization system at HKM CNHU is critically needed in order to prevent hospitalized acquired infections.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors acknowledge the participants who have contributed to the study.


