All published articles of this journal are available on ScienceDirect.
Prevalence and Correlates of Metabolic Syndrome Among Adults Attending Healthcare Facilities in Eastern Cape, South Africa
Abstract
Background and Aim:
Urbanisation and westernisation have greatly influenced the metabolic health of individuals in South Africa, with resultant increase in metabolic syndrome (METs) components. This study aimed to determine the prevalence and factors associated with METs among adults in Buffalo City Municipality, East London, South Africa.
Methods:
This was a healthcare facility-based cross-sectional, descriptive study. The World Health Organisation STEPwise demographic and lifestyle behavioural questionnaire was used to collect relevant data from 998 participants. Anthropometric measurements, blood pressure and fasting blood glucose were measured using standardised protocols. Metabolic syndrome was diagnosed using the International Diabetes Federation criteria.
Results:
The prevalence of metabolic syndrome was 21.8%; 15.6% and 24.8% among males and females, respectively. The prevalence of METs was higher among participants who were aged 56 years and above, with low level of education (grade 1 – 7), married and retired. After adjusting for confounders, only age 26 and above (AOR=4.1, CI=2.0-8.4), marriage (AOR=2.3 CI=1.6-3.3), female sex (AOR=1.6, CI=1.1-2.4), alcohol use (AOR=2.0, CI= 1.3-3.1), unemployment (AOR=1.8, CI= 1.2-2.6) and earning an income below ZAR1200 (AOR= 1.1, CI= 1.1-2.4) were significant and independent predictors of METs. Participants aged 26 and above were four times more likely to have METs. Married non-alcohol users and unemployed participants were two times more likely to have METs than unmarried alcohol users and employed individuals.
Conclusion:
There was a high prevalence of metabolic syndrome among the participants which indicates a high risk for cardiovascular diseases; a leading cause of premature morbidity and mortality.
INTRODUCTION
Metabolic syndrome (METs) is a constellation of factors that promote the development of cardiovascular diseases, diabetes mellitus type 2 and all-cause mortality. The risk factors associated with METs are insulin resistance, visceral adiposity, atherogenic dyslipidaemia, endothelial dysfunction, high blood pressure, genetics, chronic stress and hypercoagulability [1, 2]. As seen in advanced countries, there is high mortality among metabolically unhealthy individuals in the middle and low-income countries [3]. Africa is no exception to the metabolic syndrome menace. There is documented evidence of increased prevalence of METs in Africa, with increasing age [4-7]. The rapid increase in the prevalence of METs may be attributed to urbanisation, consumption of westernised diets and physical inactivity [8, 9].
An increase in the components of METs, especially obesity, has been documented in South Africa with a consequential high burden of METs, thus, constituting a severe epidemiological health problem [10-12]. Prevention and modification of the components of METs are important strategies in the prevention of cardio-metabolic diseases [13, 14]. Metabolic health screening will help identify therapeutic targets to improve cardiometabolic diseases at the population level. Very few studies have been published on the prevalence of metabolic syndrome in the Eastern Cape province of South Africa. The aim of this study was to determine the prevalence and factors associated with metabolic syndrome among adults in Buffalo City Metropolitan Municipality, Eastern Cape, South Africa. This epidemiological data could then be used to guide public health education programme at the primary healthcare settings in the Eastern Cape.
METHODS
Study Area and Design
This descriptive, cross-sectional survey is a part of the cardio-metabolic risk factors’ assessment project carried out in Buffalo City Municipality Metropolitan (BCMM), South Africa. The study screened cardio-metabolic risk factors among adults attending the largest out-patient clinics in the rural and semi-urban communities of Buffalo City Metropolitan Municipality (BCMM): Cecilia Makiwane hospital, Nontyatyambo and Empilweni-Gompo Community Healthcare Centres. These healthcare facilities serve a total population of 755,200 residents in the BCMM [15]
Participants and Sample Size
The appropriate sample size was estimated using the following formula:
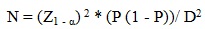 |
Where Z is the confidence level, P is the expected proportion of individuals with cardio-metabolic risk factors, and D is the margin of error. P was set at 0.40 and D at 0.05. The calculation was performed at the 95% confidence level. The required sample size per study site was 369 participants and a total of 1107 participants were included in the study. A total of 109 participants were excluded due to incomplete data, resulting in a final sample size of 998. Participants were included in the study if they were 18 years and above, attending the out-patient clinics of the selected hospital and Community Health Centres, and had fasted in the preceding eight hours prior to recruitment into the study. Acutely ill, psychotic, debilitated, pregnant or patients with physical disability were excluded.
Data Collection
The participants were questioned using the previously validated WHO STEPwise questionnaire [16], which comprises three major items; demographic and behavioural data, and measurements. Details regarding the methodology of the study have been published elsewhere [17]. Briefly, demographic variables included items on sex, age, marital status, level of education, employment status and average monthly income. The socioeconomic factors were measured by assessing the average monthly income, level of education and employment status.
The following behavioural variables were obtained by self-reporting: cigarette smoking, alcohol use, physical activity, and fruit and vegetables consumption patterns. Participants were questioned on their servings of fruit and vegetables daily. The smoking categories include primary smokers (smoking directly) or secondary smokers (if living with a smoker) or non-smoker. Anthropometric measurements (weight, height, waist and hip circumferences) followed the International Society for the Advancement of Kinanthropometry (ISAK) guidelines [18]. Waist-to-hip ratio (WHR) and the waist-to-height ratio (WHtR) were derived from the height, waist and hip circumferences.
Blood Pressure
Blood pressure was measured with a validated Microlife BP A100 Plus model which provided an average of two readings for each participant. Before the first blood pressure measurement was done, participants rested in sitting position for at least five minutes; feet on the ground and arm supported on the table. The second blood pressure reading was taken after another five minutes of rest and the average of the two readings was recorded. Hypertension was defined as the average of two systolic blood pressure of > 140 mmHg and diastolic of > 90 mmHg in accordance with the Eight Joint National Committee [19].
Glucose Testing
Fasting capillary blood glucose of each participant was measured with a validated ACCU-CHEK glucose monitoring apparatus. In fasting state, participants were diagnosed with diabetes if their fasting blood glucose exceeded 7.0 mmol/L or they were currently on medications for diabetes and they were defined as having pre-diabetes if the fasting blood glucose fell between 6.1-6.9 mmol/L [20].
Definition of the Metabolic Syndrome
Metabolic syndrome was determined using the International Diabetes Federation criteria [21] which stated that metabolic syndrome should be diagnosed if an individual is centrally (abdominally) obese (waist circumference of ≥ 94 cm for men and ≥ 80cm for women) and has any two of the following risk factors: (1) Raised triglycerides: Triglyceride level ≥ 150 mg/dL or 1.7 mmol/L; (2) Low HDL cholesterol: HDL cholesterol <40 mg/dL or 1.03 mmol/L in males and < 50 mg/dL or 1.29 mmol/L among females; (3) High blood pressure ≥130/85 mmHg or treatment of previously diagnosed hypertension; and (4) High fasting blood glucose ≥100 mg/dL or 5.6 mmol/L.
Ethical Consideration
Ethical approval was obtained from the University of Fort Hare Research Ethics Committee (Reference number, GOO061SOLO01). Prior to data collection, permission was obtained from the Eastern Cape Department of Health, management of the Sub-district Department of Health as well as the head of the respective health facilities. Participants were provided with information sheets detailing the purpose and process of the study. Each participant gave written, informed consent for his/her voluntary participation.
STATISTICAL ANALYSIS
Characteristics of study variables were expressed as a mean for continuous variables. Frequencies and proportions were reported for categorical variables. A chi-Square test was applied to compare ratios and multivariate logistic regression analysis was used to determine the potential determinants of metabolic syndrome and their 95% confidence interval (95% CI). A p-value of ≤ 0.05 was considered statistically significant. Statistical analyses were performed with the Statistical Package for Social Science (SPSS) version 21 for windows (SPSS Inc., Chicago, IL, USA).
RESULTS
The mean age of participants was 42.6 SD±16.5 years, with the age range of 18 to 75 years. The majority of the participants were over 35 years (59.4%), had attained grade level 8-12 (58.1%), earned R2000 and below (77.3%) and were unemployed (47.7%) Table (1).
| Variables | Male n=321 n(%) |
Female n=677 n(%) |
Total n=998 n(%) |
p-value |
|---|---|---|---|---|
| Age group (years) | ||||
| 18-25 | 40(12.5) | 143(21.1) | 183(18.3) | |
| 26-35 | 74(23.1) | 149(22.0) | 223(22.3) | |
| 36-45 | 67(20.9) | 116(17.1) | 183(18.3) | 0.009* |
| 46-55 | 57(17.8) | 110(16.2) | 167(16.7) | |
| 56-65 | 41(12.8) | 99(14.6) | 140(14.0) | |
| ≥66 | 42(14.1) | 60(8.9) | 102(10.2) | |
| Level of education | ||||
| No formal schooling | 62(19.3) | 84(12.4) | 146(14.6) | |
| Grade 1-7 | 57(17.8) | 99(14.6) | 156(15.6) | 0.008* |
| Grade 8-12 | 171(53.3) | 409(60.4) | 580(58.1) | |
| Tertiary | 31(9.7) | 85(12.6) | 116(11.6) | |
| Monthly income (Rands) | ||||
| No income | 134(41.7) | 300(44.3) | 445(44.6) | |
| R150-2000 | 89(27.7) | 248(36.6) | 326(32.7) | 0.000* |
| R2001-5000 | 74(23.1) | 100(14.8) | 174(17.4) | |
| R5001and above | 24(7.5) | 29(4.3 | 53(5.3) | |
| Marital status | ||||
| Single | 193(60.3) | 444(65.6) | 637(63.9) | |
| Married | 115(35.9) | 185(27.3) | 300(30.1) | |
| Separated | 1(0.3) | 5(0.7) | 6(0.6) | 0.002* |
| Divorced | 9(2.8) | 13(1.9) | 22(2.2) | |
| Widowed | 2(0.6) | 30(4.4) | 32(3.2) | |
| Racial group | ||||
| Black | 313(97.5) | 666(98.4) | 979(98.1) | |
| Coloured | 8(2.8) | 9(1.3) | 17(1.7) | 0.260 |
| White | 0(0.0) | 2(0.3) | 2(0.2) | |
| Type of employment | ||||
| Government employee | 30(9.3) | 33(4.9) | 63(6.3) | |
| Non-government employment | 98(30.5) | 133(19.7) | 231(23.2) | |
| Self-employment | 30(9.3) | 32(4.7) | 62(6.2) | |
| Student | 19(5.9) | 80(11.8) | 99(9.9) | 0.000* |
| Unemployed | 115(24.2) | 361(53.4) | 476(47.7) | |
| Retired | 29(9.0) | 37(5.5) | 66(6.6) |
Table (2) shows the prevalence of total abdominal obesity (WC, WHR and WHTR), hypertension and fasting glucose stratified by gender and age. Men had higher prevalence of hypertension 175(54.5%) compared to women, 316(46.7%). Likewise, there was a linear association between hypertension and ageing. Higher risk blood glucose level (diabetes mellitus) was found more among females (130; 19.2%) compared to males (59; 18.4%) and increased with age. High risk glucose level (pre-DM) was observed among the younger age group (18-25 years) and the middle (46-55 years), with 31(16.9%) and 26(15.6%), respectively. The prevalence of abdominal obesity was 47.5%, 65.2% and 72.5% using waist-to-hip ratio, waist circumference and waist-to-height ratio, respectively. Irrespective of the diagnostic criteria, females had a higher prevalence of abdominal obesity, which increased with increasing age. Overall, the prevalence of MetS among the study participants was 21.8%. Metabolic syndrome was seen more in females (24.8%) compared to males (15.6%) (Table 2).
| Gender | Age (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Components of MetS | Male n(%) | Female n(%) | Total n(%) |
18-25 n(%) |
26-35 n(%) |
36-45 n(%) |
46-55 n(%) |
56-65 n(%) |
>65 n(%) |
| Abdominal obesity | |||||||||
| WHR | 78(24.3) | 394(58.5) | 472(47.5) | 56(30.9) | 87(39.2) | 88(48.4) | 90(53.9) | 94(67.1) | 57(55.9) |
| WC | 107(33.3) | 542(80.3) | 649(65.2) | 91(50.0) | 142(63.7) | 120(65.9) | 110(65.9) | 116(82.9) | 70(68.6) |
| WHTR | 175(54.5) | 549(81.1) | 724(72.5) | 100(54.6) | 159(71.3) | 137(74.9) | 121(72.5) | 126(90.0) | 81(79.4) |
| Hypertension | |||||||||
| Pre-HTN | 173(53.9) | 364(53.8) | 537(53.8) | 91(49.7) | 109(48.9) | 78(42.6) | 79(47.3) | 62(44.3) | 42(41.2) |
| HTN | 175(54.5) | 316(46.7) | 491(49.2) | 35(19.1) | 66(29.6) | 72(39.3) | 113(67.7) | 117(83.6) | 88(86.3) |
| Blood glucose level | |||||||||
| Pre DM | 49(15.3) | 87(12.9) | 136(13.6) | 31(16.9) | 25(11.2) | 26(14.2) | 26(15.6) | 13(9.3) | 15(14.7) |
| DM | 59(18.4) | 130(19.2) | 189(18.9) | 6(3.3) | 24(10.8) | 26(14.2) | 37(22.4) | 55(39.3) | 41(40.2) |
| MetS# | |||||||||
| Yes | 50(15.6) | 168(24.8) | 218(21.8) | 10(5.5) | 17(7.6) | 26(14.2) | 50(29.9) | 69(49.3) | 46(45.1) |
| No | 271(84.4) | 509(75.2) | 780(78.2) | 173(94.5) | 206(92.4) | 157(85.8) | 117(70.1) | 71(50.7) | 56(54.9) |
Sex, age, marital status, level of education and employment status were the significant demographic factors associated with MetS Table (3). The prevalence of MetS was higher among participants who were aged 56 and above (49.3%) compared to those aged 18 – 25 years (5.5%), indicating an upward trend with advancing age. Participants with an average level of education, that is, grade 1 – 7, had a higher prevalence of MetS (32.7%) compared to better-educated participants (17.2%). The prevalence of MetS among married participants was more than two times (34.1%) than that of never married participants (14.0%), while among retirees (45.5%), it was more than six times than that of students (7.1%), three times than that of self-employed (12.9%) and about two times than that of unemployed participants (26.5%).
| Variables | Metabolic syndrome n(%) |
No metabolic syndrome n(%) | RR | CI | p-value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 50(15.6) | 271(84.4) | |||
| Female | 168(24.8) | 509(75.2) | 0.5 | 0.4-0.7 | 0.001* |
| Age (years) | |||||
| ≤ 25 | 10(5.5) | 173(94.5) | |||
| 26-35 | 17(7.6) | 206(92.4) | - | - | |
| 36-45 | 26(14.2) | 157(85.8) | |||
| 46-55 | 50(29.9) | 117(70.1) | 0.000* | ||
| 56-65 | 69(49.3) | 71(50.7) | |||
| ≥ 66 | 46(45.1) | 56(54.9) | |||
| Level of education | |||||
| No formal schooling | 29(19.9) | 117(80.1) | |||
| Grade 1 to 7 | 51(32.7) | 105(67.3) | |||
| Grade 8 to 12 | 118(20.3) | 462(79.7) | 0.004 | ||
| Tertiary | 20(17.2) | 96(82.8) | - | - | |
| Marital status | |||||
| Never married | 89(14.0) | 548(86.0) | |||
| Ever Married | 112(34.1) | 216(65.9) | 0.3 | 0.2-0.4 | 0.000* |
| Employment | |||||
| Government employee | 13(20.6) | 50(79.4) | |||
| Non-government employee | 33(14.3) | 198(85.7) | - | - | |
| Self-employed | 8(12.9) | 54(87.1) | |||
| Student | 7(7.1) | 92(92.9) | |||
| Unemployed | 126(26.5) | 350(73.5) | 0.000* | ||
| Retired | 30(45.5) | 36(54.5) | |||
| Income | |||||
| R2000 and below | 115(32.2) | 38(16.7) | |||
| R2001 and above | 242(67.8) | 189(83.3) | 2.4 | 1.6-3.6 | 0.000* |
The relationship between MetS and behavioural factors such as smoking, alcohol consumption and fruit and vegetable consumption is presented in Table (4). Smoking, alcohol use, fruits and vegetables’ consumption was significantly associated with MetS. Prevalence of metabolic syndrome was higher among non-smokers (24.1%), non-consumers of alcohol (27.7%) compared to smokers (9.3%) and alcohol consumers (10.0%). Participants who did not eat fruits (18.4%) and those who met the fruit consumption recommendation (five to seven times a week) (18.1%) had higher prevalence of MetS compared to those who ate fruits one to four times a week (9.6%). For vegetable consumption, participants who did not eat vegetables and those who ate vegetables five to seven times a week had a higher prevalence of MetS, 38.3% and 26.4%, respectively, compared to those who ate vegetables one to four times a week.
| Variables | Metabolic syndrome n(%) |
No metabolic syndrome n(%) |
RR | CI | p-value |
|---|---|---|---|---|---|
| Current smoker | |||||
| Yes | 14(9.3) | 136(90.7) | |||
| No | 204(24.1) | 644(82.6) | 0.3 | 0.2-0.6 | 0.000* |
| Smoking categories | |||||
| Primary smokers | 26(10.7) | 124(82.7) | |||
| Secondary smokers | 58(19.4) | 241(80.6) | - | - | 0.082 |
| Non-smokers | 134(61.5) | 415(53.2) | |||
| Ever drank alcohol? | |||||
| Yes | 32(10.0) | 287(90.0) | |||
| No | 185(27.7) | 484(72.3) | 0.3 | 0.2-0.4 | 0.000* |
| Ever consumed alcohol in the past 12 months? | |||||
| Yes | 28(10.0) | 251(90.0) | |||
| No | 74(29.6) | 176(70.4) | 0.3 | 0.2-0.4 | 0.000* |
| Fruit servings | |||||
| None | 7(18.4) | 29(80.6) | |||
| 1-4 | 57(9.6) | 538(90.4) | - | - | 0.000* |
| 5-7 | 65(18.1) | 295(81.9) | |||
| Vegetable servings | |||||
| None | 12(38.3) | 24(66.7) | |||
| 1-4 | 111(18.7) | 484(81.3) | - | - | 0.005 |
| 5-7 | 95(26.4) | 265(73.6) |
In the logistic regression, after adjusting for confounders, only age 26 and above (AOR=4.1, CI=2.0-8.4), married (AOR = 2.3 CI=1.6-3.3), female sex (AOR=1.6, CI=1.1-2.4), alcohol use (AOR=2.0, CI= 1.3-3.1), unemployment (AOR=1.8, CI= 1.2-2.6) and income level below ZAR1200 (AOR= 1.6, CI= 1.1-2.4) were significant and independent predictors of MetS.
Participants aged 26 and above were four times more likely to have MetS than participants below age 26 years. Married, non-alcohol users and unemployed participants were twice more likely to have MetS than never married, alcohol users and employed individuals. The odds for having MetS among females and those earning below ZAR1200 were 1.6 relative to males and participants earning more than ZAR1200 (Table 5).
| Variables | Beta | Wald | AOR(CI) | p-value |
|---|---|---|---|---|
| Age (years) | ||||
| 26 and above | ||||
| ≤ 25 (reference) | 1.41 | 14.68 | 4.1(2.0-8.4) | 0.000* |
| Marital status | ||||
| Ever married | ||||
| Never married (reference) | 0.85 | 22.32 | 2.3(1.6-3.3) | 0.000* |
| Sex | ||||
| Female | ||||
| Male (reference) | 0.49 | 6.10 | 1.6(1.1-2.4) | 0.014* |
| Alcohol consumption | ||||
| No | ||||
| Yes | 0.69 | 9.82 | 2.0(1.3-3.1) | 0.002* |
| Employment status | ||||
| Unemployed | ||||
| Employed (reference) | 0.58 | 9.49 | 1.8(1.2-2.6) | 0.002* |
| Income | ||||
| 1200 and below | ||||
| Above 1200 (reference) | 0.50 | 7.07 | 1.6(1.1-2.4) | 0.008* |
DISCUSSION
This study presents a high prevalence (21.8%) of metabolic syndrome among the study participants. Comparing the prevalence of metabolic syndrome (MetS) across studies is challenging due to the various definitions of metabolic syndrome in the literature. Metabolic syndrome mostly includes the combination of any three of these five criteria: abdominal obesity, hypertension, hypertriglyceridemia, low HDL-cholesterol level, and hyperglycemia [22]. The prevalence of MetS in this study is lower (21.8% vs. 42%) compared to the study conducted in Soweto, Johannesburg, South Africa [10].
The findings in this study is somewhat similar to several previous findings reported both in developed and developing countries [23-25]. However, a higher prevalence has been recorded in some other studies in the USA (33%) [26], Central America (31%) [27]; Latin American countries (Argentina, Chile and Uruguay) (37%) [28]. The high prevalence of MetS among the population in the present study is not surprising, as the components of MetS (obesity, hypertension and diabetes) are highly prevalent among the study population [17]. This high prevalence has been linked to urbanisation, westernisation, nutritional and epidemiological transition and this calls for urgent action by the policy makers and health managers to further emphasise the need for routine screening for all the components of MetS at the primary health care level.
Of all the demographic factors, sex, age, marital status, level of education and employment status were the only demographic factors statistically associated with MetS. In the logistic regression, after adjusting for confounders, age 26 and above years, marriage, female sex, alcohol use, unemployment and an income level below ZAR1200 were significant and independent predictors of MetS. These findings are in accordance with some other studies [29-31].
The significant gender variation in the prevalence of METs found in this study is comparable to other studies [32-34]. This is not surprising since METs is a clinical condition resulting from the constellation of several related risk factors which often vary across gender [33]. In this study, abdominal obesity and dysglycemia were higher among females. Studies have shown that females are more prone to abdominal obesity compared to males [35-37] as a result of hormonal regulation of body fat distribution [34]. Also, physical inactivity has been reported to be higher among females than males in this setting [17].
Physical activities which contributes to obesity, a leading prognostic factor for diabetes and consequently METs [38, 39]. Given that abdominal obesity often precedes the development of other components of MetS [40, 41], the higher prevalence of METs among women in this present study is not surprising. As such, abdominal obesity should be regarded as a vital component of cardiovascular risk evaluation in routine clinical practice, particularly, among females; with a health education follow-up on the importance of lifestyle modifications.
In this study, old age was a significant predictor of METs. This has been documented in some other studies [25, 29, 31]. The prevalence of MetS was found to be higher among participants who were aged 56 years and above (49.3%) compared to those between 18 and 25 years (5.5%).
Ageing is often associated with a higher predisposition towards cardio-metabolic risk factors such as obesity, hypertension and dyslipidemia [17, 42-45]. Ageing is often associated with lesser physical activity, a predisposing factor to obesity with its consequential metabolic health compromise [42]. Also, ageing is associated with a decline in the functioning of the islet cells which bring about insulin resistance and a new onset of diabetes [46, 47]. Similarly, changes in body system, including the cardiovascular system; the arteries and the heart often accompany ageing, resulting in elevated blood pressure, another component of METs. This also explains why the prevalence of METs is higher among the retirees who constitute the older age group. Thus, there is a need to pay closer attention to the metabolic health status of the populace as age advances.
Also, participants with a lower level of education had a higher prevalence (28.2%) compared to those with higher levels of education (6.9%). Hajian-Tilaki et al. [30] also reported a higher prevalence of METs among participants with lower educational qualification. The relationship between education and knowledge seems to be complex, education has been documented to increase knowledge [48]. We therefore can presume that the more educated individuals are, the more knowledgeable they are about their health and the significance of self-care and healthy lifestyle. However, whether this assumption is true among our study participants is rather speculative. Also, it has been reported that more educated individuals are likely to be more receptive to new ideas, developments and information provided regarding their health [49, 50]. Low levels of education, unemployment and low income are all associated with a higher likelihood of developing METs [51].
In addition, the prevalence of MetS was found to be more than three times higher among married participants (23.2%) compared to those who had never been married (6.0%). Bhanushali et al. [52] also reported similar finding of a higher prevalence of METs among married people. However, Hosseinpour-Niazi et al's study [53]did not find any significant difference between marital status and MetS. Although there is no clear explanation as to why married women should have a higher prevalence of METs than those who are single, Troxel et al. [54] identified marital quality as an important mediator between marital status and METs. The reason behind the higher prevalence of METs among married participants could also be ascribed to higher prevalence of the components of METs (obesity, diabetes and hypertension) found among the married participants [17].
Smoking, alcohol use, fruit and vegetable consumption were the statistically significant behavioural risk factors for metabolic syndrome. Participants who did not consume alcohol, either life time (16.9%) or in the past 12 months (20.4%), were found to have a higher prevalence of METs compared to those who did (5%). Although the association between MetS and alcohol consumption seems complex, light-to-moderate alcohol use is often associated with reduced risk for METs, while heavy drinking increases the risk [55, 56]. The lower prevalence of MetS found among the alcohol users might be due to the protective and beneficial effects of light-to-moderate alcohol consumption on metabolic health.
Finally, participants who consumed fruits were found to have a higher MetS (19.4%) compared to those who did not. This is contrary to the findings of other studies which have reported a lower prevalence of MetS in association with fruit consumption [57, 58] This finding should be interpreted with caution. It is possible that the decision of the participants in this study to start taking fruits was informed by advice from health practitioners on the importance of fruit consumption after they had been told their health was metabolically compromised.
Strengths and Limitations
This was a cross sectional study conducted among adults attending three large out-patient healthcare facilities located in Buffalo City Metropolitan Municipality, Eastern Cape Province of South Africa. It had enrolled mainly those patients who hailed from the Province and, therefore, might not necessarily have represented the entire South African population. Also, given that METs include the combination of five criteria namely abdominal obesity, hypertension, hypertriglyceridemia, low HDL-cholesterol level, and hyperglycaemia [22]; and our study assessed only three criteria, there is, therefore, a possibility of underestimating the prevalence of METs among the populations. Also, the use of capillary blood used in this study is not considered a diagnostic test for diabetes, although it is a convenient and acceptable method in epidemiological surveys.
Despite these limitations, in the absence of data on the clustering of these cardiometabolic risk factors among adults in BCMM, this study provides a snapshot of the metabolic health of the population. It also serves as comparable baseline data for health policy makers and researchers. Furthermore, our study includes a large sample size and multi-sites with highly standardised methods as well as a validated WHO STEPwise tool.
CONCLUSION
Irrespective of the defining criteria, our study revealed 21.8% prevalence of metabolic syndrome among adults attending the selected out-patient healthcare facilities in Buffalo City Metropolitan Municipality, Eastern Cape, South Africa. This is worrisome given that the separate components of the metabolic syndrome are shown to be associated with a higher risk of coronary heart diseases and stroke. Also, old age, marital status, female sex, alcohol use, unemployment and low income were independent predictors of metabolic syndrome among the participants. The district and provincial health policies should integrate preventive strategies toward addressing the cardiometabolic risk factors at the primary health care facilities and population level. Women, those in the older age group, individuals with low level of education and the unemployed should be targeted for interventions aimed at reducing METs. There is a need for educating patients on lifestyle changes in order to reduce the cardiometabolic risk factors, particularly among those with more than one risk factor. Finally, future studies should endeavor to screen for all the components of METs in the Eastern Cape Province in order to ascertain the exact prevalence which will inform public health policies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained in accordance with the Helsinki II Declaration from the University of Fort Hare Research Ethics Committee and the Eastern Cape Department of Health (Reference number; GOO061SOLO01). The management of the sub-district Department of Health as well as the heads of the respective health facilities gave permission prior to data collection. All participants provided written informed consent to participate in this study. Anonymity and confidentiality were ensured.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
Not applicable.
FINANCIAL SUPPORT
This study received financial support from the National Research Foundation (NRF) and the Health and Welfare Sector Education and Training Authority (HWSETA), South Africa.
AUTHORS’ CONTRIBUTIONS
EOO, DTG and OVA conceptualised, designed and drafted the paper. AOA and ES participated in data collection and gave intellectual contributions into the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval was obtained from the University of Fort Hare Research Ethics Committee. Informed consent was obtained from participants prior to data collection.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to the management and the nursing staff of the three health facilities for their support towards the successful completion of the study.


