All published articles of this journal are available on ScienceDirect.
Nutritional Status of HIV Clients Receiving HAART: Its Implication on Occurrence of Opportunistic Infection
Abstract
Purposes:
We aimed to assess the effects of nutritional status on occurrences of opportunistic infection in HIV/AIDS patients using antiretroviral therapy at Jimma University Specialized Hospital.
Methods:
We conducted a retrospective study on 340 adults who were taking antiretroviral therapy and the patients were followed for 2 years after they commence treatment. Medical Chart review was done from January 30 to February 28, 2014. SPSS for windows version 21 was used to analyze the data. The data was analyzed by SPSS for windows version 21.Time to occurrence of opportunistic infection was estimated by Kaplan-Meier analysis and Cox-proportional Hazard model was used to identify predictors of opportunistic infections.
Results:
Eighty three [24.4%] patients developed opportunistic infection after initiation of highly active anti-retroviral therapy. Fifty five [66.3%] patients were from malnourished group. Malnutrition, Stavudine based regimen, Zidovudine based regimen and taking isoniazid prophylaxis were associated with greater hazard of developing opportunistic infections.
Conclusions:
Malnutrition was significant predictor of opportunistic infections. Malnourished patients were associated with high risk and early development of opportunistic infections.
1. INTRODUCTION
The impact of HIV infection on nutritional status of patients was identified during the early times of HIV epidemic [1]. Wasting is the commonest sign of malnutrition in patients with HIV/AIDS especially as patients progress to severe disease states [1]. Increasing energy requirements, reducing food intake, and adversely affecting nutrient absorption and metabolism is the mechanism by which HIV affects nutritional status [2]. If nutritional needs of the patient are not met, the patient may have decreased immunity and increased opportunistic infections (OIs), which may further lead to malnutrition. Disease progression, increases morbidity, and reduces survival time are the outcome of poor nutritional status in PLHWA [3].
Highly active antiretroviral therapy (ART) decreases death, increases CD4 lymphocyte counts and decreases occurrences of opportunistic infections [4, 5]. Immunologic recovery differs from person to persons. It is slow in some patients, leading to incomplete recovery of immune function which in turn leads to greater risk of developing opportunistic infections and death compared to those who have fast immune recovery [6]. Some patients may die even with sufficient CD4 count and low viral load [5]. Therefore, adjunctive treatments might be important in promoting CD4 recovery and promoting survival in patients taking HAART.
Studies confirmed malnutrition was major determinant of death and poor immunologic recovery in patients taking HAART [7-11]. However, only few studies examined the effect of malnutrition on occurrence of opportunistic infections globally. Studies are lacking in developing countries including Ethiopia.
Malnutrition may negatively affect immunologic recovery to HAART, increase risk of opportunistic infections and shortens survival. Therefore, Malnutrition might be potential risk factor for increased mortality in patients initiating ART.
2. METHODS
2.1. Study Design and Participants
Retrospective cohort was done at Jimma University Hospital ART Clinic. The Hospital has a bed capacity of 450 and is the only referral and teaching hospital in western part of Ethiopia. The Hospital serves around 80,000 outpatients and 9000 inpatients each fiscal year. The Hospital ART clinic started service in 2002. The Clinic is serving 3700 patients [11]. The primary data was filled by assigned health professionals between September 11, 2006 and September 10, 2011. We extracted the data from the medical charts of the patients from January 30 to February 28, 2014. Single proportion formula was used to calculate the sample size, which assumes proportion of mortality [common measure of treatment outcome] in malnourished group to be 61.8% and proportion of mortality in well-nourished group to be 46.8. The final calculated sample size was 340 patients; one hundred seventy (170) patients in both groups. We isolated medical charts of all adult patients who started ART between September 2006 and September 2011 was isolated. We categorized the charts into malnourished and well-nourished groups based on their BMI at the beginning of HAART. Malnutrition was defined as a BMI <18.5, while BMI ≥18.5 was defined as a well-nourished. Patients with incomplete data on weight, height and outcome variables, transferred-out during follow-up, pregnant women’s (BMI and nutrient metabolism vary during pregnancy), were excluded from the study. Clinically trained health professionals were used for collecting the data. The endpoint of this study was occurrence of opportunistic infections.
We followed the patients till occurrence of event (opportunistic infection/s) or 2 years (end of study). We excluded those who did not develop opportunistic infections at the end of the 2nd years. Patients who did not develop opportunistic infection/s at the end of study were also excluded. The survival time was calculated in days using date of starting treatment and date of an event or date excluded.
2.2. Statistical Methods
Epi data version 3.1was used to edit, clean and complete the data. The data was then exported to SPSS for windows version 21 for statistical analysis. We did survival analysis. Variables with p value of <0.25 in bivariate analysis and BMI were selected and exported to multivariable cox-proportional analysis model to identify predictors of opportunistic infection/s. Survival time was estimated by Kaplan–Meier survival analysis. Cox proportional hazard model was used to identify predictors of opportunistic infection/s over a period of time t. The level of significance was set at p value less than 0.05 and 95% confidence intervals (CI) were used throughout. We checked multi-collinerity.
3. RESULTS
3.1. Baseline Characteristics
All patients were followed for 2 years. Eighty three patients developed opportunistic infections. Fifty five (66.26%) patients were from malnourished group. The median baseline CD4 count was 144.5 [IQR 89–209] cells/mm3 The mean age of the participants and the median age were 34 [IQR 26–38] years and 30 years respectively. Two hundred (58.8%) patients were females. Mean baseline BMI was 1.50 [IQR 1.0–2.00] kg/m2 (Tables 1, 2).
| Characteristics | Number of patients [%] | Number of deaths[%] | Number of OI [%] |
|---|---|---|---|
| Sex | |||
| Male | 140[41.2] | 19[45.2] | 38[45.8] |
| Female | 200[58.8] | 23[54.8] | 45[54.2] |
| BMI | |||
| <18.5 | 170[50] | 25[59.5] | 55[66.3] |
| ≥18.5 | 170[50] | 17[40.5] | 28[33.7] |
| Religion | |||
| Orthodox | 203[59.7] | 29[69] | 44[53] |
| Muslim | 100[29.4] | 13[31] | 26[31.3] |
| Protestant | 31[9.1] | 0 | 11[13.3] |
| Catholic | 2[0.6] | 0 | 1[1.2] |
| Others | 2[0.6] | 0 | 0 |
| Age(years) | |||
| <30 | 174[51.2] | 21[50.0] | 46[55.4] |
| 30-39 | 94[27.6] | 11[26.2] | 21[25.3] |
| 40-49 | 57[16.8] | 6[14.3] | 12[14.5] |
| >50 | 15[4.4] | 4[9.5] | 4[4.8] |
| Marital status | |||
| Single | 43[12.6] | 7[16.7] | 10[12.0] |
| Married | 116[34.1] | 16[38.1] | 36[43.4] |
| Widowed | 22[6.5] | 1[2.4] | 5[6.0] |
| Divorced | 44[12.9] | 8[19.0] | 9[10.8] |
| Occupation | |||
| Gov’t employee | 98[28.8] | 14[33.3] | 19[22.9] |
| Merchant | 5[1.5] | 0[0] | 2[2.4] |
| Unemployed | 189[55.6] | 25[59.5] | 51[61.4] |
| Private org | 37[10.9] | 2[4.8] | 10[12.0] |
| NGO’s | 5[1.5] | 1[2.4] | 0[0] |
| Educational status | |||
| Not educated | 69[20.3] | 4[9.5] | 24[28.9] |
| Primary | 122[35.9] | 17[40.5] | 25[30.1] |
| Secondary | 106[31.2] | 14[33.3] | 21[25.3] |
| Tertiary | 42[12.4] | 7[1.7] | 12[14.4] |
| Characteristics | Number of patients[%] | Number of deaths[%] | Number of OI[%] |
|---|---|---|---|
| WHO clinical stage | |||
| Stage I | 65[19.1] | 6[14.3] | 15[18.1] |
| Stage II | 95[27.9] | 9[21.4] | 15[18.1] |
| Stage III | 144[42.4] | 17[40.5] | 44[53.0] |
| Stage IV | 36[10.6] | 10[23.8] | 9[10.8] |
| HAART regimen | |||
| Stavudine based | 219[64.4] | 19[45.2] | 60[72.3] |
| Zidovudine based | 72[21.2] | 12[28.6] | 18[21.7] |
| Tenofovir based | 49[14.4] | 11[26.2] | 5[6.0] |
| Base line CD4 | |||
| >350 | 5[1.5] | 0[0.0] | 2[2.4] |
| 200-350 | 94[27.6] | 7[16.7] | 19[22.9] |
| 100-199 | 139[40.9] | 15[35.7] | 37[44.6] |
| <100 | 102[30.0] | 20[47.6] | 25[30.1] |
| Risky behaviour | |||
| Yes | 120[35.3] | 14[33.3] | 58[69.9] |
| No | 220[64.7] | 28[67.7] | 25[30.1] |
| Cotrimoxazole Prophylaxis | |||
| Yes | 330[97.1] | 42[100.0] | 81[97.6] |
| No Fluconazole Prophylaxis |
10[2.9] | 0[0.0] | 2[2.4] |
| Yes | 20[5.9] | 8[19.1] | 5[6.0] |
| No | 320[94.1] | 34[80.9] | 78[94.0]] |
| INH prophylaxis | |||
| Yes | 62[18.2] | 7[16.7] | 20[24.1] |
| No | 278[81.8] | 35[83.3] | 63[75.9] |
3.2. Occurrence of Opportunistic Infections
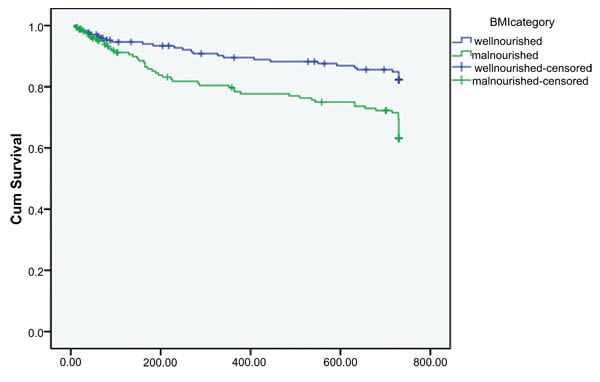
In Kaplan-Meier bivariate cox-proportional analysis, there was statistically significant difference in time of opportunistic infection/s occurrence between malnourished and well-nourished group [p=0.001]. Malnourished patients at the start of HAART develop opportunistic infections early in the course of treatment compared to well-nourished group (Fig. 1). In bivariate cox-proportional hazard model baseline nutritional status and HAART regimen were significantly associated with time of opportunistic infection/s occurrence (Table 3).
| Variables | Number of OI | Mean survival time in days [95 %CI] | P | |
|---|---|---|---|---|
| Age group (yrs) | < 30 30-39 40-49 ≥50 |
46 21 12 4 |
620.93[585.173,656.685] 632.70[587.857,677.550] 652.22[599.260,705.175] 601.58[491.012,712.155] |
0.757 |
| Sex |
Female Male |
45 38 |
634.09[603.441,664.735] 621.39[582.296,660.479] |
0.329 |
| BMI |
< 18.5 ≥ 18.5 |
55 28 |
594.27[555.615,632.922] 661.52[632.772,690.278] |
0.001 |
| Marital status |
Single Married Widowed Separated |
10 36 5 9 |
631.97[562.164,701.775] 592.67[546.266,639.070] 643.37[574.149,712.584] 612.30[580.625,643.97] |
0.615 |
| Educational level |
Not Educated Primary Secondary Tertiary |
24 25 21 12 |
566.89[503.064,630.719] 651.11[614.903,687.324] 655.27[615.672,694.875] 610.26[535.895,684.626] |
0.089 |
| CD4 count |
< 100 100-199 200-350 > 350 |
25 37 19 2 |
629.11[583.779,674.431] 617.88[578.817,656.949] 651.09[609.222,692.964] 511.40[263.608,759.192] |
0.521 |
| Risky behaviors |
Yes No |
25 58 |
647.35[608.379,686.314] 618.54[587.838,649.236] |
0.241 |
| Baseline comorbidity | Yes No |
3 80 |
675.28[587.677,762.878] 626.02[600.930,651.113] |
0.313 |
| Baseline OI |
Yes No |
62 21 |
624.29[595.897,652.684[ 642.80[597.438,688.167] |
0.818 |
| Cotrimoxazole prophylaxis | Yes No |
81 2 |
629.56[605.258,653.856] 609.00[458.693,759.307] |
0.676 |
| Fluconazole Prophylaxis | Yes No |
5 78 |
544.00[410.554,677.446] 632.73[608.431,657.035] |
0.389 |
| INH prophylaxis | Yes No |
42 41 |
577.50[512.176,642.833] 640.46[615.170,665.833] |
0.083 |
| Baseline HAART | D4T based AZT based TDF based |
60 18 5 |
614.44[583.314,645.574] 617.08[558.409,675.759] 628.86[604.828,652.887] |
0.051 |
| Baseline WHO | Stage I Stage II Stage III Stage IV |
15 15 44 9 |
649.68[598.913,700.445] 666.86[628.711,705.013] 598.24[556.866,639.611] 609.18[530.651,687.706] |
0.066 |
Variables with Hazard ratio less than 0.25 in bivariate cox-proportional hazard model were fitted to multivariable cox-proportional hazard model. BMI [HR=2.27, 95% CI: 1.416, 3.625, p=0.001], Stavudine based regimen [HR=2.93, 95% CI: 1.178, 7.309,p=0.021], Zidovudine based regimen [HR=3.15, 95% CI:1.155,8.583, p=0.025] were found to be predictors of OI in multivariable cox-proportional hazard model. Patients who took isoniazid prophylaxis were 1.69 times at hazard of developing OI [HR=1.69, 95% CI: 1.016, 2.830, p=0.043] (Table 4).
| Variable | Number of OI | HR [95% CI] | P | |
|---|---|---|---|---|
| BMI |
< 18.5 ≥ 18.5 |
55 18 |
2.27[1.416,3.625] 1 |
0.001 |
| Educational level |
Not educated Elementary Secondary Tertiary |
24 25 21 12 |
1 0.635[0.361,1.115] 0.596[0.330,1.076] 1.103[0.542,2.247] |
0.114 0.086 0.786 |
| Baseline HAART |
D4T based AZT based TDF based |
68 18 5 |
2.93[1.178,7.309] 3.15[1.155,8.583] 1 |
0.021 0.025 |
| INH Prophylaxis |
No Yes |
41 42 |
1 1.695[1.016,2.830] |
0.043 |
4. DISCUSSION
Malnutrition at the start of HAART was predictor of opportunistic infections [HR=2.93, 95% CI, 0.416, 3.625, p=0.001]. This finding is in line with reports by Simone and his colleague which shows BMI of less than 18.5 kg/m2was strongly associated with increased risk of disease progression [12]. Moreover, the study was comparable with findings reported by Zhou and his colleague which showed that lower BMI was associated with developing new AIDS defining illness [13]. Furthermore, it is consistent with finding from study conducted at South Africa where patients with low BMI, irrespective of OC, at ART initiation showed a decrease in hemoglobin, increase in AST and increase in TB at ART initiation compared to those with normal BMI (≥18.5 kg/m2) [14]. Likewise, this finding is comparable with findings from Nigeria which showed that low BMI was determinant of opportunistic infections [15]. Another study done by Yoann and his collegue confirmed that Patients with baseline BMI less than 18.5 were two times at higher risk of having opportunistic infections (p=0.016).) [16].
We identified that patients who were on Zidovudine based regimen had high risk of developing opportunistic infections compared to those patients who started their treatment by Tenofovir based regimen. This finding was in line with the study conducted in India which shows higher OI was seen in patients receiving AZT based regimen (46% vs. 31%, p=0.22) [17]. A study by Samuel and his colleagues [18] in Kenya showed that patients started on TDF based regimen had better survival than AZT based regimen (61 vs. 56.5 months) respectively. In addition, one extra opportunistic infection was prevented every 14 patients treated using this TDF/3TC/EFV regimen (p=0.026). On the contrary, AZT/3TC/EFV was the least protective regimen used in this set-up, where one patient will experience 9 episodes extra of opportunistic infections with similar course of treatment (p=0.049) [17].
In our study, patients who took prophylaxis for opportunistic infections were at high risk of developing opportunistic infections. This finding is in contrast to the study from Addis Ababa, Ethiopia which shows that Isoniazid has protective effect against development of Oppurtunistic infections. The difference could be due to small sample size and we looked at the development of all opportunistic infection. However, study done in Addis Ababa focused on the development of Tuberclosis alone.
CONCLUSION
Nutritional status at the commencement of HAART significantly predicts development of opportunistic infections in HIV patients receiving HAART.
Patients who started on Zidovudine based regimen, Stavudine based regimen were at high risk of developing opportunistic infection compared to patients who started on Tenofovir based regimen.
Isoniazid Prophylaxis contributes in the development of opportunistic infections.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by Institutional Review Board (IRB) of Jimma University.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


