RESEARCH ARTICLE
Microbial Carriage and Contamination of Mangoes by the Oriental Fruit Fly
Godfred Futagbi1, *, Nana Akosua Gyamfuah Koduah1, Benyarku Richard Ampah1, Precious Agbeko Dzorgbe Mattah2, Maxwell Billah1, James Edinam Futse3, Eric Sampane-Donkor4
Article Information
Identifiers and Pagination:
Year: 2017Volume: 10
First Page: 267
Last Page: 275
Publisher ID: TOPHJ-10-267
DOI: 10.2174/1874944501710010267
Article History:
Received Date: 13/08/2017Revision Received Date: 21/11/2017
Acceptance Date: 29/11/2017
Electronic publication date: 13/12/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Fruit flies, especially of the Family Terphritidae, are economically important pests for the horticulture industry because many species cause serious mechanical damage to a number of crops of different plant families. Studies have shown that some species of fruit flies have the potential to contaminate fruits and vegetables with enteric bacterial pathogens. However, this has not been conclusively demonstrated.
Methods:
In this study, we investigated enteric bacteria carriage by Bactrocera dorsalis and its possible role in transmission of microbes into internal tissues of fruits. Fruit flies trapped using liquid protein bait, ripe mango fruits exposed to the fruit flies and controls, as well as mangoes obtained from farms with and without fly-control traps, were analyzed for microbes, such as total aerobic bacteria, total coliforms, yeast and molds, Escherichia coli and Salmonella/Shigella spp. using direct culture methods.
Results and Discussion:
The results revealed that a high percentage of these insects carries pathogenic bacteria. This finding shows that, like B. cacuminata and B. tryoni, B. dorsalis also carries pathogenic microbes. It was also observed that mangoes sampled from fly-control farms had significantly lower microbial loads and proportions of fruits contaminated compared to those from farms without fly-control. Additionally, all microbial counts of internal tissues were significantly higher for exposed mangoes compared to unexposed mangoes. These data indicate that B. dorsalis contaminates not only the external but also internal tissues of mangoes.
Conclusion:
These findings show that B. dorsalis carries pathogenic bacteria and plays a direct role in internalization of microbes in mangoes.
1. INTRODUCTION
Fruit flies, especially, of the Family Terphritidae, are economically important pests for the fruit industry because many species are polyphagous and cause serious mechanical damage to a number of crops of different plant families [1, 2] and contribute significantly to economic losses to fruit and vegetable growers. Fruits and vegetables, especially ripened fruits and vegetables, are usually the attractants of many fruit flies including the common or vinegar fruit flies, Drosophila melanogaster and the fruit flies of the family tephritidae, such as Bactrocera species [3]. Isolation of microbial contaminants from fruit flies has been documented [4]. For example, Citrobacter freundii, Enterobacter cloacae and Klebsiella oxytoca were identified in terphrid fruit flies [4]. This raises question about the ability of fruit flies to transmit microbes into the inner tissues of fruits and vegetables. However, little is known about their role in internal contamination of fruits and vegetables with pathogenic bacteria. Studies have shown that, in addition to causing mechanical damage and just like filth flies such as Musca domestica and many blood sucking insects, such as mosquitoes, Tephritid flies have the potential to serve as vectors of pathogenic microbes [4, 5]. Though they lack the piercing and sucking mouth parts like that of typical blood feeding flies, female flies lay eggs in fruit by puncturing the skin of the fruit with their ovipositors and are likely to contaminate not only the external surface but also the internal tissues of fruits. They may be exposed to bacteria due to their habits of visiting insanitary sites and attraction to rotten foods [6]. Medfly, also known as Mediterranean fruit fly (Ceratitis capitata) has been shown in the laboratory to have the potential to carry pathogenic E. coli and transmit it to fruits [4]. Other pathogenic bacteria were also isolated from midgut of field collected wild tobacco fruit-flies (Bactrocera cacuminata) and Queensland fruit fly (Bactrocera tryoni) [5].
Bactrocera dorsalis and Ceratitis cosyra are thought of as the two most harmful out of the many terphritid species that damage mangoes in West Africa [3, 7]. However, microbial carriage and potential of these species to contaminate internal tissues of fruits and vegetables have not been established. Additionally, there has been an increasing trend of consumption of raw produce and in the light of the increasing outbreaks of infections associated with fresh produce consumption [8-11]; there is the need to investigate not only the microbial carriage of these terphritid organisms but also whether they could be directly responsible for outbreaks of infections associated with consumption of raw fruits. For example, ingestion of fresh produce contaminated with pathogenic Escherichia coli (E. coli), Salmonella, Campylobacter, Listeria and Shigella species was found to be the primary cause for outbreaks of foodborne illnesses [8, 9]. We also observed, in a previous study, that more than 70% of mangoes sampled from selected markets were contaminated with microbes, including faecal coliforms. The microbial counts were found to increase with the number of scars or punctures [12], indicating a possible role for terphritid fruit flies in microbial contamination of mangoes. This study therefore aimed at finding out the potential of B. dorsalis to carry microbial contaminants and their ability to introduce contaminants into the internal tissues of fruits and vegetables.
2. MATERIAL AND METHODS
2.1. Study Areas and Sample Collection
Twenty-four mangoes from a farm without fly-control and 8 from a farm with fly-control were used for the study. The farms were located in the Greater Accra Region of Ghana. In the fly-control farm, Ecoman protein bait (Ecoman Biotech, Beijing, China) was sprayed on plants that formed a boundary around it. The protein bait spraying was done once a week to attract and kill both male and female flies. Stop Mating pheromone fly traps (Splendid Agro Products, Accra) baited with Stop Mating block (Splendid Agro Products, Accra) were also hanged in the trees. The Stop Mating block baits were suspended on wires projecting from the lid into the traps. There were ten traps per acre as against five prescribed by the manufacturer. The pheromone traps attract only male flies. This helps to keep the female flies away from the mangoes. The purpose of the pheromone traps was to eliminate the male flies from the population and thereby preventing breeding by, as the name denotes, stopping mating. Though both farms practiced orchard weeding and pruning of trees, these were more evident in the fly-control farm than the farm without fly-control. Fruits were collected into aseptic bags. The well-managed farm was established purposely to grow and export its produce to the international market but the fruits analyzed in this study were those thought be unwholesome for export.
All mangoes obtained were unripe. Mangoes exposed to fruit flies were kept to ripe before hanging. Fruit flies, on the other hand were sampled from three markets and three sites on the University of Ghana campus (Legon Hall, Link gate and the Department of Animal Biology and Conservation Science (DABCS), which are also situated in the Greater Accra Region of Ghana. Samples were collected between October, 2015 and April, 2016.
2.2. Trapping of Fruit Flies
Five fruit fly traps were used in trapping the fruit flies. The fruit fly traps were cleaned with 70% alcohol before being taken to the various sites for trapping. Each fruit fly trap consisted of four holes in its lid which served as the entrance for the fruit flies but they cannot easily escape through. At the bottom of the holes were firmly affixed small sacks made of mosquito net which was disinfected in 70% alcohol and dried. This was done to ensure that fruit flies entering the trap fell into the net without contaminating or picking contaminants from inner surface of the trap. Fruit flies were trapped using the Ecoman Biotech® Fruit Fly Trap (Ecoman Biotech, Beijing, China) and a liquid protein bait, the Great® Fruit Fly Bait (Ecoman Biotech Co., Ltd., Beijing, China). The traps were baited by dipping paper cards in the liquid protein bait and placing the cards inside the traps as described above.
2.3. Exposure of Mangoes to B. Dorsalis for Possible Microbial Contamination
This experiment was designed to find out the possible role for B. dorsalis in transmission of microbe to fruits. Twelve ripe mangoes (controls) were kept out of reach of fruit flies in the laboratory-and another twelve from the same source were exposed to fruit flies by hanging them on mango trees for three or four days depending on the stage of ripeness. The fruits were observed from early morning to late evening, at least six times in 12 hours, from 6am to 6pm while hanging on the trees for the presence of fruit flies. For the first three or four days only B. dorsalis was seen, however, beyond that as the mango became softer and exudates started appearing, due to the punctures, Drosophila sp. were seen on the mangoes. Mangoes on which other flies were spotted or pecked by birds were replaced.
2.4. Analysis of Bactrocera Dorsalis for Microbes
One hundred and thirty-nine flies, 103 from Link gate and 17, 13 and 6 from DABCS, the markets and Legon Hall, respectively, were analyzed for external microbial contaminants. Thirty female flies were further analyzed for their internal Salmonella/Shigella (SS) carriage. The female flies were selected and evaluated for internal microbial carriage because they puncture the fruit to lay eggs in them.
To estimate the level of microbes on external surfaces, fruit flies were washed thoroughly by shaking vigorously in 200 uL of sterile saline solution and 100 uL was added to 900 uL of the sterile saline solution. All flies collected in a single net were washed together. For internal microbial analysis, each female fly was first washed with 70% ethanol and saline solution to remove any external contaminants and ethanol residue, respectively. The fly was then placed in an eppendorf tube containing 200 uL of the saline solution; gently crush using sterile pipette tip and100 uL of the resulting solution was added to 900 uL of the sterile saline solution. Four serial dilutions were made for all samples.
Direct culture methods were employed in the analyses as described previously [13]. Aerobic count, Potato Dextrose, Violet Bile Red Glucose and Salmonella/Shigella agars were used in the detection and enumeration of total aerobic plate counts (TPC), yeast and molds (YM) counts, Escherichia coli (E. coli) counts and SS counts respectively. Ten (10) uL of the various dilutions of samples were pipetted and transferred into respective dishes and the prepared agars were gently poured into appropriate dishes containing the samples and swirled gently. Each dilution was plated in triplicate. The set-ups were left on the working slab for the agar to set after which incubation was done for 24 hours at 37 ̊ C for TPC, YM and 44 ̊ C for SS and E. coli. After incubation the microbes present in samples formed colonies and were counted using a colony counter and microbial counts of the samples computed.
2.5. Analysis of Mangoes for Microbes
This analysis was done for TPC, TCPC, SS and E. coli counts. Agars used included those stated above in addition to Eosin Methylene Blue agar for TCPC. Firstly, mango fruits were disinfected with 70% ethanol at portions where incisions were made. The fruits were opened up aseptically using sterile blades and 100µL of juice from inside the fruits was pipetted using sterile pipette tips. The 100uL fruit juices were added to 900µL of standard saline solution. First dilutions were prepared for the cultures. To enumerate microbes on the external surfaces of the mangoes, the outer surface area of 1cm2 of each mango was swabbed using a cotton swab. The swab was then dipped into 100uL of standard saline solution, from which first and subsequent dilutions were made. 10uL of the diluted samples were pipetted and transferred into dishes and the prepared agars added. The dishes were swirled gently to ensure mixing and the set-ups were left on the working slab for the agars to set after which incubation was done as described above.
2.6. Statistical Analysis
Data from the laboratory analyses were analyzed using Graph Prism Statistical software (Prism, GraphPad Software, San Diego, CA, USA). A One Way ANOVA and an unpaired t-test were used to compare microbial loads of B. dorsalis among and between sampling sites, respectively. Mann-Whitney test was used to do pairwise comparisons of microbial load of mangoes. A z- test was also used to compare proportions for various categories.
3. RESULTS
3.1. Proportion of Bactrocera Dorsalis Carrying Microbes
Analysis of B. dorsalis for the carriage of microbes showed that B. dorsalis carried microbes, including enteric and pathogenic bacteria, both externally and internally.
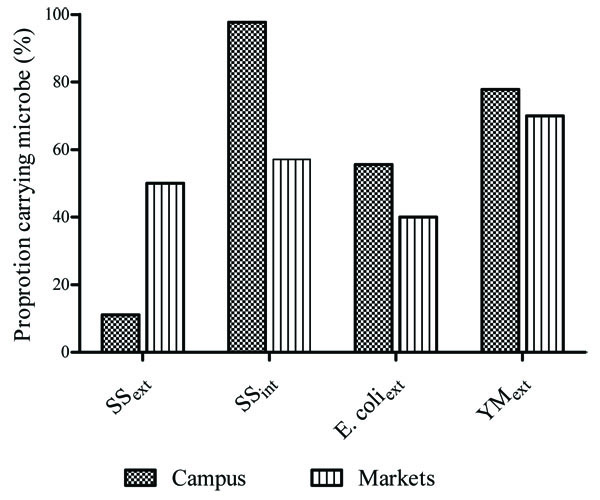
The proportion of flies carrying SS differed significantly between University campus and the selected markets. The proportion of flies carrying SS externally was significantly higher for flies caught in the markets than flies caught on campus (z=4.7715 p=0.0001). On the contrary, the proportion of flies with internal SS was significantly higher for flies sampled on campus compared to those obtained from the markets (z=6.4227 p=0.0001). However, no significant differences were observed in the proportion of flies carrying E. coli and YM between the two sites (z=1.4514, p=0.1471; z=0.8543, p=0.39532; respectively, (Fig. 1).
3.2. Microbial Load of External Surfaces and Internal Body of B. Dorsalis
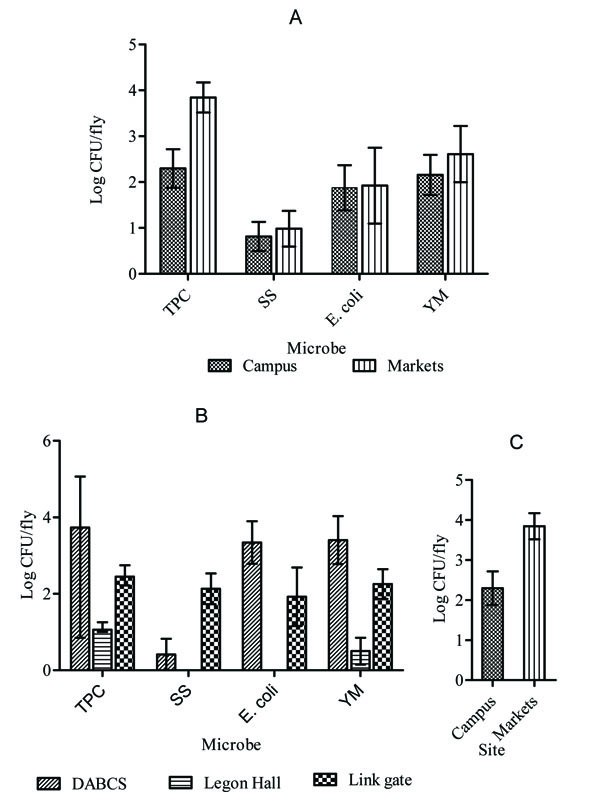
External microbial loads of flies were compared between flies sampled from campus and flies caught in selected markets. Except total aerobic counts (TPC), which were significantly higher in market samples than in flies sampled from campus {Mean difference (95%CI): 1.21 (0.07 to 2.34) log CFU/fly, p=0.0379}, no significant differences were observed in SS, E. coli and YM counts between the two sites (p>0.05; Fig. 2A). External microbial counts were compared also among the sites on campus. Mean TPC, SS, E. coli and YM counts per fly differed significantly among the sites (p=0.0195, 0.0162, 0.0147 and 0.0144, respectively), mainly due to very low counts in Legon Hall samples (Fig. 2B).
Internal pathogenic microbial loads were also analyzed. No significant differences were observed in mean SS counts in internal bodies of B. dorsalis among the three sites on campus (p=0.73800), or between the campus and Markets (p=0.0871; Fig. 2C).
3.3. Microbial Loads of Mangoes from Fly-Control Farm were Lower Than Those from Farms Without Controls
In order to determine whether these fruit flies play any role in microbial contamination of fruits, we sampled fruits harvested from the fly-control farm and those from farms without fly-control measures. The median TPC, TCPC and E. coli counts of both external surface and internal tissues of mangoes from farm with B. dorsalis control were significantly lower than those of mangoes from farm without fly-control (p<0.05; Table 1). Likewise, the proportion of mangoes from the fly-control farm had significantly lower coliform and E. coli contamination compared to those from farms without fly-control (p<0.05; Fig. 3).
| Part | Microbial Counts | Fly-control (n=8) | No Fly-control (n=10) | p-value | ||
|---|---|---|---|---|---|---|
| Median counts (25% and 75% interquartiles) | ||||||
| External (cfu/cm2) |
TPC | 60 | (50 and 190) | 2600 | (700 and 5100) | 0.0019 |
| TCPC | 10 | (0 and 30) | 950 | (100 and 1820) | 0.0225 | |
| E. coli counts | 1 | (0 and 7) | 85 | (42 and 150) | 0.0191 | |
| Internal (cfu/mL) |
TPC | 4.0 | (1.5 and 6.5) | 27.0 | (4.2 and 39.2) | 0.0022 |
| TCPC | 0.0 | (0.0 and 0.0) | 7.5 | (6.0 and 14.5) | 0.0116 | |
| E. coli counts | 0.0 | (0.0 and 2.5) | 7.0 | (3.5 and 10.1) | 0.0208 | |
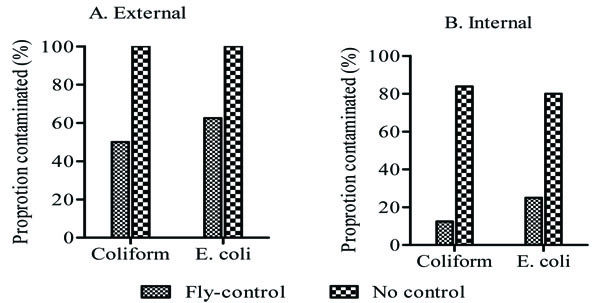 |
Fig. (3). Proportions of contaminated mangoes compared between fly-control farm and farm without controls. |
3.4. Microbial Load of Mangoes Exposed to Flies Were Lower Than Those of Controls
There were no significant differences in external TPC and TCPC between exposed and unexposed mangoes (p=0.6012, 0.1564, respectively). However, external E. coli and SS counts were significantly higher in exposed mangoes compared to unexposed ones (p=0.0191, 0.0401, respectively). Also, all microbial counts of internal tissues were significantly higher for exposed mangoes compared to unexposed mangoes (p<0.05; Table 2).
| Part | Microbial Count | Control Mangoes (n=12) | Exposed Mangoes (n=12) | p-value | ||
|---|---|---|---|---|---|---|
| Median counts ×103 (25% and 75% interquartiles) | ||||||
| External (cfu/cm2) |
TPC | 3.00 | (1.25 and 6.75) | 3.50 | (1.00 and 10.50) | 0.6012 |
| TCPC | 1.00 | (0.00 and 2.00) | 2.00 | (1.00 and 7.50) | 0.1564 | |
| E. coli counts | 0.85 | (0.42 and 1.50) | 4.70 | (0.97 and 8.85) | 0.0191 | |
| SS counts | 0.20 | (0.10 and 0.20) | 0.90 | (0.50 and 1.10) | 0.0401 | |
| Internal (cfu/mL) |
TPC | 2.50 | (1.30 and 3.70) | 13.10 | (6.85 and 21.15) | 0.0078 |
| TCPC | 7.8 | (6.5 and 1.45) | 19.0 | (9.5 and 43.5) | 0.0207 | |
| E. coli counts | 7.0 | (3.5 and 1.07) | 95.0 | (10.5 and 146.0) | 0.0061 | |
| SS counts | 5.0 | (3.0 and 1.25) | 43.0 | (11.7 and 111.3) | 0.0078 | |
4. DISCUSSION
B. dorsalis is a well-known invasive fly that causes spoilage of fruits and vegetables making them insects of high economic importance [1]. It is a common fruit fly species found in Ghana. Microbial carriage by B. dorsalis has not been established; however, studies have shown that the Mediterranean fruit fly, Wild tobacco fruit fly, and Queensland fruit fly carry microbial contaminants [4, 5]. Also, in our previous study, we observed internal contamination of mangoes with microbes, including faecal coliforms, which was associated with stage of ripeness and the number of scars and punctures [12], indicating involvement of fruit flies such as B. dorsalis. Our quest to find an explanation for our previous results led to the current investigation and this study has shown that high percentage of these insects carries microbes, including enteric pathogenic bacteria. Variations were observed in proportion of flies contaminated externally and internally with aerobic bacteria, SS, E. coli and YM from the various sites. External microbial load also varied among the sampling sites, especially, the sites on campus. The variations in the microbial load and the proportion of flies contaminated among the sites could be attributable to differences in environmental sanitary conditions. It has been shown that sites play a role in determining the microbial load carried by flies; the filthier the environment, the higher the microbial load carried by filth flies [14-17]. Significantly higher microbial loads were observed at two sites on campus, DABCS and Link gate. Though these sites do not look filthy, they are close to bushy areas of campus. It may be interesting to know what happens in the bushes around campus.
Although the specific strains of E. coli were not determined in this study, the fact that our B. dorsalis samples carried E. coli, implies that they could carry E. coli O157:H7 as well if it is in the environment, just as demonstrated in Ceratitis capitata [4]. More interestingly, they carry enteric pathogens such as SS.
The proportion of fruits contaminated, as well as low microbial load of fruits from fly-control farms, indicates that B. dorsalis promotes contamination of mangoes. But we could not attribute a direct role at this stage. However, our subsequent experiment to determine a possible role for B. dorsalis in transmission of microbes to fruits shows a remarkable increase in both proportion of fruits contaminated internally and externally and the microbial loads of mangoes exposed to the flies. Microbes isolated from the flies including E. coli and SS were also seen in the inner tissues of the mangoes. This points to direct introduction of microbes into the inner tissues of fruits by B. dorsalis. Despite the limitation of not constantly observing the hanged mangoes for the entire period, we could draw the above conclusion because we did not expose the fruits analysed in this report long enough to produce exudates that could attract other flies including Drosophila sp. Moreover, Drosophila sp. are diurnal just as many species of flies. Additionally, Drosophila sp. could not directly contaminate the inner tissues of the fruits. Terphritid flies can directly introduce the microbes into the inner tissues of fruits. B. dorsalis and other terphritid species pierce the fruits with their long and sharply pointed ovipositors creating wounds and transmitting pathogens on their ovipositors into fruits during oviposition. In our earlier work on mangoes pathogenic E. coli was not observed as a contaminant of mangoes [13], suggesting that the current E. coli isolates might not be pathogenic. But the presence of E. coli and, more interestingly, SS in mangoes must be of concern.
Infections from melons, tomatoes, green leafy vegetables and sprouts [10, 18-20] are some of the documented outbreaks associated with consumption of raw produce. For instance, fresh tomatoes contaminated with Salmonella species, spinach with pathogenic E. coli, and melons exposed to Salmonella enterica, Campylobacter jejuni, pathogenic E.coli and norovirus, were associated with outbreak of infections [18-20]. Fruit flies that carry those microbes could be responsible for contamination of fresh fruits and vegetables. This study therefore indicates that B. dorsalis is not only associated with economic loss but can pose a serious health risks to humans. The data indicate that B. dorsalis can contribute to the spread of infections known to be transmitted by filth flies, including cholera. Contamination of fruits by these terphritid flies must be considered seriously because microbes introduced into the inner tissues of fruits cannot be removed by washing. As efforts are made to reduce or eliminate foodborne infections, activities of fruit flies must be looked at as, not only economic challenge to the horticulture industry but also, a public health problem. Farmers therefore need to adopt fruit fly control measures to improve not only the quality of their produce but also minimize microbial contamination. Additionally, public education is needed to create the awareness about microbial contamination of mangoes by fruit flies and health risks that may be associated with consumption of contaminated mangoes.
CONCLUSION
E. coli, Salmonella spp. and Shigella spp. have been identified for the first time in B. dorsalis and this is an indication that other Bactrocera sp., as well as other tephritid fruit flies, could harbor and contaminate food and vegetables with such microbes. These findings further buttress our earlier suggestion that consumers should avoid patronising mangoes with scars and punctures.
LIST OF ABBREVIATIONS
| CFU | Colony forming units |
| TCPC | Total coliform plate counts |
| TPC | Total plate counts |
| SS | Salmonella/Shigella |
| YM | Yeast and molds |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Mr Jonathan Quaye for technical support.
REFERENCES
| [1] | Clarke AR, Armstrong KF, Carmichael AE, et al. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu Rev Entomol 2005; 50(1): 293-319. |
| [2] | Badii KB, Billah MK, Afreh-Nuamah K, Obeng-Ofori D. Species composition and host range of fruit-infesting flies (Diptera: Tephritidae) in northern Ghana. Int J Trop Insect Sci 2015; 35(3): 137-51. |
| [3] | Potter FM, Fruit flies. Agricultural Science Center North, Lexington, KY 40546-0091. Available at: https//entomology.ca.uky.edu/ef621. 2010.https//entomology.ca.uky.edu/ef621 [online]. [cited 2016]. |
| [4] | Sela S, Nestel D, Pinto R, Nemny-Lavy E, Bar-Joseph M. Mediterranean Fruit Fly as a Potential Vector of Bacterial Pathogens. Appl Environ Microbiol 2005; 71(7): 4052-6. |
| [5] | Thaochan N, Drew RA, Hughes JM, Vijaysegaran S, Chinajariyawong A. Alimentary tract bacteria isolated and identified with API-20E and molecular cloning techniques from Australian tropical fruit flies, Bactrocera cacuminata and B. tryoni. J Insect Sci 2010; 10: 131. |
| [6] | Hendrichs J, Katsoyannos BI, Prokopy RJ. Bird faeces in the nutrition of adult Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) in nature. Mitt Dtsch Ges Allg Angew Entomol 1993; 8: 703-7. |
| [7] | The ACP-EU Technical Centre for Agricultural and Rural Cooperation(CTA). How to Control the Mango Fruit Fly. CTA Practical Guide Series, No. 142013. Available at: https://publications.cta.int/media/publications/downloads/1770. Accessed on 4 June, 2016. |
| [8] | Olsen AR, Hammack TS. Isolation of Salmonella spp. from the housefly, Musca domestica L., and the dump fly, Hydrotaea aenescens (Wiedemann) (Diptera: Muscidae), at caged-layer houses. J Food Prot 2000; 63(7): 958-60. |
| [9] | Kirk MD, Fullerton K, Gregory J. Fresh produce outbreaks in Australia 2001-2006. Board 21. 2008 International Conference on Emerging Infectious Diseases Program and Abstracts Book 2008; 49-50. |
| [10] | Mohle-Boetani JC, Farrar J, Bradley P, et al. Salmonella infections associated with mung bean sprouts: epidemiological and environmental investigations. Epidemiol Infect 2009; 137(3): 357-66. |
| [11] | Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol Infect 2009; 137(3): 307-15. |
| [12] | Futagbi G, Addo M, Mattah PA, Donkor ES. Microbial Quality of Mangoes from selected markets in Accra, Ghana. N Y Sci J 2016; 9(3): 32-7. |
| [13] | Marshall RT. Standard methods for the examination of diary & vegetable products 16th ed. 1992. |
| [14] | Adeyemi O, Dipeolu OO. The numbers and varieties of bacteria carried by filth flies in sanitary and unsanitary city area. Int J Zoonoses 1984; 11(2): 195-203. |
| [15] | Fotedar R, Banerjee U, Singh S, Shriniwas , Verma AK. The housefly (Musca domestica) as a carrier of pathogenic microorganisms in a hospital environment. J Hosp Infect 1992; 20(3): 209-15. |
| [16] | Rahuma N, Ghenghesh KS, Ben Aissa R, Elamaari A. Carriage by the housefly (Musca domestica) of multiple-antibiotic-resistant bacteria that are potentially pathogenic to humans, in hospital and other urban environments in Misurata, Libya. Ann Trop Med Parasitol 2005; 99(8): 795-802. |
| [17] | Barreiro C, Albano H, Silva J, Teixeira P. Role of flies as vectors of foodborne pathogens in rural areas. ISRN Microbiol 2013; 2013: 718780. |
| [18] | Hedberg CW, Angulo FJ, White KE, et al. Outbreaks of salmonellosis associated with eating uncooked tomatoes: Implications for public health. Epidemiol Infect 1999; 122(3): 385-93. |
| [19] | Bowen A, Fry A, Richards G, Beuchat L. Infections associated with cantaloupe consumption: A public health concern. Epidemiol Infect 2006; 134(4): 675-85. |
| [20] | CDC. Update of Multistate Outbreak of E.coli O157: H7 Infections from Fresh Spinach [Online]. 2006, [cited 2016]. Available from: https://www.cdc.gov/ecoli/2006/spinach-10-2006.html. |