All published articles of this journal are available on ScienceDirect.
Preventive Services Utilization Among Cancer Survivors Compared to Cancer-free Controls
Abstract
Purpose:
To summarize the current evidence on preventive services utilization in cancer survivors.
Methods:
A systematic literature review and meta-analysis was conducted in February 2016. Studies were included if they compared the utilization of influenza vaccination, cholesterol/lipid testing, bone densitometry, or blood pressure measurement among survivors of adulthood cancer to cancer-free controls. Random effects meta-analyses were conducted to pool estimates.
Results:
Literature search identified 3740 studies of which 10 fulfilled the inclusion criteria. Cancer survivors were significantly more likely to utilize bone densitometry (OR=1.226, 95% CI: 1.114 – 1.350, p<0.001) and influenza vaccination (OR=1.565, 95% CI: 1.176 – 2.082, p=0.002) than cancer-free controls. No statistically significant differences were detected for blood pressure measurement and cholesterol/lipid testing (OR=1.322, 95% CI: 0.812 – 2.151, p=0.261; OR=1.046, 95% CI: 0.96 – 1.139, p=0.304).
Conclusions:
Cancer survivors were more likely to receive influenza vaccinations and bone densitometry. Future studies should evaluate underlying mechanisms and whether the utilization of preventive services translates into prolonged survival of cancer survivors.
Implications for Cancer Survivors:
Our meta-analysis demonstrated cancer survivors to be more likely to receive the preventive services such as influenza vaccination and bone densitometry than cancer free controls. Still, these results should be interpreted in the context of suboptimal utilization of preventive services in general, and for cancer survivors in specific. Future research should evaluate the underlying mechanisms and whether utilization of preventive services is associated with overall survival in cancer survivors.
1. INTRODUCTION
Over the last decades, the number of patients surviving cancer, commonly referred to as cancer survivors, has increased rapidly, reaching approximately 11.9 million US survivors in 2008 [1]. A high prevalence of both acute and chronic medical comorbidities, such as influenza infections, hypertension, or metabolic syndrome, are observed in cancer survivors and may be attributable to treatment side effects and a detrimental lifestyle [2, 3]. A recent literature review has shown that these conditions negatively influence survival of cancer patients [4].
In light of the crucial role of comorbidities in the growing number of cancer survivors, several publications analyzed the utilization of preventive services, such as influenza vaccination, or bone densitometry for early detection of osteoporosis, showing contradicting results: both increased and decreased utilization of preventive services were reported [5, 6].
So far, two systematic reviews examined the utilization of screening services among cancer survivors to detect cancer recurrence or secondary malignancies. A systematic review by Wilkins et al. [7] concluded that cancer survivors were less likely to adopt cancer screening than cancer-free controls. Conversely, a systematic review and meta-analysis by Corkum et al. [8] reported that cancer survivors were screened more frequently for new primary breast, cervical, and colorectal cancers compared to cancer-free controls. However, so far no review has focused on the utilization of preventive services other than cancer screening.
Therefore, the aim of this study was to summarize the evidence on utilization of preventive services in cancer survivors compared to cancer-free controls.
2. METHODS
2.1. Literature Search
In February 2016, a systematic literature search without date and language restrictions was conducted using Medical Subject Heading (MeSH) terms and title/abstract keywords related to preventive services, cancer, and cancer survivorship. The full search algorithm is provided in the Appendix.
The electronic database MEDLINE (PubMed) was searched for peer-reviewed observational epidemiological studies. The EMBASE electronic database was searched with according search terms via the DIMDI portal (Deutsches Institut für Medizinische Dokumentation und Information). To identify additional studies and gray literature, conference proceedings were screened and public health professionals at the Harvard TH Chan School of Public Health contacted.
2.2. Study Selection
For inclusion in this review, the following criteria had to be fulfilled: Observational study design, adult cancer survivors (of any cancer type), cancer-free controls (defined as adults without any history of cancer, except for non-melanoma skin cancer). We considered studies for inclusion reporting on at least one of the following outcomes: Blood pressure measurement, cholesterol/lipid testing, bone densitometry, or immunization vaccines.
Studies of childhood cancer survivors, secondary survivors such as family members of cancer survivors and those with no cancer-free control group or controls matched on screening services were excluded. In addition, review articles, commentaries, and editorials were excluded from this review.
Two authors independently reviewed each study for inclusion by first assessing titles and abstracts, and then the full text. Disagreements in study selection were resolved by consensus. Reasons for exclusion were documented.
2.3. Data Extraction
A standardized form was utilized to extract data. Information on each study included authors, publication year, study design, data source, cancer and preventive service ascertainment, inclusion and exclusion criteria, country of study population, type of cancer included, time under observation, total sample size, sample size for cases and controls, population characteristics such as age, gender, race, ethnicity, insurance, and socioeconomic status, preventive services, and statistical analyses used.
For specific preventive services, the following data was extracted: Definition of preventive service completion including time frame, eligibility criteria for preventive service, total number among cancer survivors and controls, Odds Ratios (ORs) and 95% Confidence Intervals (CIs) comparing cancer survivors to cancer-free controls, as well as confounders considered in the analyses.
At least two authors independently extracted data using the standardized data extraction form and compared their results. Discrepancies were resolved by consensus.
2.4. Assessment of Study Quality
Two independent authors performed a quality assessment of each eligible study using the Newcastle-Ottawa quality assessment Scale (NOS) [9]. The scale includes 8 questions, grouped under 3 broader categories: Group selection, comparability of groups, and outcome ascertainment. A single point is awarded for each question, and a maximum of two points may be awarded for comparability of groups. Study quality is reported on a scale from 0 points (greatest bias) to 9 points (least bias). The NOS is widely applied for study quality assessment because of its easy implementation and recommendation by the Cochrane Collaboration Handbook [10].
2.5. Statistical Analyses
Meta-analyses were calculated for influenza vaccination, cholesterol/lipid testing, bone densitometry and blood pressure measurement using STATA, version 13, (StataCorp LP, College Station, TX, USA). Meta-regressions, influence meta-analyses and other sensitivity analyses, as well as tests of publication bias were conducted separately for each preventive service.
All ORs and 95% CIs compared utilization of preventive services among cancer survivors versus cancer-free controls. For studies reporting crude numbers or percentages, crude ORs and 95% CIs were calculated from raw data. All ORs and CIs extracted from original studies were log transformed before analysis. The 95% CIs were used to calculate the natural logarithm of the standard error as ln(SE) = [ln (upper 95% CI) - ln(lower 95% CI)]/3.92.
A random effects model using the DerSimonian-Laird method was chosen due to heterogeneity among study designs and populations, as well as potential variability in definitions of cancer survivorship and preventive service utilization [11]. For one study reporting separate ORs for cancer subgroups, a random effects model was used to provide one study-wide pooled estimate of comparison.
To assess between-study heterogeneity in each analysis, forest plots were assessed for overlapping confidence intervals. In addition, heterogeneity was evaluated utilizing the I-squared statistic and associated p-value from a χ2 test [12]. Subgroup analyses were computed to identify potential sources of heterogeneity based on study characteristics. Subgroups were defined a priori and included study design (matched case-control vs. cross-sectional), study quality (NOS>=7 vs. <7), cancer type (breast cancer vs. all cancer types), preventive service ascertainment (self-reported vs. medical records/insurance claims data) and matching procedure (comorbidity matched controls vs. non-comorbidity matched controls).
Sensitivity analyses were conducted based on the inclusion of one study by Snyder et al. [13]. This study had been primarily excluded due to matching on mammography.
To assess the influence of individual studies on the overall effect size, a meta-influence plot was calculated. For these influence analyses, one study at a time was left out to assess to which extend the overall pooled estimate changed. This method allows to assess, whether the overall results are merely driven by one study.
For assessment of potential publication bias, Egger´s tests were conducted and funnel plots of the log OR vs. its standard error were visually inspected [14].
All p-values provided are two-sided. An alpha level of 0.05 was chosen for statistical significance.
3. RESULT
3.1. Study Selection and Study Characteristics
PubMed and EMBASE database searches yielded 3740 publications after removal of duplicates. 541 publications were identified after screening of titles and abstracts. Of these, 10 studies fulfilled the prespecified inclusion criteria. A total of 71.564 cancer survivors and 241.683 cancer-free controls were included. One study by McBean et al. [15] reported no differences in utilization of influenza vaccination and bone density testing, but failed to provide multivariable adjusted odds ratios and was therefore excluded from further analyses. Fig. (1) shows the record flow chart of this review.
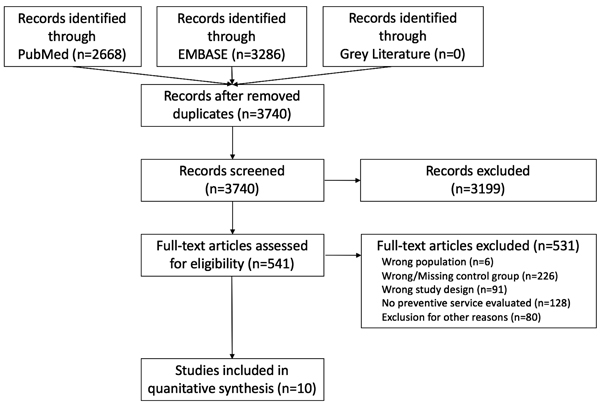
Table 1 details study characteristics. Of 10 included studies, 2 were cross sectional [16, 17], and 8 were matched case control studies [18, 19, 5, 6, 20-23] Among the latter, 2 matched on comorbidities (scale from 0-2) as one of the matching factors [22, 23].
Four studies ascertained the utilization of preventive services using self-reported data such as questionnaires or interviews [5, 16, 7, 20], 5 used insurance claims data [18, 19, 21-23], and one study utilized medical records [6]. One study was conducted in the United Kingdom [6]; 9 studies were localized in the United States of America.
| Author/Year | Country | Type of Cancer | Study Design | Study Population | Preventive Service Ascertainment | Number of Participants | Proportion of Female [%] | Mean Age [years] | Preventive Services | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Survivors / Controls | ||||||||||
| Earle et al. 2003 | USA | Breast cancer | Matched case-control | 1997-98 SEER*-Medicare | Insurance claims data | 5965 / 6062 |
100% / 100% |
78.7 / 78.8 |
During 1997-1998: Influenza vaccination, cholesterol screening, bone densitometry | 8 |
| Earle et al. 2004 | USA | Colorectal cancer | Matched case-control | 1997-98 SEER-Medicare | Insurance claims data | 14884 / 16559 |
57.6% / 56.7% | 79.7 / 79.8 |
During 1997-1998: Influenza vaccination, cholesterol screening, bone densitometry | 8 |
| Duffy et al. 2005 | USA | Breast cancer | Matched case-control | MEPS† | Self-reported | 85 / 340 |
100% / 100% |
61.7 / 63.2 |
Within previous year: Influenza vaccination; within previous 5 years: cholesterol screening | 5 |
| Snyder et al. 2009 | USA | Stage 1-3 Breast cancer | Matched case-control (matched on comorbidities) | SEER-Medicare population | Insurance claims data | 23731 / 23396 |
100% / 100% |
75.7 / 75.7 |
During year 2 past cancer diagnosis: Influenza vaccination, cholesterol screening, bone densitometry | 8 |
| Khan et al. 2010 | UK | Breast, colorectal, prostate cancer | Matched case-control | GPRD‡ | Medical record | 29244 / 116,418 | Breast: 100% / 100% CRC: 49.5%/49.5% Prostate: 0% / 0% |
Breast: 67.7/67.7 CRC: 75.1/75.1 Prostate: 76.9/76.9 |
Annually: influenza vaccination, every 3 years: Cholesterol testing, bone density scan, blood pressure measurement | 6 |
| Fairley et al. 2010 | USA | All, except non-melanoma skin cancer | Cross-sectional | 2006 Massachusetts BRFSS§ survey data | Self-reported | 716 / 7375 |
62.1% / 52.4% | median <64 / <54 | Past 12 months: Influenza vaccination | 7 |
| Bishop et al. 2010 | USA | Leukemic or breast cancer | Matched case-control | CIBTR ǂ | Self-reported | 662 / 158 |
62% / 70% |
49.1 / 50.1 | Within previous year: Influenza vaccination, blood pressure measurement | 7 |
| Snyder et al. 2011 | USA | Prostate cancer | Matched case-control (matched on comorbidities) | 2000 SEER-Medicare | Insurance claims data | 10482 / 10482 |
0% / 0% |
74.6 / 74.6 | During month 49-60 from diagnosis: Influenza vaccination, cholesterol screening, | 8 |
| Lowenstein et al. 2015 | USA | All, except non-melanoma skin cancer | Cross-sectional | 2003 Medicare Current Beneficiary Survey | Self-reported | 1882 / 10133 |
60.6% / 57% |
median >75 / <75 | Within previous year: Influenza vaccination; within previous 6 months: cholesterol measurement, blood pressure measurement; ever received: bone mineral density, pneumonia vaccination | 3 |
| Lafata et al. 2015 | USA | Breast, colorectal cancer | Matched case-control | Tumor registries of four non-profit health systems∆ | Insurance claims data | 5273 / 50759 |
CRC: 50% Breast: 100% |
median <74 / <74 | Annually: Lipid profile, bone densitometry for age >65 | 6 |
3.2. Preventive Service Utilization
Fig. (2) depicts that, overall, cancer survivors were significantly more likely to utilize preventive services than their cancer-free controls (OR=1.279, 95% CI: 1.145 – 1.430, p<0.001).
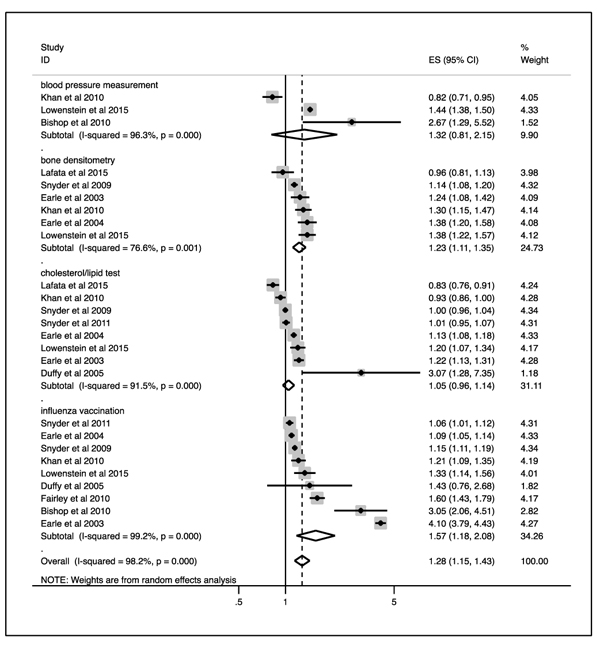
Comparably, in separate analyses of each preventive service, cancer survivors were significantly more likely to utilize bone densitometry (pooled OR=1.226, 95% CI: 1.114 – 1.350, p<0.001) and influenza vaccination (pooled OR=1.565, 95% CI: 1.176 – 2.082, p=0.002) than cancer-free controls. No statistically significant difference between cancer survivors and controls was evident for blood pressure measurement and cholesterol/lipid testing (pooled OR=1.322, 95% CI: 0.812 – 2.151, p=0.261; pooled OR=1.046, 95% CI: 0.96 – 1.139, p=0.304).
3.3. Heterogeneity Assessment and Stratified Analyses
For each preventive service, heterogeneity was analyzed in subgroups by study design, study quality, cancer types included, ascertainment of preventive service utilization, and control matching. Results are separately presented in Tables (2a and 2b).
| Heterogeneity | Bone Densitometry | Influenza Vaccination | Cholesterol/Lipid Testing | Blood Pressure Measurement |
|---|---|---|---|---|
| Overall | 76.6 (p=0.001) | 99 (p<0.001) | 91.5 (p<0.001) | 96.3 (p<0.001) |
| Study Design | - | - | - | - |
| Matched case control | 74.4 (p=0.004) | 99.4 (p<0.001) | 92.1 (p<0.001) | 89.7 (p=0.002) |
| Cross sectional | NA | 71.5 (p=0.061) | NA | NA |
| Study Quality | - | - | - | - |
| NOS>=7 | 71.8 (p=0.029) | 99.5 (p<0.001) | 91.5 (p<0.001) | NA |
| NOS<7 | 81.4 (p=0.002) | 0.0 (p=0.579) | 90.8 (p<0.001) | 98.1 (p<0.001) |
| Cancer Types | - | - | - | - |
| Unrestricted | 78.9 (p=0.003) | 93.3 (p<0.001) | 92.5 (p<0.001) | 96.3 (p<0.001) |
| Breast cancer | 21.8 (p=0.258) | 99.8 (p<0.001) | 93.2 (p<0.001) | NA |
| Ascertainment | - | - | - | - |
| Record/claims data | 74.4 (p=0.004) | 99.6 (p<0.001) | 92.8 (p<0.001) | NA |
| Self-reported | NA | 80.6 (p<0.001) | 77.1 (p=0.037) | 63.9 (p=0.096) |
| Matching | - | - | - | - |
| Comorbidity matched | NA | 81.2 (p=0.021) | 0.0 (p=0.735) | NA |
| General population | 72.1 (0.006) | 99.3 (p<0.001) | 93.1 (p<0.001) | 96.3 (p<0.001) |
| Stratified Analysis | Bone Densitometry | Influenza Vaccination | Cholesterol/Lipid Testing | Blood Pressure Measurement | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | 1.23 (1.11-1.35) | 1.57 (1.18-2.08) | 1.05 (0.96-1.14) | 1.32 (0.81-2.15) | ||||
| Study Design | p=0.390 | p=0.826 | p=0.489 | p=0.983 | ||||
| Matched case control | 1.19 (1.08-1.33) | 1.60 (1.14-2.25) | 1.03 (0.94-1.12) | 1.40 (0.44-4.42) | ||||
| Cross sectional | 1.38 (1.21-1.57) | 1.47 (1.23-1.76) | 1.20 (1.07-1.34) | 1.44 (1.38-1.5) | ||||
| Study Quality | p=0.852 | p=0.493 | p=0.632 | p=0.380 | ||||
| NOS>=7 | 1.23 (1.09-1.38) | 1.69 (1.18-2.44) | 1.08 (0.99-1.18) | 2.67 (1.29-5.52) | ||||
| NOS<7 | 1.21 (0.99-1.47) | 1.25 (1.15-1.37) | 1.03 (0.84-1.26) | 1.09 (0.63-1.89) | ||||
| Cancer Types | p=0.673 | p=0.4 | p=0.409 | - | ||||
| Unrestricted | 1.25 (1.08-1.45) | 1.33 (1.15-1.53) | 1.01 (0.90-1.13) | NA | ||||
| Breast cancer | 1.16 (1.08-1.24) | 1.91 (0.68-5.34) | 1.16 (0.94-1.43) | - | ||||
| Ascertainment | p=0.390 | p=0.627 | p=0.205 | p=0.347 | ||||
| Record/claims data | 1.19 (1.08-1.32) | 1.46 (0.99-2.14) | 1.01 (0.93-1.11) | 0.82 (0.71-0.95) | ||||
| Self-reported | 1.38 (1.21-1.57) | 1.69 (1.30-2.20) | 1.73 (0.71-4.25) | 1.75 (0.99-3.09) | ||||
| Matching | p=0.559 | p=0.258 | p=0.687 | - | ||||
| Comorbidity matched | 1.14 (1.08-1.19) | 1.11 (1.03-1.19) | 1.00 (0.97-1.03) | NA | ||||
| General population | 1.25 (1.11-1.40) | 1.75 (1.08-2.83) | 1.08 (0.94-1.23) | - | ||||
There was substantial (50-75%) or considerable (75-100%) between-study heterogeneity for most subgroups, except for breast cancer patients receiving bone densitometry, NOS<7 studies for influenza vaccination and comorbidity matched controls receiving cholesterol/lipid tests (not statistically significant at the chosen alpha-level).
For several subgroups, only one study was available, resulting in non-calculable between-study heterogeneity (NA).
Stratified analyses for each preventive service were calculated for subgroups by study design, study quality, cancer type, ascertainment of preventive service utilization, and control matching. Pooled odds ratios and p-values for subgroup comparisons are depicted in (Tables 2a and 2b). There was no statistically significant effect measure modification in subgroup pooled odds ratios at the chosen alpha level.
3.4. Sensitivity Analyses
For sensitivity analyses, we included one study by Snyder et al. that was primarily excluded due to matching of breast-cancer patients on controls receiving mammography [13].
Pooled estimates changed to OR=1.32 (95% CI: 0.81-2.15, p=0.261) for blood pressure measurement, OR=1.208 (95% CI: 1.126-1.297, p<0.001) for bone densitometry, OR=1.037 (95% CI: 0.967-1.112, p=0.307) for cholesterol/lipid test, and OR=1.508 (95% CI: 1.201-1.893, p<0.001) for influenza vaccination.
Influence analyses showed substantial changes in the pooled estimate towards smaller odds ratios upon exclusion of Earle at al [18]. for influenza vaccination, Earle at al [19]. for cholesterol/lipid test, and Lowenstein at al [17]. for blood pressure measurement, as well as towards a larger odds ratio upon exclusion of Snyder at al [13]. for bone densitometry.
4. DISCUSSION
This systematic literature review and meta-analysis demonstrates that cancer survivors are more likely to receive certain preventive services than their cancer-free counterparts: The observed pooled estimates were OR=1.23 (95% CI:1.11-1.35, p<0.001) for bone densitometry and OR=1.57 (95% CI: 1.18-2.08, p=0.002) for influenza vaccination. At the same time, no statistically significant difference was evident for cholesterol/lipid tests (OR=1.05, 95% CI: 0.96-1.14, p=0.261) and blood pressure measurement (OR=1.32, 95% CI: 0.81-2.15, p=0.304).
One study matching breast cancer-survivors to cancer-free controls on receipt of mammography supports the results of our review [13]. This may indicate that the utilization of preventive services, such as bone densitometry, is independent from screening for primary malignancies. On an individual study level, only Lafata et al. [21] and Khan et al. [6] reported estimates of less than OR=1 for bone densitometry, blood pressure measurement, and cholesterol/lipid tests. These studies were based on patient populations from the United Kingdom and Non-governmental organization databases, thus indicating that the pooled estimates of this review might not be generalizable to all populations.
For most preventive services, there was considerable to substantial heterogeneity, which was consistently high across subgroups, except for bone densitometry in breast cancer patients.
This heterogeneity is inherent to the outcomes evaluated in the different studies and reflects the inconsistent results reported by earlier reviews on cancer screening services [7, 8]. In our study, heterogeneity might originate from differing timeframes and definitions of preventive services. Further, differences in the study´s underlying patient populations might explain the observed heterogeneity: For example, Synder et al. exclusively included older male patients with prostate cancer [22], while Bishop et al. evaluated predominately younger, female patients with breast cancer or leukemia [20]. On the individual study level, demographics of cancer-survivors and controls were well balanced using matching procedures or multivariable statistical models.
In stratified analyses the pooled estimates showed consistent effects across all subgroups. There was no evidence of effect measure modification, although the tests might be statistically underpowered due to small sample sizes. In sensitivity analyses, the pooled estimates proved robust upon inclusion of one study by Snyder et al. [13]. There was no evidence for publication bias.
Several explanations for differing utilization of preventive and screening services among cancer survivors and their cancer-free counterparts have been suggested, both on the health care provider and individual patient level.
Providers might realize that cancer survivors are at high risk for comorbidities, leading to an increase in examinations. In addition, cancer patients may frequently interact with the health care system. Thus, they might have a higher awareness and eagerness to utilize preventive services. On the contrary, providers may focus on malignancies, ignoring other potential comorbidities. Cancer-survivors may also suffer from their diagnosis emotionally, discouraging further interaction with the health care system [24]. Finally, cancer survivors may face financial challenges due to health-care costs related to cancer treatment, deterring them from further examinations.
Throughout the last decade, recommendations for annual influenza vaccinations have changed in the US. As of 2010, the US Advisory Committee on Immunization Practices recommends influenza vaccinations for all ages [25]. Still, older versions recommend influenza vaccinations only for patients older than 50 years [25]. Since many studies were conducted prior to 2010, one might argue that the observed differences in influenza vaccination arise from an elder population of cancer survivors compared to cancer-free controls. Further, racial and ethnic disparities in US influenza vaccination rates have been reported [26]. Racial/ethnic population imbalances among included studies might explain the observed differences between cancer survivors and cancer-free controls. However, in most of the included studies, cancer survivors and controls were of comparable age and race/ethnicity [5, 6, 18-23]. Since “cancer survivorship” was consistently defined as living after treatment for cancer, it is unlikely that influenza vaccinations given during cancer treatment have biased our results.
In the general population, bone densitometry is routinely utilized as a screening tool for osteoporosis, while in cancer-survivors bone densitometry can be used to detect cancer-treatment related osteoporosis as well: For women undergoing hormone therapy for breast cancer bone densitometry might be utilized to detect treatment related osteoporosis. Most included studies evaluated bone densitometry from medical claims data without reporting on the indication for this preventive service. Therefore, the difference in bone densitometry utilization between breast cancer survivors and cancer-free controls might be biased by diagnostic rather than preventive use. Still, our findings were not limited to this specific subgroup and therefore indicate more generalizable results.
Blood pressure measurement and cholesterol/lipid testing showed non-significant differences between cancer survivors and cancer free controls. In contrast to bone densitometry and influenza vaccination, these preventive services are highly established in general practice and comparably inexpensive [27, 28]. Completion rates of these preventive services are probably much higher, although literature search yielded no explicit US/European rates during the last years. Therefore, the absolute difference in utilization between cancer survivors and controls might be lower in the highly prevalent preventive services blood pressure measurement and cholesterol/lipid testing than for less frequently applied bone densitometry and influenza vaccination. This might contribute to the observed lack of statistical significance in our meta-analysis, aggravated by small sample sizes.
The main limitation of this study is a low sample size and considerable heterogeneity of those studies included, although mitigated to a certain degree by implementation of random effects meta-analyses. Included studies evaluated highly divergent populations such as survivors of prostate, breast or colorectal cancer.
Further, timeframes for preventive services were inconsistent across studies, and often failed to follow national guidelines. Only one study was conducted outside the US, and thus our results might not be generalizable to a broader population or different health care systems. In addition, with only 10 studies included, subgroup analyses were potentially underpowered to detect effect measure modification. Moreover, including only 3 studies evaluating blood pressure measurement limits the interpretability of these results.
The random effects models calculated in this review only estimate one parameter for τ2 (between study variability) obtained via the DerSimonian-Laird method. This method neglects potential variability of τ2, and thus might provide pooled estimates with confidence intervals too narrow, and p-values too small. Moreover, with random effects models, small studies yield a relatively high weight when compared to large studies, as opposed to fixed effects models. In this review, sample sizes vary from 425 to 145.000 patients, and thus the relatively homogenous weighting of random effects models might be questioned. Finally, only few studies provided separate measures of preventive service utilization distinguishing follow-up care by primary care physicians and oncologists, and subgroup analyses were not feasible [18, 19, 21-23]. It remains unclear, whether the higher preventive service utilization for cancer survivors observed in our study is mainly attributable to specialists´ follow-up.
Still, this review has several strengths. A rigid literature search was conducted, identifying 541 potential publications, of which 10 fulfilled the inclusion criteria. Two authors separately screened and extracted data, thereby minimizing errors. Moreover, statistical analyses and subgroups were chosen a priori.
CONCLUSION
In conclusion, this review demonstrates that cancer-survivors are more likely to utilize the preventive services influenza vaccinations and bone densitometry than their cancer-free counterparts. Our findings must be critically viewed in light of suboptimal screening rates in the general US population, and cancer-survivors in particular [29]. Future research should evaluate the underlying mechanisms, whether results are generalizable to other populations and if utilization of preventive services is translates into prolonged overall survival.
AUTHORS CONTRIBUTIONS
Annemarie Uhlig, Johannes Uhlig, Arne Strauss, Lutz Trojan, Joachim Lotz and Ali Seif Amir Hosseini contributed to conception, design, data gathering, evaluation and analysis, and promoted the manuscript. The final manuscript was approved by all authors.
FUNDING
None.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not required (meta-analysis of IRB approved studies).
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are bases of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


