All published articles of this journal are available on ScienceDirect.
Comparative Study on the Clinicopathological Profiles of Breast Cancer Among Iraqi and British Patients
Abstract
Background:
Breast cancer is the most common cancer in Iraq and the United Kingdom. While the disease is frequently diagnosed among middle-aged Iraqi women at advanced stages accounting for the second cause of cancer-related deaths, breast cancer often affects elderly British women yielding the highest survival of all registered malignancies in the UK.
Objective:
To compare the clinical and pathological profiles of breast cancer among Iraqi and British women; correlating age at diagnosis with the tumor characteristics, receptor-defined biomarkers and phenotype patterns.
Methods:
This comparative retrospective study included the clinical and pathological characteristics of (1,940) consecutive female patients who were diagnosed with invasive breast cancer from 2014 to 2016 in Iraq (Medical City Teaching Hospital, Baghdad: 635 cases) and UK (John Radcliffe, Oxford and Queen's, BHR University Hospitals: 1,305 cases). The studied parameters in both groups comprised the age of the patient at the time of diagnosis, breast cancer histologic type, grade, tumor size, lymph node status, clinical stage at presentation, Estrogen Receptor (ER), Progesterone Receptor (PR) and HER2 positive tumor contents and the receptor-defined breast cancer surrogate subtypes.
Results:
The Iraqi patients were significantly younger than their British counterparts and exhibited higher trend to present at advanced stages; reflected by larger size tumors and frequent lymph node involvement compared to the British (p<0.00001). They also had worse receptor-defined breast cancer subtypes manifested by higher rates of hormone receptor (ER/PR) negative, HER2 positive tumor contents, Triple Positive and Triple Negative phenotypes (p<0.00001). Excluding HER2 status, the significant differences in the clinical and tumor characteristics between the two populations persisted after adjusting for age among patients younger than 50 years.
Conclusion:
The remarkable differences in the clinical and tumor characteristics of breast cancer between the Iraqi and British patients suggest heterogeneity in the underlying biology of the tumor which is exacerbated in Iraq by the dilemma of delayed diagnosis. The significant ethnic disparities in breast cancer profiles recommend the prompt strengthening of the national cancer control plan in Iraq as a principal approach to the management of the disease.
1. INTRODUCTION
Cancer is a leading cause of morbidity and mortality worldwide [1]. As the fourth most common cause of death in the Eastern Mediterranean Region (EMR), after cardiovascular, infectious diseases and injuries [2], it was responsible for 9.4% of all mortalities and 5.1% of all disability-adjusted life years in 2015 [3]. At the global level, breast cancer ranks as the second registered cancer in both sexes, the first among women and the fifth cause of death from cancer overall; case fatality rates being highest in middle and low resource settings [1]. Within the EMR, breast cancer is by far the most common female cancer and the most frequent cause of cancer-related mortality among women in all countries of the region [3, 4] which has an estimated population of 597 million people.
It has been documented that racial disparities are reflected in the incidence, mortality, and survival of patients with breast cancer [5]. While the incidence rates of breast cancer are stable or declining in the developed countries since 2000, the frequencies of the disease are steadily rising in the EMR, with annual increases ranging between 1-5% [3, 4, 6]. The low survival from breast cancer in this region of the world is probably attributable to the late stages at presentation as a consequence of the inadequate diagnostic and treatment facilities and poor public education [2-4, 7]. Early diagnosis coupled with appropriate therapy is currently accepted as the most feasible control strategy for breast cancer in developing countries [8, 9].
In Iraq, cancer is the second killer among the general population following cerebrovascular diseases [10]. Breast cancer continues to be the most prevalent malignancy nationwide over the past three decades; accounting for 19.4% of all newly diagnosed cancers, 34.7% of female malignancies and 22.5% of cancer-related deaths among Iraqi women [1]. Although a national program for early detection and downstaging of breast cancer was initiated in 2001, local studies indicate that the highest incidence rates are most frequently observed among middle-aged Iraqi women and more than 40% of the cases are still diagnosed at advanced stages [7, 11-13].
By comparison, although breast cancer is the most commonly diagnosed cancer in the UK, forming 15.4% of newly diagnosed cases and 31.2% of female malignancies [14], yet survival from that disease is amongst the highest of all registered cancers; obviously attributable to the well organized screening programs and effective treatment policies [15].
The objective of this study was to compare the clinical and pathological profiles of breast cancer among Iraqi and British women; correlating age at diagnosis with the tumor characteristics, receptor-defined biomarkers and phenotype patterns to determine whether there are significant differences in the tumor biology that reflect the survival disparities in both countries. The findings of this study will also serve to define a benchmark for monitoring and future evaluation of the breast cancer control activities in Iraq in line with International standards.
2. MATERIALS AND METHODS
2.1. Study Settings and Data Collection
This comparative retrospective descriptive study included the clinical and pathological characteristics of (1,940) female patients diagnosed with invasive breast cancer in two countries; Iraq (635 cases) and UK (1,305 cases).
2.1.1. The Iraqi Group
This group enrolled 635 consecutive female patients who were diagnosed with breast cancer at the Referral Training Center for Early Detection of Breast Tumors, Medical City Teaching Hospital in Baghdad over a three-year period from 2014 to 2016. Data was extracted from an established information system database developed by the principal investigator under the direct supervision of the International Agency for Research on Cancer (IARC); utilizing the information registered in the clinical records and histopathology reports belonging to patients with histologically confirmed infiltrative breast carcinoma. Only followed up cases with valid documented data were included.
2.1.2. The British Group
The analyzed data were retrieved retrospectively from the histopathology records of consecutive anonymous cases of histologically confirmed infiltrative breast carcinoma. The studied cases belonged to 1,305 female patients who were diagnosed with the disease during the period from 2014 to 2016 in two major hospitals in the UK:
- John Radcliffe, Oxford University Hospitals, NHS Foundation Trust, Oxford (576 cases; data source: Department of Cellular Pathology).
- Queen's BHR University Hospitals, NHS Trust, Essex (729 cases; data source: Departments of Cellular Pathology and Oncology).
Cases were eligible for inclusion in this study if they were diagnosed with invasive breast cancer during the defined period of time and were over 20 years of age. Cases with carcinoma in situ and those showing recurrent disease or incomplete data were excluded from the study.
The ethical approval was initially obtained by the Ethical Research Committee of the National Cancer Research Center, Baghdad University, in accordance with the ethical standards laid down by the Declaration of Helsinki. The study protocol is part of a Regional Comparative Breast Cancer Research Project, approved by IARC Ethics Committee, WHO in 2016 (IEC 16-11, 26/1/16; NCRC 19/10/16).
2.2. Compared Variables
The analyzed parameters in both groups comprised the age of the patient at the time of diagnosis, breast cancer histologic type, grade, tumor size, lymph node status, stage of the disease at presentation, Estrogen Receptor (ER), Progesterone Receptor (PR), Human Epidermal growth factor Receptor 2 (HER2) contents of the primary tumors and the receptor-defined breast cancer surrogate subtypes. The types of breast carcinoma were classified according to WHO [16] while the grades of the disease were assessed by applying the modified Nottingham Bloom-Richardson system [17]. The Tumor size (T), Nodal status (N) and the clinical stage were defined in accordance with the TNM Classification System [18].
Immunohistochemical (IHC) staining for molecular marker studies was carried out on the formalin fixed paraffin-embedded blocks containing the breast cancer tissue specimens to evaluate ER, PR and HER2 contents of the primary tumors. Nuclear expression of hormone receptor proteins was detected by specific monoclonal antibodies through the immune-peroxidase method; utilizing Dako Envision kits (Iraq, UK/Queen's), Leica kits (UK/Oxford), Ventana automated immunostainer (UK/Oxford and Queen's) and Dako autostainer (Iraq). The stained specimens were assessed through visual examination in a semi-quantitative fashion incorporating the staining intensity and the percentages of the positively stained tumor cells. ER and PR were considered positive when the staining was reflected in at least 10% of the tumor cells; graded as +3 (strong), +2 (moderate) and +1 (weak). For HER2 assessment complete dark membrane staining in 30% of the tumor cells was scored as positive.
The IHC stained breast carcinoma specimens were then classified into four main receptor-defined surrogate subtypes (phenotypes):
- HR Positive / HER2 Negative (Luminal A): ER/PR (+) and HER2 (-)
- HR Positive / HER2 Positive (Luminal B / Triple Positive): ER/PR (+) and HER2 (+)
- HR Negative / HER2 Positive (Non-luminal / HER2 Enriched): ER/PR (-) and HER2 (+)
- HR Negative / HER2 Negative (Non-luminal / Triple Negative): ER/PR (-) and HER2 (-)
As the management of breast cancer in Queen's Hospital depends on the determination of ER and HER2 tumor contents (without PR), the comparison between the aforementioned breast cancer subtypes among the Iraqi and British groups was carried out using the observed findings from John Radcliffe, Oxford University Hospital. On the other hand, as displayed above, Queen's hospital provided data from the Oncology department as well which allowed the comparative analysis between the clinical stages of the disease in the two countries according to the TNM classification system which relies on the availability of information regarding Metastasis (M), in addition to T and N.
2.3. Statistical Analysis:
Data were analyzed by exploring the descriptive statistics in the two studied groups. Categorical data were summarized by using frequencies and percentages, while the information regarding age was presented as age groups, means and Standard Deviations (SD). Statistical analysis was performed to correlate the corresponding findings related to the clinical an pathological features among the Iraqi and British women utilizing SPSS version 16.0 statistical program (Chicago) and Chi-Square test to compare categorical variables. P values less or equivalent to 0.05 were considered significant.
3. RESULTS
The clinical and tumor characteristics of 635 female patients diagnosed with invasive breast carcinoma from Iraq were compared with the corresponding findings of 1,305 female patients from UK (Table 1).
| VARIABLESs | IRAQI Patients N=635 (%) | BRITISH Patients N=1,305 (%) | Chi square P value |
|---|---|---|---|
| AGE range (years) | 24 - 78 | 26 - 95 | < .00001 |
| Mean (SD) | 49.4 (9.62) | 61.7 (12.92) | |
| Age Category | |||
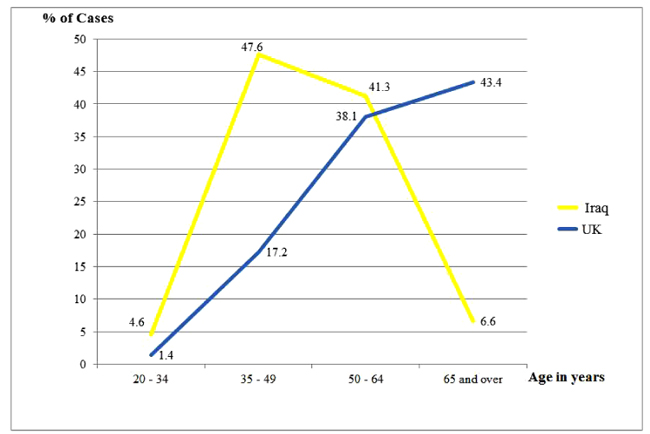
| 20 - 34 | 29 (4.6) | 18 (1.4) | |
| 35 - 49 | 302 (47.6) | 224 (17.2) | |
| 50 - 64 | 262 (41.3) | 497 (38.1) | |
| 65 and over | 42 (6.6) | 566 (43.4) | |
| HISTOLOGIC TYPE | 0.00114 | ||
| IDC (NOS)* | 481 (86.5) | 1015 (81.5) | |
| ILC** | 49 (8.8) | 125 (10.0) | |
| Mixed | 12 (2.2) | 19 (1.5) | |
| Others | 14 (2.5) | 86 (6.9) | |
| Unknown/Missing | 79 | 60 | |
| TUMOR SIZE | < .00001 | ||
| T1 | 120 (20.3) | 723 (63.2) | |
| T2 | 351 (59.3) | 369 (32.3) | |
| T3 | 94 (15.9) | 40 (3.5) | |
| T4 | 27 (4.6) | 12 (1.0) | |
| Unknown/Missing | 43 | 161 | |
| LYMPH NODE | < .00001 | ||
| N0 | 184 (31.7) | 842 (76.9) | |
| N1 | 185 (31.8) | 214 (19.5) | |
| N2 | 126 (21.7) | 25 (2.3) | |
| N3 | 86 (14.8) | 14 (1.3) | |
| Unknown/ Missing | 54 | 210 | |
| HISTOLOGIC GRADE | < .00001 | ||
| I | 32 (6.7) | 204 (16.6) | |
| II | 324 (67.6) | 594 (48.3) | |
| III | 123 (25.7) | 431 (35.1) | |
| Unknown/Missing | 156 | 76 | |
| CLINICAL STAGE | (Queen's, n=729) | < .00001 | |
| I | 63 (12.0) | 369 (60.8) | |
| II | 250 (47.5) | 220 (36.2) | |
| III | 168 (31.9) | 14 (2.3) | |
| IV | 45 (8.6) | 4 (0.7) | |
| Unknown/Missing | 109 | 122 |
**Invasive Lobular carcinoma
Highly significant differences were noted with respect to age of the patients at presentation among the two studied groups (p<0.00001); which were reflected clearly in the age range and mean values (49.4 and 61.7 years among the Iraqi and British patients, respectively). The average age at diagnosing the Iraqi patients was about 12 years earlier than their British counterparts who showed that the oldest patients presented at the age of 95 years as opposed to 78 years among the Iraqi group. While the age frequencies plateau and drop down after the age of 50 years among the Iraqis, they continued to rise reaching a peak after 65 years among the British (Fig. 1).
The most common diagnosed histologic type was infiltrative invasive Ductal carcinoma (not otherwise specified NOS) followed by infiltrative Lobular carcinoma in both groups (86.5% and 8.8% respectively compared with 81.5% and 10.0% respectively among Iraqi and British tumors). Nevertheless, the differences between the two populations were still significant (p<0.01). A statistical difference was displayed regarding histologic grades as well; while a lower rate of grade I breast carcinomas was observed in the Iraqi group as opposed to the British (6.7% versus 16.6%), 67.6% and 25.7% respectively were graded as II and III among the former compared with 48.3% and 35.1% respectively among the latter.
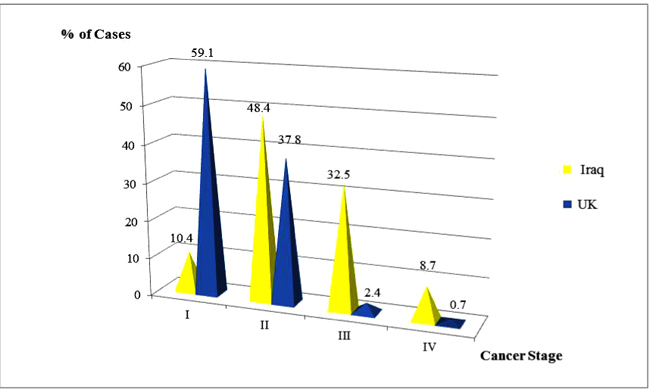
Regarding the maximum tumor diameter at histologic diagnosis, the Iraqi patients presented with significantly larger tumor sizes (4 and 5 times higher for T4 and T3 subsets respectively). Merely 20.5% of their breast tumors were small at the time of detection measuring 2cm or less compared with 63.2% among the British (p<0.00001). Likewise, the British patients exhibited a remarkably significant higher proportion of node-negative disease (p<0.00001) which was registered in 76.9%, in contrast, N0 was noted in less than one-third of the Iraqi patients (31.7%). That was obviously reflected in the highly significant favorable clinical stage at presentation of breast cancer among the British patients compared to the Iraqis (60.8%, 36.2%, 2.3% and 0.7% respectively for Stages I, II, III and IV versus 12%, 47.5%, 31.9% and 8.6% respectively) as displayed in Fig. (2) (p<0.00001).
The obvious differences in the percent distribution of the receptor-defined molecular biomarkers among the two studied groups were significantly reflected in the displayed breast cancer subtypes (p<0.00001). IHC examinations of the studied tumor specimens revealed that the percentages of ER, PR positive tumor contents were higher in the British group, while the HER2 expressions were lower; accounting for 86.5%, 79.7% and 12.6% respectively versus 69.2%, 66.7% and 29.2% respectively in Iraqi patients (Table 2).
| VARIABLES |
IRAQI Patients N = 635 (%) |
BRITISH Patients N = 1,305 (%) | Chi square Test P value |
|---|---|---|---|
| ESTROGEN RECEPTOR | N = 561 | N = 1272 | < .00001 |
| Positive | 388 (69.2) | 1100 (86.5) | |
| Negative | 173 (30.8) | 172 (13.5) | |
| Unknown | 74 | 33 | |
| PROGESTERONE RECEPTOR | N = 561 | N = 543 | . < .00001 |
| Positive | 374 (66.7) | 433 (79.7) | |
| Negative | 187 (33.3) | 110 (20.3) | |
| Unknown | 74 | 33 | |
| HER2 STATUS | N = 541 | N = 1138 | < .00001 |
| Positive | 158 (29.2) | 143 (12.6) | |
| Negative | 383 (70.8) | 995 (87.4) | |
| Unknown | 94 | 167 | |
| BREAST CANCER SUBTYPES | N = 532 | N = 441 | < .00001 |
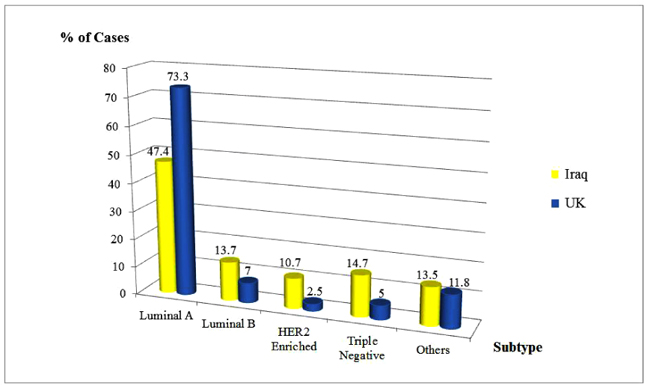
| Luminal A (E+/P+/HER2-) | 252 (47.4) | 325 (73.7) | |
| Luminal B (E+/P+/HER2+) | 73 (13.7) | 31 (7.0) | |
| HER2 Enriched (E-/P-/HER2+) | 57 (10.7) | 11 (2.5) | |
| Triple Negative (E-/P-/HER2-) | 78 (14.7) | 22 (5.0) | |
| Others | 72 (13.5) | 52 (11.8) |
The corresponding rates of Luminal A, Luminal B, HER2 enriched and Triple Negative subtypes were 47.4%, 13.7%, 10.7% and 14.7% respectively among the British cohort compared to 73.7%, 7%, 2.5% and 5%, respectively among the Iraqis (Fig. 3).
With the objective of evaluating the impact of age at presentation on the clinical stage and receptor defined subtypes in patients from both countries, the studied parameters were stratified according to age groups (Tables 3 and 4). Focusing on the Iraqi cohort, no statistical association was noted between the discerned distributions of age and the clinical stage at presentation of the disease. Although higher rates of larger tumors (T3, T4) were encountered in younger patients below 50 years than those over 65 years (35.7% versus 7.5%), yet that relationship was not significant. Nevertheless, the correlation of age was significant with respect to nodal involvement (at p <0.01); which was more prominent among younger age groups. Regarding the IHC defined receptors, no significant association was illustrated between age and ER/PR tumor content, nevertheless, breast cancer specimens belonging to patients younger than 35 years tended to reveal lower rates of PR and statistically higher HER2 positive expressions (p < 0.1). Nevertheless, that correlation was not reflected significantly in the corresponding rates of the four main IHC breast cancer phenotypes (Table 3).
| VARIABLES |
IRAQI PATIENTS AGE / Years (%) 20-34 35-49 50-64 = / > 65 |
Total | P Value | |||
|---|---|---|---|---|---|---|
| STAGE | 19 (3.6)* | 250 (47.5) | 219 (41.6) | 38 (7.2) | 526 | - |
| I | 1 (5.3)** | 32 (12.8) | 25 (11.4) | 5 (13.2) | 63 | 0.4248 |
| II | 8 (42.1) | 111 (44.4) | 117 (53.4) | 14 (36.8) | 250 | |
| III | 9 (47.4) | 86 (34.4) | 58 (26.5) | 15 (39.5) | 168 | |
| IV | 1 (5.3) | 21 (8.4) | 19 (8.7) | 4 (10.5) | 45 | |
| TUMOR SIZE | 28 (4.7) | 283 (47.8) | 241 (40.7) | 40 (6.8) | 592 | - |
| T1 | 6 (21.4) | 56 (19.8) | 49 (20.3) | 9 (22.5) | 120 | 0.2207 |
| T2 | 12 (42.9) | 163 (57.6) | 148 (61.4) | 28 (70) | 351 | |
| T3 | 9 (32.1) | 49 (17.3) | 34 (14.1) | 2 (5.0) | 94 | |
| T4 | 1 (3.6) | 15 (5.3) | 10 (4.1) | 1 (2.5) | 27 | |
| LYMPH NODE | 24 (4.1) | 279 (48) | 240 (41.3) | 38 (6.5) | 581 | - |
| N0 | 4 (16.7) | 86 (30.8) | 83 (34.6) | 11 (28.9) | 184 | 0 .0095 |
| N1 | 8 (33.3) | 85 (30.5) | 84 (35) | 8 (21.1) | 185 | |
| N2 | 3 (12.5) | 70 (25.1) | 44 (18.3) | 9 (23.7) | 126 | |
| N3 | 9 (37.5) | 38 (13.6) | 29 (12.1) | 10 (26.3) | 86 | |
| ER STATUS | 25 (4.5) | 265 (47.2) | 234 (41.7) | 37 (6.6) | 561 | - |
| ER Positive | 19 (76) | 170 (64.2) | 172 (73.5) | 27 (73) | 388 | 0 .1121 |
| ER Negative | 6 (24) | 95 (35.8) | 62 (26.5) | 10 (37) | 173 | |
| PR STATUS | 25 (4.5) | 266 (47.4) | 233 (41.5) | 37 (6.6) | 561 | - |
| PR Positive | 14 (56) | 168 (63.2) | 167 (71.7) | 25 (67.6) | 374 | 0 .1449 |
| ER Negative | 11 (44) | 98 (36.8) | 66 (32.3) | 12 (32.4) | 187 | |
| HER2 STATUS | 24 (4.4) | 260 (48.1) | 220 (40.7) | 37 (6.8) | 541 | - |
| HER2 Positive | 12 (50) | 74 (28.5) | 59 (26.8) | 13 (35.1) | 158 |
0.0968 |
| HER2 Negative | 12 (50) | 186 (71.5) | 161 (73.2) | 24 (64.9) | 383 | |
| SUBTYPES | 19 (4.1) | 222 (48.3) | 186 (40.4) | 33 (7.2) | 460 | - |
| Luminal A | 8 (42.1) | 112 (50.5) | 114 (61.3) | 18 (54.5) | 252 | 0.2296 |
| Luminal B | 5 (26.3) | 35 (15.8) | 27 (14.5) | 6 (18.2) | 73 | |
| HER2 Enriched | 3 (15.8) | 26 (11.7) | 24 (12.9) | 4 (12.1) | 57 | |
| Triple Negative | 3 (15.8) | 49 (22.1) | 21 (11.3) | 5 (15.2) | 78 | |
**Percent of Total Column
| VARIABLES |
BRITISH PATIENTS AGE / Years (%) (20-34) (35-49) (50-64) (= / > 65) |
Total | P Value | |||
|---|---|---|---|---|---|---|
| STAGE | 9 (1.5)* | 101 (16.6) | 242 (39.9) | 255 (42) | 607 | - |
| I | 2 (22.2)** | 45 (44.6) | 153 (63.2) | 169 (66.3) | 369 | < .00001 |
| II | 3 (33.3) | 53 (52.5) | 82 (33.9) | 82 (32.2) | 220 | |
| III | 3 (33.3) | 2 (1.9) | 6 (2.5) | 3 (1.2) | 14 | |
| IV | 1 (11.1) | 1 (0.9) | 1 (0.4) | 1 (0.4) | 4 | |
| TUMOR SIZE | 17 (1.5) | 203 (17.8) | 431 (37.7) | 493 (43.1) | 1144 | - |
| T1 | 5 (29.4) | 110 (54.2) | 291 (67.5) | 317 (64.3) | 723 | 0.00001 |
| T2 | 7 (41.2) | 84 (41.4) | 125 (29) | 153 (31) | 369 | |
| T3 | 4 (23.5) | 7 (3.4) | 12 (2.8) | 17 (3.4) | 40 | |
| T4 | 1 (5.9) | 2 (1.0) | 3 (0.7) | 6 (1.2) | 12 | |
| LYMPH NODE | 15 (1.4) | 198 (18.1) | 417 (38.1) | 465 (42.5) | 1,095 | - |
| N0 | 7 (46.7) | 137 (69.2) | 321 (77) | 377 (81.1) | 842 | 0.0008 |
| N1 | 5 (33.3) | 55 (27.8) | 82 (19.7) | 72 (15.5) | 214 | |
| N2 | 2 (13.3) | 4 (2.0) | 9 (2.2) | 10 (2.2) | 25 | |
| N3 | 1 (6.7) | 2 (1.0) | 5 (1.2) | 6 (1.3) | 14 | |
| ER STATUS | 15 (1.2) | 215 (16.9) | 487 (38.3) | 555 (43.6) | 1272 | - |
| ER Positive | 12 (80) | 180 (83.7) | 425 (87.3) | 483 (87) | 1100 | 0.5049 |
| ER Negative | 3 (20) | 35 (16.3) | 62 (12.7) | 72 (13) | 172 | |
| PR STATUS | 7 (1.3) | 100 (18.4) | 197 (36.3) | 239 (44) | 543 | - |
| PR Positive | 6 (85.7) | 82 (82) | 159 (80.7) | 186 (77.8) | 433 | 0 .7701 |
| PR Negative | 1 (14.3) | 18 (18) | 38 (19.3) | 53 (22.2) | 110 | |
| HER2 STATUS | 14 (1.2) | 192 (16.9) | 440 (38.7) | 492 (43.2) | 1138 | - |
| HER2 Positive | 5 (35.7) | 44 (22.9) | 56 (12.7) | 38 (7.7) | 143 | < .00001 |
| HER2 Negative | 9 (64.3) | 148 (77.1) | 384 (87.3) | 454 (92.3) | 995 | |
| SUBTYPES | 7 (1.8) | 73 (18.8) | 144 (37) | 165 (42.4) | 389 | - |
| Luminal A | 4 (57.1) | 54 (74) | 124 (86.1) | 143 (86.7) | 325 | 0.0681 |
| Luminal B | 1 (14.3) | 11 (15.1) | 11 (7.6) | 8 (4.8) | 31 | |
| HER2 Enriched | 1 (14.3) | 3 (4.1) | 4 (2.8) | 3 (1.8) | 11 | |
| Triple Negative | 1 (14.3) | 5 (6.8) | 5 (3.5) | 11 (6.7) | 22 | |
**Percent of Total Column
On the other hand, among the British cohort, there was a highly statistical significant association between age and the stage of the disease at diagnosis (p < 0.00001). Table 3 reveals that 81% of the patients over 65 years did not have nodal involvement and approximately two thirds had small tumors presenting at stage I. While ER and PR did not correlate statistically with the age of the patient, the relationship with HER2 was highly significant (p<0.00001); only 7.7% of breast cancer among the British women older than 65 years exhibited HER2 positive overexpression compared to 35.7% among those under the age of 35 years (Table 4).
A sub-set analysis was carried out to discern the distribution of tumor characteristics among the Iraqi and British patients who were younger than 50 years. Accordingly, Table 5 included 331 patients out of 635 (52.1%) from Iraq and 242 out of 1,305 (18.5%) from UK. Interestingly, while the mean age at diagnosis was very close in both populations (42.1 and 43.3 among Iraqi and British women respectively), and no statistical difference was noted with respect to the histological types of breast carcinomas in both series, nevertheless, the variations between the two sub-sets were still highly significant. That statistical difference was evident regarding the distributions of tumor size, nodal involvement, histologic grade, stage of the disease, hormone receptor contents and breast cancer phenotypes. Compared to the Iraqi cohort, the British patients exhibited significantly higher rates (p<0.00001) of small size tumors < 2cm (52.3% versus 20.2%), axillary node-negative disease (67.9% versus 29.7%) and early stage (I) presentation (43.1% versus 12.2%). Although, as displayed in Table 2, breast cancer of the Iraqi series exhibited more than twice the rate of HER2 than did the British, Table 5 shows that there was an obvious rise in the rate of HER2 positive tumor contents among the British patients younger than 50 years (23.7%) as compared to the overall British (12.6%); thus yielding insignificant statistical difference between patients in the two series regarding the overexpression of that biomarker. Nevertheless, the percentage of Triple Negative breast cancer remained almost three times higher among the Iraqi patients than their British counterparts (21.6% versus 7.7%).
| Tumor Characteristics | IRAQI Patients | BRITISH Patients | Chi square Test |
|---|---|---|---|
| - | N= 331 (%) | N= 242 (%) | P value |
| MEAN AGE (SD) | 42.1 (6.01) | 43.3 (5.11) | - |
| HISTOLOGIC TYPE | - | - | 0.6182 |
| IDC (NOS) | 250 (85.0) | 187 (83.5) | |
| ILC | 25 (8.5) | 16 (7.1) | |
| Mixed | 7 (2.4) | 7 (3.1) | |
| Others | 12 (4.1) | 14 (6.2) | |
| TUMOR SIZE | - | - | < .00001 |
| T1 | 62 (19.9) | 115 (52.3) | |
| T2 | 175 (56.3) | 91 (41.7) | |
| T3 | 58 (18.6) | 11 (5.0 | |
| T4 | 16 (5.1) | 3 (0.9) | |
| LYMPH NODE | - | - | < .00001 |
| N0 | 90 (29.7) | 144 (67.9) | |
| N1 | 93 (30.6) | 60 (28.3) | |
| N2 | 73 (24.1) | 6 (2.8) | |
| N3 | 47 (15.5) | 3 (0.9) | |
| HISTOLOGIC GRADE | - | - | 0 .00003 |
| I | 18 (7.2) | 28 (12.3) | |
| II | 156 (63) | 95 (42) | |
| III | 74 (29.8) | 103 (45.6) | |
| CLINICAL STAGE | Queen's : 128 cases | < .00001 | |
| I | 33 (12.2) | 47 (43.1) | |
| II | 119 (44.2) | 56 (51.4) | |
| III | 95 (35.3) | 5 (4.6) | |
| IV | 22 (8.2) | 2 (0.9) | |
| ESTRORECEPTOR (ER) | - | - | < .00001 |
| Positive | 189 (65.2) | 192 (83.5) | |
| Negative | 101 (34.8) | 38 (16.5) | |
| PROGESTERONE RECEPTOR | - | - | 0.0001 |
| Positive | 182 (62.5) | 88 (82.2) | |
| Negative | 109 (37.5) | 19 (17.8) | |
| HER2 STATUS | - | - | 0.1121 |
| Positive | 86 (30.3) | 49 (23.7) | |
| Negative | 198 (69.7) | 157 (76.2) | |
| BREAST CANCER SUBTYPES | - | - | 0 .0016 |
| Luminal A (E+/P+/HER2-) | 120 (49.8) | 58 (74.3) | |
| Luminal B (E+/P+/HER2+ ) | 40 (16.6) | 12 (14.1) | |
| HER2 Enriched (E-/P-/HER2+) | 29 (12) | 4 (3.8) | |
| Triple Negative (E-/P-/HER2-) | 52 (21.6) | 6 (7.7) |
4. DISCUSSION
Almost 70% of cancer-related mortality is registered in low- and middle-income countries where patients present at late stages due to inaccessible diagnosis and treatment [1, 8]; only 20% of those have the necessary data to drive cancer policy [19]. The incidence rates of breast cancer have increased by 3-folds during the last 30 years in selected countries in the EMR [6]. It has been documented that early detection of breast cancer, when linked with prompt and adequate therapy, could significantly reduce mortality [2, 8, 9] irrespective of the biological nature of the disease [20]. Based on that rationale, some nations belonging to EMR have established public awareness programs coupled with early diagnostic facilities to downstage breast cancer at presentation [7, 13, 21]. Collecting good quality data from cancer patients, through a comprehensive information system and well-conducted descriptive studies, is essential for monitoring the changing trends with the introduction of new public health initiatives.
This study demonstrated significant differences in the clinical profiles and tumor characteristics between the Iraqi and British patients. It has been reported that variations in the presentation, morphology and molecular markers of breast cancer among patients in different ethnic groups could reflect disparities in genetic, social and economic experience which ultimately affect tumor behavior and prognosis [5, 22-27].
Previous reports have described the burden of breast cancer in Iraq, as the most prevalent malignancy among the community in general and the second cause of cancer-related deaths, emphasizing the dilemma of younger age and late stage at diagnosis [10-13, 28]. The latest published Iraqi cancer registry revealed that 4,529 new female breast cancer cases were registered in 2013 forming an incidence rate of 26/100,000 female population, while 909 women died from that disease [11]. On the other hand, UK has one of the highest rates of breast cancer worldwide [1, 6]. Cancer Statistics Registration, England documented that breast cancer was the most widespread malignancy recording 45,764 new cases in 2015 with an incidence rate of 170/100,000 among females [14]. Yet the survival rates of the disease was higher than any other registered cancer in the UK forming an average of 96% for all stages of breast cancer combined [15].
Our data revealed that the average age of the Iraqi patients at the time of diagnosis was 12 years younger than their British counterparts. Similar observations were reported in comparative studies on the characteristics of breast cancer among Saudi versus Swiss women [25], and Sudanese versus German [26] and Italian women [27]. In our study, 43.4% of the British patients were diagnosed after the age of 65 years compared to 6.6% among Iraqis. Focusing on the structure of the two populations, it is observed that in 2016 among an estimated Iraqi population of 37.2 million merely 2.9% were aged 65 years and over versus 18% among the estimated 65.6 million in UK. Earlier studies from Iraq and other low-middle income countries have pointed out to the propensity for the disease to affect younger women in their forties and thirties emphasizing the displayed plateau in the incidence rates followed by the decline after the age of 50 [11-13, 28, 29]. It has been postulated that the rapid rise in breast cancer incidence before menopause followed by the slowing down afterward in developing countries, in contrast to the steady increase in the developed nations, might be attributable to the diminishing levels of circulating estrogens in women of young age population structure [30]. On the other hand, breast cancer often affects elderly patients in UK. In 2012-2014, 48% of breast cancer cases were registered in British women over 65 years; the incidence rates were highest among those aged 85 and over [14]. The Second All Breast Cancer report, which focused on inequalities and variations of outcomes with age and deprivation, showed that 46% of breast cancer among black women was diagnosed under the age of 50, whereas among white women 30% of the cases were detected in those aged 70 and over [31].
The study revealed that Iraqi patients exhibited significantly higher trend to present at advanced stages reflected by larger size tumors and frequent lymph node involvement compared to their British counterparts. The Iraqi group as well had worse receptor defined subtypes manifested by higher rates of hormone receptor negative, HER2 positive tumor contents and Triple Negative phenotypes. Excluding HER2 positive tumor content, such significant differences in tumor characteristics between the two populations persisted after adjusting for age among patients younger than 50 years in both series.
Despite the fact that the age of the Iraqi patients had no statistical relationship with the breast cancer stage at diagnosis, the correlation was highly significant among the British series (p<0.00001); where two thirds of their patients over 65 years presented at stage I. It is discouraging to observe that 40.5% of the Iraqi patients still present at advanced stages III and IV as opposed to merely 3% among the British group. Nevertheless, it is worthwhile mentioning that the observed rates of advanced stage breast cancer among Iraqi patients in this study are significantly lower than those published in a former study performed in the same geographic setting few years after the introduction of the national program for early detection of breast cancer [28]. Unfortunately, that Iraqi initiative was retarded following years of successive wars, civil conflicts and displacements which resulted in disruption of the health care structures and supplies, financial strains and deficiency of qualified professionals [32].
On the contrary, the Office for National Statistics, England reported that 8% and 5% of British women registered with breast cancer during 2012-2014 presented at stages III and IV respectively, while those who were diagnosed at stages I or II had a one year survival which is very similar to the general population [15]. That reflects clearly the advantages of operating well-organized screening mammography programs on all women aged 50 years and over till the age of 70 [22]. The recorded differences in the stage distribution of breast cancer among the UK population have been shown to represent socioeconomic and ethnic disparities [22, 31]. It has been demonstrated that black African and Caribbean women were twice likely to present at later stages than white women [33].
Comparable findings were reported in previous surveys that investigated the influence of ethnic disparities on the detection and prognosis of breast cancer among patients from developed versus developing countries [25-27, 34-36]. It was displayed that women from low-middle income settings experience issues related to access to care. Earlier studies have highlighted the variations of health care priorities in developing countries emphasizing the dilemma of global inequalities in the management of breast cancer and the importance of allocating effective resources when implementing the relevant services [37, 38]. The problems associated with access to medical care and affordability disappear when poverty and insurance status are controlled [39].
Cohort studies that compared the clinical and pathological characteristics of breast cancer between Sudanese and German women [26] and between Africans from Central Sudan and Europeans from Northern Italy [27] reported that Sudanese patients were diagnosed at advanced stages exhibiting aggressive breast cancer behavior illustrated in very large tumor sizes and poorly differentiated grades than the German and Italian patients who had significantly lower proportion of positive lymph nodes. A former survey conducted to identify potential molecular differences between breast cancers in Europe and the Middle East demonstrated that the incidence of low-grade disease was 14 times lower in Saudi than Swiss women suggesting differences in genetic susceptibility [25]. Further research from developing countries showed that the African Tanzanian women had poorly differentiated breast cancers that expressed higher ER and/or PR negative contents and presented at significantly late stages compared to the Caucasian Italian women [35]. Identical findings were presented in a descriptive survey that analyzed the pathological behavior of breast cancer among Ghanaian Sub-Saharan African women versus the Norwegian coordinate [36].
In a study focusing on challenges to early diagnosis and treatment of breast cancer in developing countries, it was observed that 30 -80% present at stages III and IV [40]. The displayed significant gaps in the knowledge, attitudes and practices towards breast cancer among individuals from low-middle income countries including Iraq, prompt the potential to elevate the level of awareness through public education campaigns to address the burden of that cancer in the community and the means for its early detection and control [41, 42].
The molecular subtype classification of breast cancer has been strongly accepted as an independent prognostic marker in the management of the disease [20, 43]. A highly significant difference in the rates of the IHC phenotypes between the two populations was demonstrated in this study and maintained in the comparative profiles among those under the age of 50 (p<0.001). Whereas age in this study did not correlate statistically with the hormone receptor status (ER, PR) and the receptor-defined phenotypes in both populations, other studies indicated that Luminal A was more common among older patients [44]. On the other hand, the association of age with HER2 overexpression was evident among both groups in this study and most significant in the British series (p<0.0001). That was illustrated by a sharp rise in the rate of that biomarker in younger women under 50 years; thus supporting prior studies which documented that young patients frequently present with HER2 overexpression when compared to their older counterparts [45]. A recent survey showed that HER2 positive frequencies increased when the patients were diagnosed at yes stages, with larger tumors, poorly differentiated grades, estrogen receptor-negative disease and younger ages (46). Nevertheless, other studies failed to demonstrate a statistical relationship between HER2 and age [47, 48].
Our study disclosed clearly that breast cancer tissues belonging to British patients expressed significantly higher hormone receptor-positive contents but lower HER2 over expressions compared to the Iraqi specimens; reflecting a higher frequency of Luminal A phenotype and lower rates of Luminal B, Triple Negative and Triple Positive subtypes. That was in agreement with the presented findings from earlier studies which revealed that patients from developed western societies were more likely to have ER/PR positive / HER2 negative tumor contents than their African and Middle Eastern counterparts [25-27, 34-36]. The documented evidence indicates that IHC markers exhibit significant variations in their expressions in terms of race, ethnicity and geographic distributions reflecting genetic susceptibility, tumor heterogeneity, demographic characteristics, biologic or reproductive factors.
In accordance with relevant retrospective surveys, the current study has limitations represented by the lack of population-based sampling and the reported frequency of unknown data. Since the study settings represent large clinic-based tertiary referral centers serving the surrounding population, a selection bias is not assumed. Our analysis was based on the registered information of the Iraqi patients and the available clinical records of those who were followed up and on the data extracted from the histopathology and oncology reports of the British cohort. Nevertheless, the missing data did not preclude the comparative evaluation of the clinical and tumor parameters due to the adequate number of cases included in the study from both populations.
CONCLUSION AND RECOMMENDATIONS
The remarkable differences in the clinical and tumor characteristics between Iraqi and British patients suggest heterogeneity in the underlying biology of breast cancer which is exacerbated by the dilemma of access to care and delayed diagnosis in Iraq. These ethnic disparities emphasize the importance of initiating genotype expression studies and recommends comprehensive assessment of the breast cancer surrogate subtypes as a feasible approach in the management of the disease. In order to reach the distinguished profile of breast cancer presented by the British patients, there should be a determined political will to invest in the health care system of Iraq through supporting the infrastructure, building capacities of the professionals and strengthening the national cancer control plan.
FUNDING
Declared none.
AUTHORS' CONTRIBUTION
The principal corresponding author designed the study, analyzed the results, wrote the manuscript and presented the final version. The second author supported the study design and worked with the rest of the team in providing relevant information, manuscript revision, data entry and statistical analysis.
ETHICS APROVAL AND CONSENT TO PARTICIPATE
The ethical approval was initially obtained by the Ethical Research Committee of the National Cancer Research Center, Baghdad University. The study protocol is part of a Regional Comparative Breast Cancer Research Project, approved by IARC Ethics Committee, WHO in 2016 (IEC 16-11, 26/1/16; NCRC 19/10/16).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
Informed written consent was obtained from all the participants.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest that competes with any of the contents of the manuscript.
ACKNOWLEDGEMENTS
We thank gratefully Dr. Derek Roskell, Mr. Kieron White (Department of Cellular Pathology, John Radcliffe Hospital, Oxford University, UK) and Ms. Albena Naydenova, Mr. Robert Lucas (Departments of Cellular Pathology and Oncology, Queens, BHR University Hospital, UK) for their assistance in providing the requested anonymous data. Special thanks are due to Dr. Wieeam Saleh, Dr. Sana Nadir, Dr. Wigdan Hamza and Dr. Inaam Aziz (Referral Center for Early Detection of Breast Tumors, Oncology Teaching Hospital, Baghdad) and the research team of the National Cancer Research Center of Baghdad University.


