All published articles of this journal are available on ScienceDirect.
Prevalence of Methicillin-resistant Staphylococcus Aureus in Saudi Arabia Revisited: A Meta-analysis
Abstract
Background:
The dramatic increase in the prevalence of methicillin-resistant Staphylococcus aureus as a source of nosocomial and community-associated infections in Saudi Arabia has attracted the attention of many researchers and public health workers. Hence, the aim of this meta-analysis is to assess the extent of the problem in Saudi Arabia at large.
Methodology:
PubMed database was searched for articles about the prevalence of MRSA in Saudi Arabia, and the relevant data from all eligible studies were analyzed to assess the overall prevalence rate by ProMeta3.
Results:
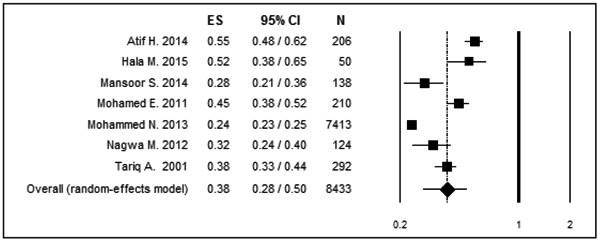
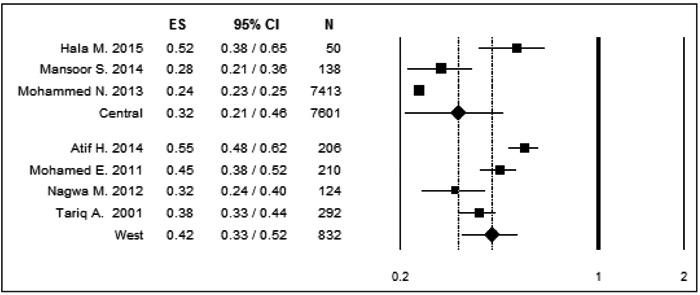
Seven articles were included in this meta-analysis with the sample size of 8433 individual. The overall prevalence of MRSA was 38% (95% CI: 28 – 50). The prevalence of MRSA according to the region was 32% (95%CI: 21 – 46) for the central region and 42% (95% CI: 33 – 52) for the western region.
Conclusion:
The findings of this study indicate that the overall prevalence of MRSA in Saudi Arabia is relatively high, with the western region showing the highest prevalence rates, which necessitates the urgent implementation of preventive and educative strategies.
1. INTRODUCTION
Antibiotic resistance of a significant number of bacterial pathogens has emerged as a growing public health threat, attracting the attention of many health organizations, as it hinders the efforts of effectively preventing and treating the increased bacterial infections [1]. The medical and economic implications of the spread of both hospital and community-associated multidrug-resistant bacteria were reported by a number of studies [2-5].
Staphylococcus aureus is a common skin normal flora that causes less common infections following breaks in the skin or mucous membrane, ranging from superficial skin and soft tissue infections to life-threatening toxic shock syndrome, endocarditis, and sepsis [6, 7].
Historically, the introduction of penicillin as a treatment regimen for S. aureus infections was a very effective method, however, by the mid of the twentieth century, the bacteria had already developed resistance to the drug by producing beta-lactamase enzyme which inactivates penicillin, ampicillin, and amoxicillin. This necessitated the production of beta-lactamase stable antibiotics such as methicillin and cloxacillin [1]. In the early sixties of the last century, the first Methicillin-Resistant Staphylococcus Aureus (MRSA) was identified [8], initially, as a Hospital-Acquired (HA-MRSA), and later, Community-Acquired (CA- MRSA) started to emerge and dramatically increase in number in many countries [1]. Both asymptomatic and opportunistic forms of MRSA were reported in animals, though they occur transiently, they present a potential source of community-acquired infection [9]. HA-MRSA and CA-MRSA have typically different microbiological characteristics, risk factors, and clinical manifestations. For instance, the CA-MRSA causes less severe disease for the majority of the infections [10]. The MRSA resistance is highly attributed to the presence of mecA or mecC genes in the bacterial chromosome. These genes code for different versions of penicillin-binding proteins that interfere with the binding of beta-lactam antibiotics to the bacterial cell wall [9].
The prevalence of MRSA infections shows a range of 13% - 74% worldwide [11], while in Europe, it ranged from 0.9% to 56% in 2014 [12]. In the US, the Center for Disease Control and Prevention (CDC) reported an approximately 50% of S. aureus nosocomial infections in the intensive care units were caused by MRSA [13].
A considerable variation was reported in the prevalence of MRSA in the Gulf Corporation Council Countries (GCC), with Saudi Arabia showing the highest prevalence rate of 29.9%, while Kuwait showed the lowest of 3.3% [14]. Numerous studies from Saudi Arabia have also reported an increase in the prevalence rate of MRSA [15-17]. This increase in the MRSA cases was attributed to the large influx of expatriates and pilgrims from all over the world. The present study included all recently published studies, which add strength and weight to the result, considering that the only previous attempt to pool the prevalence rates of MRSA in Saudi Arabia included studies date at least decade ago. Determination of the country’s overall epidemiology and the epidemiological parameters of antibiotic-resistant bacteria play a crucial role in setting effective prevention and control measures. Hence, the aim of the current meta-analysis is to pool the data from relevant published studies to determine the overall MRSA prevalence in Saudi Arabia.
2. MATERIALS AND METHODS
2.1. Literature Search Strategy
For the current meta-analysis we followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) to search the Medline Database for all relevant citations using the following keywords (“MRSA”, “Methicillin-Resistant Staphylococcus aureus”, “ORSA”, “Oxacillin-Resistant Staphyloccous aureus” along with the keywords “prevalence”, “epidemiology”), we limited the search to studies published in English on MRSA. This search yielded 714 publications, which were screened by title and abstract, to yield 61 publications. A full-text review was carried out by the two authors independently to identify 18 publications that met our eligibility criteria. In addition, manually screening of references of all potentially eligible studies resulted in 6 additional publications. Finally, we excluded all studies that do not fall within the time frame set for the meta-analysis. Ultimately, seven studies were used for the quantitative synthesis of the pooled prevalence rate. The process of study selection is shown in Fig. (1).
2.2. Eligibility Criteria
All included studies in this meta-analysis met the following inclusion criteria: the study should be of cross-sectional design, published in English language, conducted on Saudi or non-Saudi study population living in Saudi Arabia of all age groups, the antibiotic studied are either methicillin or oxacillin or both, and studies carried out in both community or hospital settings.
The study should also provide the total number, the percentage and the confidence interval of MRSA or enough data to calculate these values. Studies were excluded if conducted on specimens from non-human sources, duplicate reports, reviews, meta-analyses, letter to the editor and editorial articles. The eligibility criteria were assessed by both reviewers and disagreements between reviewers were resolved by consensus
2.3. Data Extraction
Data from eligible studies were extracted by one reviewer and cross-checked by the second reviewer using data extraction table, disagreements were resolved by discussion and consensus. The extracted data included PubMed identifier number, the name of first author, year of publication, study design, the population studied, type of settings (community or hospital), age, province or city, the total number of subjects, number of MRSA, 95%CI (confidence interval).

2.4. Quality Assessment
The quality of each eligible study was assessed using a validated quality assessment tool for cross-sectional studies [18]. To calculate a total quality score, we assessed six quality items; 1) clearly defined target population, 2) the use of probability sampling to identify potential respondent, 3) the matching of respondent characteristics and the target population, 4) standardization of data collection methods, 5) reliability of survey instruments 6) validation of survey instruments. The score for each item was either ‘0' for ‘no' and ‘1' for ‘yes'. The total score for each study ranged from 0 to 6, with scores ranging between 0 - 3 regarded as a low quality, and scores between 4 - 6 regarded as high-quality studies.
2.5. Statistical Analysis
The meta-analysis for the current study was performed with ProMeta3 software, using a random-effects model (DerSimonian Laird method) [19] to determine the overall pooled prevalence and 95% confidence interval. The heterogeneity was estimated using Cochran Q statistic and I2, heterogeneity was considered statistically significant with I2>50%. We also carried out subgroup analyses, according to the region of the study (western, and central).
Publication biases were assessed with funnel plot, Begg’s rank correlation test [20] and Egger’s linear regression test [21], with P value ˂ 0.1 indicates potential bias. We carried out the sensitivity analysis to assess the influence of each individual study. The significance of the overall effect of the subgroup was assessed using the Z test. The statistical analysis was carried out using the ProMeta3 software.
3. RESULTS
The present meta-analysis included seven studies according to the selection criteria with a sample size of 8433, one reported data from community settings, five from hospital settings, and one from mixed settings. While according to the region, three studies were from the central region, four from the western region.
The characteristics of the eligible studies including first author, year of publication, sample size, study settings, region, prevalence, 95% CI, and the quality score of the studies (Table 1).
| First author | Year | Sample Size | Study Settings | Region | MRSA Prevalence | 95%CI | Quality Score | Reference |
|---|---|---|---|---|---|---|---|---|
| Atif H. | 2014 | 206 | Hospital | Western | 55% | 48 – 62 | 5 | [22] |
| Hala M. | 2015 | 50 | Hospital | Central | 52% | 38 – 65 | 4 | [23] |
| Mansoor S. | 2014 | 138 | Hospital | Central | 28% | 21 – 36 | 6 | [24] |
| Mohamed E. | 2011 | 210 | Hospital | Western | 45% | 38 – 52 | 4 | [25] |
| Mohamed N. | 2013 | 7413 | Hospital | Central | 24% | 23 – 25 | 5 | [26] |
| Nagwa M. | 2012 | 124 | Mixed | Western | 32% | 24 – 40 | 4 | [27] |
| Tariq A. | 2001 | 292 | Mixed | Western | 38% | 33 – 44 | 5 | [28] |
The pooled prevalence of MRSA in Saudi Arabia was estimated to be 38% (95%CI: 28-50, T2=0.40) for a sample size of 8433 (Fig. 2). These values were obtained using the random effect method due to the significant heterogeneity among the eligible studies (Q = 179.39; P ˂0.001; I2= 96.6).
The pooled MRSA prevalence and corresponding 95%CI were also assessed for different subgroups according to the region, where the prevalence of MRSA in the central region including 3 studies, was 32% (95%CI: 21-46) and the western region including 4 studies, was 42% (95%CI: 33-52) (Fig. 3). Studies among these regional subgroups also showed a significant heterogeneity, the western subgroup (Q = 22.31; P˂0.001; I2 = 86.55), while the central subgroup (Q = 20.90; P ˂ 0.001; 90.43).
3.1. Publication Bias
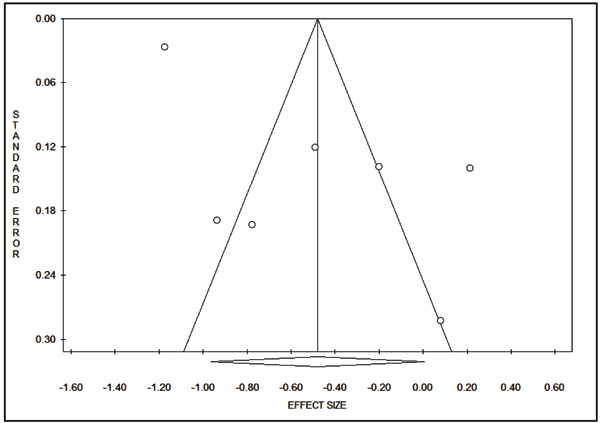
The publication bias was assessed using funnel plot, Begg’s and Mazumdar’s rank correlation test, and Egger’s test. The funnel plot did not show any evidence of publication bias, (Fig. 4). The results of Begg’s test (Z = 0.45, P = 0.652) and Egger’s test (t = 3.29, P = 0.22) indicating absence of publication bias.
4. DISCUSSION
The selective pressure for mutated and possibly antibiotic-resistant bacteria which is created by the use, overuse, and misuse of antibiotics for treating both human and animal infections has proven to be worrisome both medically and economically on the global level [29]. The nosocomial and community-associated MRSA strains have emerged as a serious threat, as they acquire genes that make them resistant to nearly all beta-lactam antibiotics, and are becoming increasingly resistant to non-beta-lactam antibiotics [9]. In the Arabian Peninsula as general and Saudi Arabia in particular, MRSA is emerging as a serious health issue, with a number of studies reporting prevalence rates ranging between 8-49% [30-35]. The results of meta-analysis reveal that the prevalence rate of MRSA in Saudi Arabia is 38% which falls well within that range, and indicate a significant increase from the previously reported 29.9% [14]. This relatively large prevalence can be attributed to the fact that Saudi Arabia is a destination for a large number of immigrants and pilgrims including potential carriers of the drug-resistant bacteria. Although it is normal for a bacterial strain to undergo mutations and genetic transformations leading to the development of drug resistance, but the problem is aggravated by a number of contributing factors, such as antibiotics misuse, and the prescription of the drug based on clinical judgment only with no or seldom use of antibiotic sensitivity tests or other appropriate diagnostic procedures. It worth noting that the significant heterogeneity observed in the present meta-analysis can be explained by the differences in the population of the included studies. In an attempt to resolve the heterogeneity issue, we calculated the pooled prevalence based on the region. The included studies focused on two main regions in Saudi Arabia, namely, central and western region. A prevalence rate of 42% was noted in the western region and 32% in the central region. The high prevalence rate in the western region can be explained by the fact that this region is over-populated by immigrants from a number of Asian and African countries. The region also witnesses a large influx of pilgrims during the time of the Islamic pilgrimage, among whom a significant number might be infected or carriers of MRSA. Thus, it is evident that MRSA constitutes a major public health concern in Saudi Arabia, which necessitate the application of strict infection control guidelines, and community-oriented policies and campaigns to increase the public awareness of the risks of the uncontrolled use of antibiotics.
CONCLUSION
In this study, the MRSA prevalence rate in Saudi Arabia is considerably high, with a significant proportion of the cases detected in the western region of the country, given the multi-national nature of the population in that region and the fact that this region represents a very busy commercial and religious hub for people from all over the world. The findings of this study emphasize the urgent need for comprehensive screening, preventive, and educative strategies to control the increasing MRSA prevalence rate. Since this current study included studies that covered the central and western region of the country, future studies that cover the entire country are recommended to show the comprehensive picture of MRSA prevalence in Saudi Arabia.
LIST OF ABBREVIATIONS
| CA-MRSA | = Community-Acquired Methicillin-Resistant Staphylococcus aureus |
| CDC | = Center for Disease Control |
| CI | = Confidence Interval |
| HA-MRSA | = Hospital-Acquired Methicillin-Resistant Staphylococcus aureus |
| MOOSE | = Meta-analysis of Observational Studies in Epidemiology |
| MRSA | = Methicillin-Resistant Staphylococcus aureus |
| ORSA | = Oxacillin-Resistant Staphylococcus aureus |
| PRISMA | = Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| S. aureus | = Staphylococcus aureus |
| US | = United States |
CONSENT FOR PUBLICATION
Not applicable
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are highly indebted to the dean and staff of faculty of Applied Medical Sciences, University of Bisha, for their help and support.


