All published articles of this journal are available on ScienceDirect.
Diagnosing Diabetes Mellitus With Glycated Haemoglobin in Newly Diagnosed HIV-positive Patients in Buffalo City Municipality, South Africa: A Cross-sectional Study
Abstract
Background:
The HbA1c estimates long-term glycaemic control in individuals. However, scanty data exist on the determination of Diabetes Mellitus (DM) in newly diagnosed HIV patients using the HbA1c screening tool in the South African context. Thus, this study examines the prevalence of diabetes mellitus in newly diagnosed HIV-positive patients in Buffalo City Municipality, East London, South Africa.
Methodology:
This was a cross-sectional study of 335 newly HIV-diagnosed patients between August 2016 and July 2017. Demographic (age, gender, residence, employment status and level of education) and behavioural variables (smoking and alcohol use (past 30 days)) were by self-reporting. Information on HbA1c and other clinical variables were obtained from the medical records of the patients. Diabetes mellitus was defined based on the Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) 2017 guideline of HbA1c of above 6.5%. Weight and height were measured using standard protocols. Logistic regression analyses were applied to determine the predictors of abnormal glycated haemoglobin.
Results:
Majority of the participants were female (72%). The prevalence of patients with HbA1c greater than 6.5% was 6%. The multivariate analysis indicates only age (p=0.031) and race (0.019) significantly shows a correlation to increase the risk of development of DM in newly diagnosed HIV positive patients. The binary logistic regression analysis shows that age (above 46 years) (p=0.001; AOR (6.60); CI (2.08-20.9) was directly related to the development of DM.
Conclusion:
Consistent with other studies, the exclusive non-fasting HbA1c, which is a marker of glycaemic control, only underestimate glycemia in HIV patients with diabetes in this present study. Notwithstanding, HIV patients who are over 40 years are likely to develop DM. As such, screening older individuals diagnosed with HIV is crucial in offering a timely point of care and interventions.
1. INTRODUCTION
Diabetes Mellitus (DM) is a global major public health challenge. Diabetes mellitus, which was a rare disease in rural African settings, has now become a disturbing health issue in subSaharan Africa. In 2017, the International Diabetes Federation (IDF) estimates that the global number of adults with DM will increase by 48% in 2045, and with Africa region projected to have the highest increase of 156% [1]. Similarly, in 2015, an estimated analysis of DM prevalence in Africa shows an increase from 3.2% to 3.7% by 2040 [2]. In South Africa, IDF estimated that 2.3 million people were living with DM in 2015, while a large proportion of people had un diagnosed DM [2]. Perhaps, the surging increases in DM in developing regions of the world could be linked to the ageing population and urbanisation [3]. Again, in 2015, global mortality attributed directly to DM was 5.1 million deaths [2]. Worryingly, over 80% DM associated mortality is prevalent in less developed countries [4] where HIV is hyperendemic [5].
Diabetes mellitus prevalence amongst HIV infected individuals can range from 3.8% [6] to about 15.1% [7]. The literature has indicated that older age, a higher BMI, pre-existing condition such as hypertension [7], the role of antiretroviral therapy (ART) [8, 9], and gender, duration of HIV infection, CD4 count, viral burden, socioeconomic status, culture and body composition indicators [10] are the determinants of increased risk of acquisition of DM in HIV infected individuals. The longer the period of HIV infection, higher viral load of above 5 log copies and a low CD4 count are a direct contributor to the development of DM in HIV positive patients [11]. In addition, co-infection of hepatitis C virus with HIV predisposes one to the development of DM; which is attributed to the production of intrahepatic tumor-necrosis factor with subsequent hepatic steatosis that leads to the development of insulin resistance [12].
Patients could actually present in different ways with HIV and DM; patients diagnosed with DM at the onset of HIV infection, and with hyperglycaemia upon initiating therapy [13]. HIV infected individuals suffer from insulin resistance and not insulin deficiency. It is observed that impaired glucose tolerance and insulin resistance precede the development of weight loss in such patient [14].
HIV-infected individuals who develop the metabolic syndrome (inclusive of DM) exhibit inflammatory and adipocytes problems (elaboration of high levels of C-reactive protein (CRP) and leptin) and lower adiponectin levels which likely result to the pathogenesis of diabetes [9]. Thus, some guidelines advice for the use of fasting and postprandial glucose values for screening and during monitoring of therapy are encouraged [13]. The Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guideline has advocated for the use of HbA1c for screening for DM because it offers them to make immediate management decisions based on glycaemic control that the patient has achieved over the preceding three months [14], and provides an accurate measure in a non-fasting state. The SEMDSA recommends diagnostic criteria of HbA1c≥6.5% for diabetes [15]. The use of glycated haemoglobin (HbA1c) increases diagnostic accuracy, but the extent of association between HIV and DM might not be clearly delineated [16]. The twin burden of DM and HIV would place a significant economic strain on regions with already poor constrained resources, particularly, in the Eastern Cape Province, which is one of the poorest provinces in South Africa. In addition, the economic implications for patients and their families are enormous. Therefore, diagnosis of DM in newly diagnosed HIV patients is important to inform health managers for optimal management strategies to reduce twin prevalence of diabetes and HIV related morbidity and mortality. In this context, this study aims to determine the proportion of newly diagnosed HIV patients with diabetes mellitus using the HbA1c screening tool in low resource settings in the Eastern Cape, South Africa.
2. MATERIALS AND METHODS
Data from this study was from the ‘Genetic Characteristics of HIV-1, and Determinants of Late Presentation for Care and Prevalence of Diabetes Mellitus among Newly Diagnosed HIV Patients Cohort Study’ in the Eastern Cape, South Africa. The details regarding the setting, design, sample and sampling procedure of this present study have been previously published [17]. Briefly, the study involved 335 randomly selected new HIV-diagnosed patients attending health facilities in the study setting between August 2016 and July 2017. Participants’ demographic information includes age, gender, place of residence, employment status and level of education. In addition, behavioural variables: smoking and alcohol use (past 30 days) was obtained by self-reporting. Information pertaining to HbA1c and other clinical variables was obtained from the medical records of the patients.
The International Society for the Advancement of Kinanthropometry protocol [18] was used to measure the weight and height of the participants. Body weight was measured in light clothing without shoes using a calibrated digital electronic weighing scale (Seca 813, Seca, UK) to the nearest 0.1 kilogram. A calibrated vertical stadiometer (Seca Portable 217 Seca, UK) was used to measure height to the nearest 0.1 centimetre. Body mass index (BMI) was calculated by dividing body weight (kg) by height squared (m2). Overweight and obesity were defined as >25.0kg/m-29.9kg/m2 and ≥30kg/m2, respectively [19]. Diabetes mellitus was defined as HbA1c of ≥6.5% according to the 2017 SEMDSA guideline [15].
2.1. Ethics Approval
The University of Fort Hare Ethical Committee gave ethical approval for the study (REC 270710028RA level 01). Permission was obtained from the Eastern Cape Health Department (Ref: EC_2016RP22_139) prior to data collection. The aim and nature of the study were explained to the participants; and only participants who signed the informed consent form were selected to participate in the study.
2.2. Data Analysis
Descriptive statistics of frequency counts and percentages were applied to summarize the variables. Multiple logistics regression was performed to calculate odds ratios (OR) and 95% confidence interval (CI) to determine the association of demographic variables and HbA1c. A p-value of 0.05 was considered statistically significant. All the statistical analyses were done using the Statistical Package for Social Sciences (SPSS) version 22.0, Chicago, IL, USA.
3. RESULTS
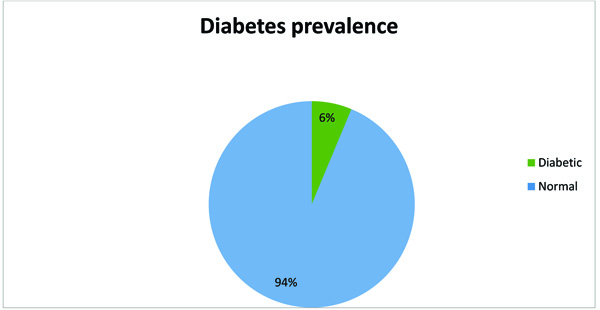
Of the 335 participants, surveyed analysis was conducted on 330 participants who had completed glycated HbA1c result. The majority of the participants were women (226/69%), single (271/81.4%), unemployed (199/59.6%), and had secondary education (180/54.1%). Of the participants surveyed, 9.6% (32) were under-weight, 155 (46.9%) had normal weight, 60 (18.1%) and 40 (12.1%) were overweight and obese, respectively. Twenty-six percent of the participants consumed alcohol, and less than 20% (57) were smokers (data not shown). The prevalence of newly diagnosed HIV patients with an HbA1c ≥6.5% was 6% Fig. (1). Over half the patients were asymptomatic of any underlying HIV related illness, and about 45% of the patient had a stage 3 or 4 AIDS-defining illness.
Of all the factors in the multivariate analysis, only age (p=0.031) and race (0.019) show a significant association of increased risk of development of DM in newly diagnosed HIV positive patients (Table 1). However, the binary logistic regression analysis (Table 2), shows that age (above 46 years) (p=0.001; AOR (6.60); CI (2.08-20.9) was directly related to the development of DM.
| Variables | HbA1c <6.5 | HbA1c ≥6.5 | P-value |
|---|---|---|---|
| Age | - | - | - |
| <46 years | 265 (95.0) | 14 (5.0) | 0.031 |
| ≥46 years | 45 (86.5) | 7 (13.5) | - |
| Sex | - | - | - |
| Male | 95 (91.3) | 9 (8.7) | 0.179 |
| Female | 214 (94.7) | 12 (5.3) | - |
| Level of Education | - | - | - |
| No formal education | 39 (92.9) | 3 (7.1) | 0.738 |
| Primary | 50 (96.2) | 2 (3.8) | - |
| Secondary | 168 (94.4) | 10 (5.6) | |
| Tertiary | 53 (91.4) | 5 (8.6) | - |
| Alcohol use | - | - | - |
| Yes | 82 (92.1) | 7 (7.9) | 0.278 |
| No | 227 (94.6) | 13 (5.4) | - |
| Smoking | - | - | - |
| Yes | 52 (91.2) | 5 (8.8) | 0.361 |
| No | 257 (94.5) | 15 (5.5) | - |
| Race | - | - | - |
| Black | 299 (94.0) | 19 (6.0) | 0.019 |
| White | 4 (66.7) | 2 (33.3) | - |
| Coloured | 7 (100.0) | 0 (0.0) | - |
| Employment status | - | - | - |
| Unemployed | 185 (93.9) | 12 (6.1) | 0.495 |
| Employed | 125 (93.3) | 9 (6.7) | - |
| Body mass index (kg/m2) | - | - | - |
| Underweight | 28 (87.5) | 4 (12.5) | 0.528 |
| Normal weight | 145 (93.5) | 10 (6.5) | - |
| Overweight | 57 (95.0) | 3 (5.0) | - |
| Obesity | 38 (95.0) | 2 (5.0) | - |
| WHO clinical stage | - | - | - |
| Stage 1 | 160 (94.7) | 9 (5.3) | 0.724 |
| Stage 2 | 8 (88.9) | 1 (11.1) | - |
| Stage 3 | 113 (93.4) | 8 (6.6) | - |
| Stage 4 | 27 (90.0) | 3 (10.0) | - |
| Stage at presentation | - | - | - |
| Late presentation with advanced disease | 108 (92.3) | 9 (7.7) | 0.728 |
| Late presenter | 77 (95.1) | 4 (4.9) | - |
| Early presenter | 123 (93.9) | 8 (6.1) | - |
| CD4 Count | - | - | - |
| 501 and above | 63 (92.6) | 5 (7.4) | 0.531 |
| 350-500 | 56 (98.2) | 1 (1.8) | - |
| 100-349 | 113 (93.4) | 8 (6.6) | - |
| 1-99 | 56 (93.3) | 4 (6.7) | - |
| Variables | Beta | Wald | AOR | CI | p-value |
|---|---|---|---|---|---|
| Age | - | - | - | - | - |
| ≥46 years | 1.89 | 10.24 | 6.60 | 2.08-20.94 | 0.001 |
| <46 years (Ref) | - | - | - | - | - |
| Sex | - | - | - | - | - |
| Male | 0.55 | 0.78 | 1.73 | 0.51-5.87 | 0.377 |
| Female (Ref) | - | - | - | - | - |
| Level of education | - | - | - | - | - |
| No formal education | -0.82 | 0.89 | 0.44 | 0.08-2.53 | 0.359 |
| Primary | -1.56 | 0.94 | 0.21 | 0.03-1.34 | 0.098 |
| Secondary | -0.96 | 0.67 | 0.38 | 0.10-1.42 | 0.152 |
| Tertiary (Ref) | - | - | - | - | - |
| Alcohol use | - | - | - | - | - |
| Yes | 0.18 | 0.07 | 1.20 | 0.32-4.47 | 0.791 |
| No (Ref) | - | - | - | - | - |
| Smoking | - | - | - | - | - |
| Yes | 0.22 | 0.08 | 1.25 | 0.27-5.67 | 0.775 |
| No (Ref) | - | - | - | - | - |
| Employment status | - | - | - | - | - |
| Unemployed | -0.39 | 0.49 | 0.68 | 0.23-2.02 | 0.48 |
| Employed (Ref) | - | - | - | - | - |
| CD4 Count | - | - | - | - | - |
| 501 and above | 0.461 | 0.30 | 1.59 | 0.30-8.29 | 0.585 |
| 350-500 | -1.16 | 0.97 | 0.31 | 0.03-3.15 | 0.325 |
| 100-349 | 0.41 | 0.35 | 1.50 | 0.39-5.80 | 0.556 |
| 1-99 (Ref) | - | - | - | - | - |
4. DISCUSSION
The present study has highlighted a DM prevalence of 6% among newly diagnosed HIV infected individuals. This is lower than Abebe et al. [20] study that compared DM prevalence between pre-ART and patient on ART and reported a higher DM prevalence in the pre-ART population. Similar findings of higher prevalence of DM have been reported in a retrospective database analysis of HIV infected individual in the USA; however, the authors failed to compare between pre-ART patient and ART using patients [6]. HbA1c reflects long-term glucose status of a person with DM. Shortcomings exist regarding its ability to estimate glycemia in an HIV infected individual [21]. Another recommendation was to avoid its use because of high HbA1c-glucose disparity in patients with high mean corpuscular volume (MCV), concurrent NRTI use (esp. abacavir) and low CD4 count [22], explain in the light of the possibility of HIV infected individuals with fast red blood cells turnover. Given the probable incongruence between HbA1c and glycaemic control, fasting blood sugar is clinically desirable DM [21]. One study found HbA1c insensitive, yet a specific diagnostic tool for diabetes in HIV-infected individuals [22]. However, we adopted the use of the HbA1c in this study based on the decreased likelihood of patient presenting to the clinic at a fasting state, and the cross-sectional nature of the study. Other reasons attributed to the poor performance of HbA1c in underestimating glycemia in HIV infected individuals include a high level of haemolysis among HIV infected individuals [20].
In this present study, the majority of the participants above 46 years of age had an increased risk of developing type 2 DM. This was similar to findings in a diverse cohort conducted in the United Kingdom where the risk of developing diabetes was significant in individuals with age above 49 years [7]. The role of ART in the development of type 2 DM has been demonstrated [23], however, our study focused mainly on patients not exposed to ART. In contrast to other studies, the present study found no association between gender, level of education, body mass index, smoking, alcohol use, stage of HIV illness and risk of developing DM [24-26].
5. LIMITATIONS
The use of HbA1c for the diagnosis of DM in HIV infected individuals might have underestimated glycaemia among the sample in this present study. In addition, the cross-sectional nature of the study limits the ability to draw an association between variables. Additionally, given the small sample size of the study, coupled with the fact that the study was conducted in only five selected health facilities in the Buffalo City Municipality, the findings cannot be generalised to the entire Province nor South Africa.
CONCLUSION
Notwithstanding, the role of HbA1c in diagnosing diabetes HIV infected individuals. The present study demonstrates a low prevalence of DM among newly diagnosed HIV patients in this understudied, and resource-limited setting. In addition, increasing age is a likely factor for the development of DM among the participants. Therefore, regular screening of newly diagnosed HIV positive patients for DM is important for timely health care interventions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The University of Fort Hare Ethical Committee gave ethical approval for the study (REC 270710028RA level 01). Permission was obtained from the Eastern Cape Health Department (Ref: EC_2016RP22_139) prior to data collection.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Wrtten informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
Financial support was received from the Discovery Foundation and South Africa Medical Research Council (#SAMRC/UFH/P790) for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the staff of the Eastern Cape Department of Health for their help rendered during data collection. Finally, the participants for agreeing to participate in this study.


