All published articles of this journal are available on ScienceDirect.
Prevalence of Self-medication and its Influence in the Labor Force in Rural Hlaing Tharyar, Yangon, Myanmar
Abstract
Background:
As self-medication is becoming the most familiar and preferred type of medical care in developing countries, this study was designed to measure the prevalence of self-medication and its influence on the labor force in rural areas of Hlaing Tharyar Township, Yangon, Myanmar.
Methods:
A cross-sectional study using structured questionnaires was conducted among 250 laborers during April 2015.
Results:
The prevalence of self-medication among the labor force was (89.2%) in which 64.0% had poor knowledge, 56.8% had poor perception, and 68.8% received poor social support for self-medication practices. A multiple logistic regression analysis revealed that three variables influenced self-medication practices: (1) decision-making role for the treatment of illness (odds ratio [OR] = 3.79, 95% confidence interval [CI] = 1.7–12.38); (2) poor perception (OR = 5.33, 95% CI = 1.66–17.08); and (3) poor social support (OR = 4.86, 95% CI = 1.61–14.63).
Conclusion:
These findings indicate the need for health education intervention and behavior change communication training for promoting rational drug use among this rural labor force.
1. INTRODUCTION
The use of drugs or medicines by patients to self-treat diseases or symptoms or the use of previously-prescribed medicine, and taking medication prescribed by unlicensed traditional and/or health staffs are defined as self-medication [1]. They may account for drug resistance, delay proper treatment and mask diseases, which can lead to life-threatening cases [2]. Despite the attempts of developing countries to achieve universal health care coverage, mainly for underserved populations, self-medication using Over-The-Counter (OTC) drugs is becoming the most familiar and preferred type of medical care. It is estimated that over 92% of all purchasers used at least one OTC medicine and 55% have used more than one in a year [3]. In the South-East Asia Region, self-medication is a progressively practiced phenomenon and the prevalence is estimated to be within the range of 12.7% to 95% [4, 5]. Nepal, India and Pakistan have reported prevalence estimates of around 50% [6-8]. In Sri Lanka, almost 64% of the families from an urban area had practiced self-medication [9]. It was found that self-medication was a low-cost alternative especially for mild illnesses when a person was dissatisfied with available medical care.
The popularity of self-medication is due to its social acceptability and low cost of treatment. Reliance on self-medication and traditional practitioners was predominant among people in rural areas and among the lower social classes for whom modern health services provided by the government were not affordable and were not acceptable [10]. In 2013, only a small portion (5%) of drug stores was handled by proper pharmacists, while almost 75% of outlets were assigned by other non-experts [11]. Self-medication practices among Myanmar villagers were highly prevalent and practiced by 97.8% of respondents. In addition, most of the respondents (83.3%) were dissatisfied with costs, waiting time, nurses’ behavior and services given at hospital or health center [10]. Low income groups used the retail pharmacy, where treatments were provided by neither pharmacists nor trained health personnel. In a qualitative approach study conducted in a peri-urban area of Yangon City, Myanmar, participants were familiar with frequently advertised drugs and thought that buying drugs from drug stores was the most convenient way. Moreover, going to clinics was viewed as the last option, as they did not like the repeated appointments and long waiting time [12].
The study area, Hlaing Tharyar Township, is a peri-urban area in the northern part of Yangon city. It has families of different socio-economic-demographic classes among the permanent residents, as well as a transient population from other parts of the country. Therefore, population of the township, particularly the labor force, has increased vastly due to the inward migration from other townships, mainly in search of better job opportunities in the industrial zone. The township comprises 20 wards in the urban area and 18 villages in the rural area. Health care is provided by one township hospital, one station hospital, one urban health center, one Rural Health Center (RHC) and five sub RHCs as government sectors. Although the sanctions are nearly full, some doctors and midwives are away for further study so there is a shortage of human resources and these facilities may not cover all the needs for health in the community [12]. There were very few studies about self-medication practices among the labor force in Myanmar and not much is known about its major contributing factors [13]. Therefore, this study will serve as baseline information for those who are interested in doing research on self-medication practices in Myanmar. Moreover, this study will help to explain the reasons for self-medication practices among the labor force and propose a new way of thinking to formulate a proper strategy for promoting rational use of drugs in their society.
2. MATERIALS AND METHODS
The present study was conducted in rural Hlaing Tharyar, Yangon Division, Myanmar. According to the National Population Commission Census of 2014, this area is inhabited by 205,672 individuals, 89% of whom are male and 11% female [14]. A structured questionnaire was used as the data collection instrument. It was initially developed in English and then translated into Myanmar by back-translation method. Prior to commencing the main study, the questionnaire was pre-tested by administering it to 30 Myanmar nationals residing in Bangkok, who were asked to provide feedback on the clarity of language used, item sequence and other aspects. Next, the Cronbach’s alpha coefficient (α) was calculated for all instrument scales, whereby 0.802 was obtained for knowledge about self-medication, 0.807 for perception related to self-medication and 0.783 for social support for self-medication.
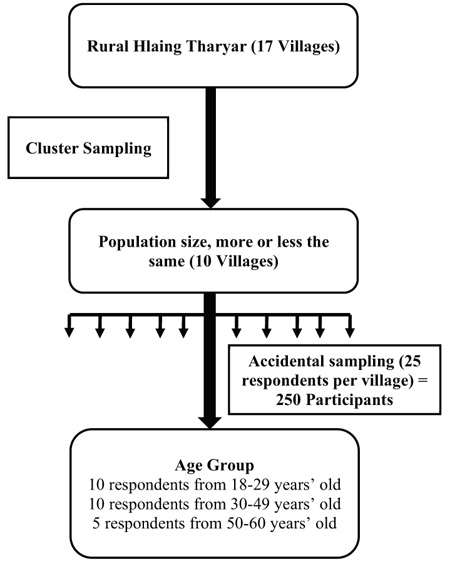
The population of interest for this investigation comprised of both males and females of working age (18 to 60 years) residing in rural Hlaing Tharyar. The cluster sampling technique was adopted to select 10 of the 17 villages located in this region. Next, 25 individuals were randomly selected from each of these 10 sampled villages (with 10 respondents aged 18−29, 10 aged 30−49, and 5 aged 50−60 years), resulting in the desired sample size of 250 participants. To increase the participation rate, the households meeting the study inclusion criteria were informed of the data collection at least one day in advance. If the randomly chosen household had no working-age members, another respondent was identified and recruited. All study participants took part in individual face-to-face interviews and completed the questionnaires. After cleaning, the data was encoded and entered into Epidata 3.1 before analyzing the responses to each part of the questionnaire using Statistical Package for the Social Sciences (SPSS), version 18 (Fig. 1).
3. RESULTS
Analysis of the 250 participants’ survey responses revealed that 89.2% of the sample (223 participants) engaged in self-medication practices while the remaining 10.8% did not, as shown in Table 1. In the former group, self-medication was mostly used for treating headache (89.2%), followed by fever (87.9%) and cough (80.7%). Around half of the respondents practiced self-medication for muscle pain (57.8%), sore throat (55.2%), minor injuries (53.8%) and running nose (50.2%). Similarly, 37.2%, 36.8%, 36.3% and 34.5% reported self-medicating due to flu, allergic rash, diarrhea and stomachache, respectively. Finally, about 10% of the sample resorted to self-medication when suffering from mouth ulcers, earache and asthma.
According to the general sample characteristics reported in Table 2, 60.0% of the respondents are aged ≤ 35 years, and 74.4% are married, while both genders are equally represented. In addition, 72% of the participants live in households with fewer than six members, and 78.4% are educated at the high school level or below. With respect to occupation, 63.2% of the sample reported working more than 8 hours per day, while 53.2% earn no more than 130,000 Kyats (˜85USD) per month. Nearly half (54.4%) of the sample stated that their living expenses exceeded their individual income. Moreover, 76.8% of the sample noted that they determined the best way to treat an illness, while the remaining 23.2% left such decisions to family members or healthcare personnel. Yet, 64.0% of the respondents stated that their self-medication knowledge was poor, while the remaining 36.0% rated it as good. In addition, 56.8% of the participants had poor level of perception related to self-medication, drug use, health services and health personnel, while the remaining 42.3% rated it as good. The results also showed that 68.8% of the sample received poor social support from their community for self-medication.
The associations were computed between self-medication practice and female (p=0.02), lower education (p=0.03), working more than 8 hours per day (p=0.011), less income (p=0.036), not enough income for living expenses (p=0.022) and decision making on treatment of illness (p=0.000). The female respondents were 2.7 times more likely to practice self-medication than males (OR= 2.72, 95% CI= 1.14-6.47). The respondents who had high school and lower education were 2.8 times more likely to practice than those with higher education (OR=2.88, 95% CI= 1.25-6.64). Moreover, the respondents who worked more than 8 hours per day practiced self-medication about 3 times more than those who worked equal to or less than 8 hours. It was also found that low income groups were 2.5 times more likely to practice self-medication than higher income groups, and those making self-decision on treatment of illness were 3 times more than the other decided by family members or health personnel. Moreover, significant associations were found between self-medication and knowledge (p=0.008), perception (p=0.001) and social support (p=0.014). The respondents with poor knowledge levels about self-medication were 2.9 times more likely to practice self-medication than those who had fair and good knowledge (OR= 2.93, 95% CI= 1.29-6.63). The respondents who had poor perception related to self-medication practiced it 4.3 times more than those who had good levels of perception (OR= 4.38, 95% CI= 1.78-10.79). It was also found that the respondents who received poor social support practiced self-medication 2.6 times more often than those who received good support (OR= 2.68, 95% CI= 1.19-6.00).
| Self-medication | Number (n) | Percent (%) |
|---|---|---|
| Self-medication first to treat illness | ||
| Yes | 223 | 89.2 |
| No | 27 | 12.1 |
| Type of illness among 223 participants who have practiced self-medication | ||
| Headache | 199 | 89.2 |
| Fever | 196 | 87.9 |
| Cough | 180 | 80.7 |
| Muscle aches/ pain | 129 | 57.8 |
| Sore throat | 123 | 55.2 |
| Minor injuries | 120 | 53.8 |
| Running nose | 112 | 50.2 |
| Flu | 83 | 37.2 |
| Allergic rash | 82 | 36.8 |
| Diarrhea | 81 | 36.3 |
| Stomachache | 77 | 34.5 |
| Itchy eyes | 47 | 21.1 |
| Mouth ulcer | 35 | 15.7 |
| Asthma | 27 | 12.1 |
| Earache | 25 | 11.2 |
| Others e.g. Arthritis, Herpes, Eczema | 11 | 4.9 |
| General Characteristics | n (%) |
Self-medication (%) |
OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Age group (years) | 0.454 | |||||
| ≤ 35 | 150 (60.0) | 88.0 | 12.0 | 1.00 | ||
| > 35 | 100 (40.0) | 91.0 | 9.0 | 1.38 | 0.59-3.20 | |
| Gender | 0.020* | |||||
| Male | 123 (49.2) | 84.6 | 15.4 | 1.00 | ||
| Female | 127 (50.8) | 93.7 | 6.3 | 2.72 | 1.14-6.47 | |
| Marital status | 0.670 | |||||
| Married | 186 (74.4) | 88.7 | 11.3 | 1.00 | ||
| Single/ Divorced/ Widowed | 64 (25.6) | 90.6 | 9.4 | 1.23 | 0.47-3.19 | |
| Total number of family member (persons) | 0.479 | |||||
| ≤ 5 | 180 (72.0) | 88.3 | 11.7 | 1.00 | ||
| > 5 | 70 (28.0) | 91.4 | 8.6 | 1.41 | 0.54-3.65 | |
| Education | 0.010* | |||||
| College/ Bachelor | 54 (21.6) | 79.6 | 20.4 | 1.00 | ||
| Primary/ Secondary/ High | 196 (78.4) | 91.8 | 8.2 | 2.88 | 1.25-6.64 | |
| Occupation | 0.446* | |||||
| Employed | 199 (79.6) | 88.4 | 11.6 | 1.00 | ||
| Dependent | 51 (20.4) | 92.2 | 7.8 | 1.54 | 0.51-4.66 | |
| Working hours per day (n=210) | 0.011* | |||||
| ≤ 8 | 52 (20.8) | 78.8 | 21.2 | 1.00 | ||
| > 8 | 158 (63.2) | 91.8 | 8.2 | 2.99 | 1.25-7.18 | |
| Average income per month (Kyats) (n=210) | 0.036* | |||||
| > 130,000 | 65 (26.0) | 81.5 | 18.5 | 1.00 | ||
| ≤ 130,000 | 133 (53.2) | 91.7 | 8.3 | 2.51 | 1.04-6.05 | |
| Income status for living/ expense (n=210) | 0.022* | |||||
| Enough with/ without saving | 62 (24.8) | 80.6 | 19.4 | 1.00 | ||
| Not enough, no debt/ with debt | 136 (54.4) | 91.9 | 8.1 | 2.73 | 1.13-6.58 | |
| Decision making role on treatment of illness | 0.006* | |||||
| Family member/ heath personnel | 58 (23.2) | 79.3 | 20.7 | 1.00 | ||
| Self-decision | 192 (76.8) | 92.2 | 7.8 | 3.08 | 1.35-7.03 | |
| Knowledge about self-medication | 0.008* | |||||
| Good | 90 (36.0) | 82.2 | 17.8 | 1.00 | ||
| Poor | 160 (64.0) | 93.1 | 6.9 | 2.93 | 1.29-6.63 | |
| Perception related to self-medication | 0.001* | |||||
| Good | 108 (42.3) | 81.5 | 18.5 | 1.00 | ||
| Poor | 142 (56.8) | 95.1 | 4.9 | 4.38 | 1.78-10.79 | |
| Social support for self-medication | 0.014* | |||||
| Good | 78 (31.2) | 7.6 | 17.9 | 1.00 | ||
| Poor | 172 (68.8) | 92.4 | 82.1 | 2.68 | 1.19-6.00 | |
*Significance at P<0.05, Degree of freedom for Chi-square test=1. CI: Confidence interval, OR: Odds ratio
| Variables | Unadjusted | Adjusted* | p-value | ||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Decision making for treatment of illness | 0.027* | ||||
| Family member/ health person | 1.00 | 1.00 | |||
| Self-decision | 3.08 | 1.35-7.03 | 3.79 | 1.17-12.38 | |
| Perception related to self-medication | 0.005* | ||||
| Good/ Fair | 1.00 | 1.00 | |||
| Poor | 4.38 | 1.78-10.79 | 5.33 | 1.66-17.08 | |
| Social support for self-medication | 0.005* | ||||
| Good/ Fair | 1.00 | 1.00 | |||
| Poor | 2.68 | 1.19-6.00 | 4.86 | 1.61-14.63 | |
*Adjusted for age, gender, education, working hours, income and decision making on treatment of illness, knowledge about self-medication, perception related to self-medication and social support for self-medication
*Significance at P<0.05, OR: Odds ratio (Unadjusted and Adjusted)
From Table 3, after adjusted multiple logistic regressions for variables in the model, the results showed that three variables were statistically associated with self-medication practice: decision making for treatment of illness (adjusted OR = 3.79, 95% CI = 1.17-12.38), perception related to self-medication (adjusted OR = 5.33, 95% CI = 1.66-17.08), social support for self-medication (adjusted OR = 4.86, 95% CI = 1.61-14.63).
4. DISCUSSION
Over several decades, the high prevalence of self-medication has been reported to exist all over the world, but particularly in developing countries [15]. This study reports a prevalence rate of 89.2% among the Myanmar labor force, a smaller rate than that found by another study conducted among Myanmar villagers (98%) [16]. Both are higher than the rates in nearby countries like South India (71%), Malaysia (62.7%), and Nepal (59%) [17, 18]. However, any significant rate of self-medication (>50%) is likely to result in a health challenge anywhere in the world [19]. The variations among rates may be attributable to different socio-economic factors, such as existing prevalence, cultural beliefs, and government drug-control regulations. They might also be due to individual perceptions of, for instance, cheapness, saving of time, ease of access, and the mildness of the illness, as well as previous experience. A study in Ethiopia found the cost of treatment to be an important motivation for self-medication [20]. Self-medication practices vary with specific diseases and different severities [21]. This study found that most of the respondents self-medicate for headache and fever, with a smaller number self-medicating for coughs and muscle pain. These results compare well with those of an Ethiopian study, as almost all of the respondents practiced self-medication for trivial illnesses like headache (60%) [20]. Another study, in India, found that more participants (73.2%) self-medicated for headache and body ache than for any other illnesses [22]. Pain was found to be the most common reason to self-medicate, with allergies and diseases of the respiratory tract next [23].
Of ten general variables, six were significantly associated with self-medication: gender, education, daily working hours, personal income, income status, and ability to decide on a treatment for illness. The results revealed that female respondents are 2.7 times more likely than male respondents to practice self-medication. Studies in Sri Lanka, Nepal, and South India, however, reported the practice to be more prevalent in males than in females [24, 25], perhaps because females are more dependent on males in those societies. In Myanmar’s female labor force, economic problems and lack of time contribute more to the women’s greater indulgence in self-medication. Although no association was found between age groups and self-medication practice, the results showed that the practice gradually decreases with increasing age. This might be because an aging person, with experience of previous episodes, would, in general, be more likely to visit a medical doctor [17]. This study reports that the practice of self-medication among groups with lower education (high school and below) is 2.8 times more than it is among those with higher education. Many studies have proved that the education level of the participants influences the likelihood of practicing self-medication [7, 18, 26]. Other studies contradict these results, finding that participants with higher education are more likely to self-medicate, possibly because they can read and understand the descriptions and dosages printed on drugs packages [23, 27].
This study found no association between respondents’ occupations and their self-medication practices. However, those with low individual monthly income (130,000 Kyats or less), earning insufficient for living expenses, self-medicate more readily than higher income groups. One study in Mexico revealed a greater likelihood for low-income respondents to self-medicate [28]. This association between income and self-medication has an economic basis. Most laborers and factory workers in the study area are very poor; even though they work hard for more than eight hours daily, their income is very low. Because they can save time and money by avoiding private clinics and hospitals except in emergency, their obvious first resort is to try self-medication to solve their health problems. Furthermore, decisive respondents are more likely to self-medicate than those whose decisions are made by significant others, such as family members and healthcare personnel. This trend possibly exists because the respondents in this study rely on their own decisions rather than allowing others to make decisions for them.
This study found a generally poor knowledge of self-medication. Most respondents lack knowledge of important facts including drug dosage, the adverse effects of drugs, and the dangers of self-medication. Those with a poor knowledge level were found to practice self-medication 2.9 times more than those with fair or good knowledge (OR= 2.93, 95% CI= 1.29-6.63). A study in Ethiopia found similar results, with more than two thirds of the respondents having no preliminary knowledge of the adverse effects of over-the-counter drugs [29]. This finding highlights their need for health and drug education. One key factor is the cost of self-medication, considered by all as reasonable and affordable. One of the studies in Brazil concluded that people tend to be more cautious of self-medication and to practice it less because of a lack of medical knowledge [30]. Nevertheless, multiple logistic regression analysis shows that the knowledge level of the respondents is not significant.
The study shows that perception of self-medication is strongly related to self-medication practice. Respondents with poor perception self-medicated 4.3 times more than those with moderate or good perception (OR= 4.38, 95% CI= 1.78-10.79). Multiple logistic regression analysis shows that perception regarding self-medication is also statistically associated with self-medication practice (p < 0.05). Of 250 respondents, more than half (56.8%) had poor perception related to self-medication and only 14% had a good perception level. Many strongly perceived self-medication as a time-saving, economical approach to minor illness and as a temporary measure. These findings align with the World Health Organization’s statement that self-medication may offer a reasonably-priced, acceptable option for relieving common non-serious illnesses [16]. Other studies confirm that these factors serve to influence self-medication practices among the community [24].
The study revealed that 68.8% had poor levels of social support for self-medication. The respondents mainly received mental support, financial support, and free drugs from their family members. A study in Jordan concluded that one main factor for choosing self-medication was receiving suggestions from drug vendors, neighbors, friends, or family members [31]. Also, from the psychological perspective, comparing with others from local surroundings effects people’s well-being [32]. This study showed that to obtain information about drugs, patients usually asked drug sellers and health personnel, followed by family members. A study in Ethiopia showed that pharmacists, rather than other health care personnel, are a major source of information in the community regarding self-medication [16]. Another report from a Nigerian study [33] showed that the main channels of information and the usual sources of medication were local drug sellers who are usually closer to the grassroots, probably due to the relatively limited number of registered pharmacists and medical doctors in developing countries. It was found that the respondents who received poor social support practiced self-medication 2.6 times more often than those who received fair and good support (OR= 2.68, 95% CI= 1.19-6.00). The association was still significant with p-value 0.005 after analyzing with multiple logistic regressions. The result was in line with the study among students in Thailand that, the less social support received, the more self-medication is practiced. This was possibly due to exceptionally reasonable prices of self-medication and availability of pharmacy shops [34].
CONCLUSION
In contrast, the study proved that there is an alarming situation with respect to self-medication practice along with its associated factors among the rural labor force. Therefore, we would like to suggest that health education and topic discussions about responsible self-medication practices in working environments and factories should be promoted. Again, behavior change communication trainings and programs should be provided in order to change workers’ misunderstandings and doubts concerning self-medication. Implementation of health personnel involvement programs should be supported with coordination and collaboration of responsible organizations and community people. It is also necessary to provide the workers with real information through interpersonal communication in addition to mass media sources in order to explore the correct information.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was reviewed and approved by The Ethical Committee of Faculty of Public Health, Mahidol University (COA. No. MUPH 2015-075).
HUMAN AND ANIMAL RIGHTS
No humans/animals were used for the studies that are basis of this research.
CONSENT FOR PUBLICATION
The researcher asked for the consent from the respondents before commencing the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
FUNDING
The authors received no financial support for this study, authorship, and/or publication of this article.
ACKNOWLEDGEMENTS
Declared none.


