All published articles of this journal are available on ScienceDirect.
Community Origin and Previous Use of Antimicrobials Increase the Risk of Nosocomial Multidrug-resistant Bacteria Colonisation in the Intensive Care Unit in a Brazilian Hospital
Abstract
Background:
Hospitalised patients are often surrounded by microorganisms, and antibiotic-resistant pathogens are a major and growing threat to public health.
Objective:
This study aimed to investigate the epidemiology and the risk factors for colonisation by multidrug-resistant organisms (MDROs) in a Brazilian hospital.
Methods:
Patients in the Intensive Care Unit (ICU) who underwent nasal and rectal swab cultures for the surveillance of colonisation by MDROs were evaluated in a retrospective study. MDROs were determined by routine microbiological cultures.
Results:
Of the 785 patients included in this study, 86 presented positive results for MDRO colonisation. Overall, the most frequently isolated organism was Klebsiella pneumoniae (41.9%), followed by Escherichia coli (33.7%). The main type of resistance was the production of extended-spectrum beta-lactamases (ESBL). The prevalence of MDRO infections was significantly associated with the patient's origin (community or hospital-acquired). Having been submitted to previous antimicrobial drug therapy was significantly associated with MDRO infection (relative risk [RR]: 4.02 [2.60 - 6.23]).
Conclusion:
MDRO ICU colonisation was variable, with similar frequencies as other centres, and important factors, including previous hospital stay and antibiotic use, were closely related to MDRO colonisation. Therefore, control interventions should reduce their rates, especially considering the particularities of each geographic centre.
1. INTRODUCTION
Hospitalised patients are often confined to hospital beds and surrounded by multiple devices, equipment and environmental surfaces that can harbour microorganisms [1]. Thus, there is a concern regarding the environmental role in antimicrobial-resistant pathogen transmission among patients [2, 3]. Epidemiologic data suggested for more than a century and a half that microbes are spread from patient to patient via contaminated hands. In this context, multidrug-resistant organisms (MDROs) deserve special attention in healthcare facilities [4-7].
MDROs are defined as microorganisms, mainly bacteria, that are resistant to one or more classes of antimicrobial agents [8]. Previous studies reported similarity between the antimicrobial susceptibility profiles of non-clinical (furniture, medical devices, and gloves) and clinical MDRO isolates. These data suggest that the hospital environment is a potential origin for MDRO persistence [1]. Additionally, MDROs, such as methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-Resistant Enterococci (VRE), and certain Gram-negative Bacilli (GNB) including the Extended-spectrum Beta-lactamase (ESBL) producers, have been isolated from hands, gloves, equipment, or healthcare workers involved in the care of infected or colonised patients [9-11]. Common body sites of infection include blood, urinary tract, nasal and rectal sites, catheters, abscesses, and secretions from ocular, pulmonary, and peritoneal regions [5].
Hospitalised patients, especially those in the Intensive Care Unit (ICU), present more risk factors for acquiring an MDRO infection. They are debilitated and surrounded by tubes and equipment; thus, ICU patients present the highest MDRO infection and mortality rates [12]. Moreover, MDRO colonisation increases a patient's risk of developing infections, such as Bloodstream Infections (BSIs), as observed in patients with VRE colonisation [13].
Notably, more than 70% of the bacteria that cause hospital-acquired infections are resistant to at least one of the drugs most commonly used to treat such infections [14, 15]. Researchers suggested that this high percentage of resistance is related to the excessive and improper use of broad-spectrum antibiotics, especially in healthcare settings [16]. Recent data demonstrated that the costs associated with nosocomial-infected patients are 4-times higher than non-infected ones; the costs are even higher in MDRO cases [17]. Moreover, the risk of hospital readmission in the first 30 days after discharge is higher in such patients [18]. Therefore, antibiotic-resistant pathogens constitute an important and growing threat to public health. In this way, the approaches for the prevention and control of these pathogens must be personalised for specific needs. Specific types of MDROs can be more prevalent, while others may represent a higher risk for hospital readmissions, depending on the particular environment or hospital [5, 9, 19, 20].
In order to improve the knowledge regarding microorganisms’ prevalence and profile in specific centres, and to analyse the risk factors for MDROs colonisation and infection in patients admitted to ICUs, a retrospective study was conducted in a hospital in southwest Paraná, Brazil.
2. MATERIALS AND METHODS
This retrospective study was conducted in an ICU in the Southwest Regional Hospital Walter Alberto Pecoits, Paraná, Brazil, a general, medium-sized hospital. All of the care is provided by the Unique Health System from the Brazilian government (SUS), which meets the demand of the entire southwest region of Paraná State and other border cities from Santa Catarina State (over 600,000 inhabitants). Samples from hospitalised patients were collected from January 2014 to February 2016. This study was approved by the National Ethics Committee - Brazil Platform (Approval number: 2.155.862).
Samples from nasal and rectal sites were collected with a sterile swab for microbiological culture at the moment of the patient’s admission to the ICU. Additionally, rectal samples were collected weekly for follow-up. Both collections are performed as usual routine procedures in the Service.
Pathogen identifications and resistance profiles were determined using routine microbiological methods following the recommendations for tests [21]. Briefly, nasal samples were cultured in blood and MacConkey agars. A rectal swab was also added to Tryptic Soy Broth (TSB) and incubated for 24 h at 36°C. Subsequently, antimicrobial resistance evaluation was performed with antibiogram tests. For MDROs in the nasal swab, the potential pathogens were S. aureus with intermediate resistance to vancomycin (VRSA), MRSA, VRE, or Acinetobacter baumannii resistant to carbapenem (Cbpnm). Rectal swab samples were examined for Cbpnm-resistant enterobacteria, third- and fourth-generation cephalosporins and non-fermenter bacteria resistant to Cbpnm and/or polymyxin. All results were obtained from the hospital infection control service database that receives notifications for all MDRO isolates from the microbiology laboratory.
Patient characteristics and risk factor data were collected from medical records; these data included age, gender, previous hospitalisation (defined as hospitalisation in another health service for more than 48 h before admission in the studied hospital) and previous use of antibiotics (determined as antimicrobial use up to 48 h prior to material collection) as potential factors associated with colonisation by MDRO.
For statistical analyses, two conditions were considered: 1) community-associated MDRO patients (CA-MDRO), who did not previously stay in hospital, and hospital-associated MDRO origin (HA-MDRO), for patients who acquired the microorganism colonisation at the studied hospital; 2) prevalent cases (those with positive culture results regardless of the period between admission and positivity) and incident cases (those with negative culture results at admission and further positivity in samples obtained 7 days post-admission). The relative risk (RR) of patient origin and previous antimicrobial use relationship to MDRO positivity was calculated. Statistical analyses and graphs were obtained using Prism 6.0 (GraphPad Software Inc., La Jolla, CA).
3. RESULTS
During the study period, 785 patients were admitted to the ICU. Of these, 86 (10.9%) presented positive results for MDRO colonisation in nasal and/or rectal samples regardless of the length of the ICU stay. Overall, Klebsiella pneumoniae had the highest prevalence among isolates (n = 36, 41.9%), followed by Escherichia coli (n = 29, 33.7%), A. baumannii (n = 9, 10.4%), Enterobacter spp. (n = 8, 9.3%), S. aureus (n = 2, 2.3%), Pseudomonas aeruginosa (n = 1, 1.2%), and Citrobacter spp. (n=1, 1.2%; Fig. 1).
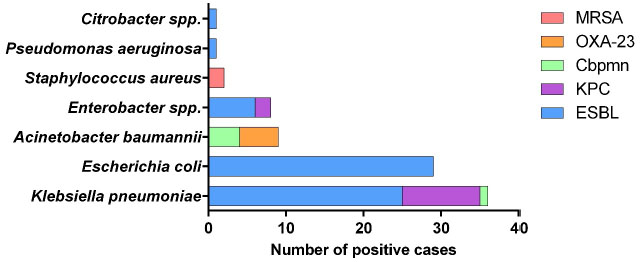
Nineteen positive cultures were from CA-MDRO and 67 were from HA-MDRO (Table 1). For ESBL-producing bacteria, E. coli and K. pneumoniae had the highest prevalence among isolates in both the community and hospital environment, followed by Enterobacter spp. A. baumannii, K. pneumoniae resistant to Cbpnm (KPC) and Enterobacter spp. resistant to Cbpnm were detected only in the hospital environment.
At the studied ICU, the MDRO incidence was 62.8% (54 new colonisation were documented). The number of colonisations varied over the weeks: 28 patients were colonised after 7-14 days in the hospital, 16 patients were colonised after 15-22 days, 5 patients between 23-30 days, and 5 after more than 30 days.
Table 2 depicts the studied population characteristics and RR to MDROs. Age and gender were not related to MDRO infection. Notably, patients transferred from another hospital presented a 2.41-fold greater chance of MDRO infection (Table 2). Likewise, having been submitted to previous therapy with antimicrobials drugs was significantly associated with infection with MDROs (RR: 4.02 [2.60 - 6.23]; Table 2).
| – |
Community-associated MDRO Patients (CA-MDRO)* (n=19) |
Hospital-associated MDRO Origin (HA-MDRO)** (n=67) |
Total (n=86) |
| ESBL | – | – | – |
| Escherichia coli | 12 (63.1) | 17 (25.4) | 29 (33.7) |
| Klebsiella pneumoniae | 5 (26.3) | 20 (29.8) | 25 (29.1) |
| Enterobacter spp. | 0 | 6 (8.9) | 6 (7.0) |
| Pseudomonas aeruginosa | 0 | 1 (1.5) | 1 (1.2) |
| Citrobacter spp. | 0 | 1 (1.5) | 1 (1.2) |
| Cbpnm | – | – | – |
| Acinetobacter baumannii | 0 | 4 (6.0) | 4 (4.6) |
| Klebsiella pneumoniae | 0 | 1 (1.5) | 1 (1.2) |
| KPC | – | – | – |
| Klebsiella pneumoniae | 0 | 10 (14.9) | 10 (11.6) |
| Enterobacter spp. | 0 | 2 (3.0) | 2 (2.3) |
| OXA-23 | – | – | – |
| Acinetobacter baumannii | 1 (5.3) | 4 (6.0) | 5 (5.8) |
| MRSA | – | – | – |
| Staphylococcus aureus | 1 (5.3) | 1 (1.5) | 2 (2.3) |
ESBL: Extended-Spectrum Beta-Lactamases, MRSA: Methicillin-Resistant Staphylococcus aureus, Cbpnm: Carbapenem, KPC: Carbapenem-Resistant Klebsiella, OXA-23: Oxacillin 23.
| – |
Positive Cultures (n=86) n (%) |
Negative Cultures (n=699) n (%) |
Relative Risk (95% Confidence Interval) |
p |
| Age (years) | – | – | – | – |
| 18-60 | 36 (41.9) | 324 (46.4) | - | 0.43 |
| >60 | 50 (58.1) | 375 (53.6) | ||
| Gender | – | – | – | – |
| Male | 49 (57.0) | 382 (54.6) | - | 0.68 |
| Female | 37 (43.0) | 317 (45.4) | ||
| Origin | – | – | – | – |
| Transferred from another health center | 28 (32.6) | 103 (14.7) | 2.41 (1.59 – 3.63) | <0.0001 |
| Community | 58 (67.4) | 596 (85.3) | ||
| Previous antimicrobials use | – | – | – | – |
| No | 26 (30.0) | 473 (67.7) | 4.02 (2.60 – 6.23) | <0.0001 |
| Yes | 60 (70.0) | 226 (32.3) |
b Relative risk: 0.83 (0.78-0.88), 95% confidence interval).
4. DISCUSSION
The risk of developing nosocomial infection or colonisation in the intensive care unit by MDRO is high. The local characteristics and optimal means for the control of antibiotic-resistant pathogens have been debated for decades.
The prevalence of MDROs in ICUs varies among regions, periods of study, and biological samples. In the present study, 10.9% of ICU patients presented MDRO. Considering specific groups of drug-resistant bacteria, the current data are consistent with Nangino et al. [22], who reported nosocomial infections in 8.9% of ICU patients. On the other hand, Magira et al. [23] reported an MDRO prevalence of 41.7% in ICU patients. However, their study evaluated more biological samples, including blood, sputum, and urine, and this factor may justify the higher prevalence. Therefore, the overall rates are difficult to estimate because most studies described specific characteristics of the searched organism and specific healthcare settings.
Our results demonstrate colonization by various microorganisms, which possibly relate to cases of infections of the population of this geographical area. These data corroborate previous reports from various national and multinational surveillance groups, all of which demonstrate a marked variability among pathogens across different geographic regions and over time [1, 5, 19, 20, 24, 25]. For example, in a recent Turkish prospective cohort study, 26.6% of ICU patients acquired nosocomial infections: P. aeruginosa (25%) was most frequent, followed by S. aureus (21.4%), E. coli (18.7%), and A. baumannii (16.9%) [26]. In Columbia, E.coli was the most prevalent gram-negative bacteria isolated in ICU patients, and the authors also showed a 4% increase in cases of infection by K. pneumoniae in 2012 [27]. Data from Brazilian centres and ICUs also diverge with regards to the percentage of cases and microorganism type. In a tertiary public hospital in São Paulo, approximately 40% of isolated strains from ICU patients were identified as gram-positive organisms and almost 50% as gram-negative. Coagulase-negative Staphylococcus (CoNS) was the most prevalent isolated microorganism (21.6%), followed by P. aeruginosa (12.4%). A. baumannii was isolated mainly in the blood and bronchoalveolar lavage, whereas P. aeruginosa predominated in bronchoalveolar lavage and urinary samples [5]. These data and others demonstrate the importance of knowing the particularities of diverse centres in order to drive decisions and protocols regarding antibiotic use and infection control and prevention [15].
According to the Global Health Report on Surveillance from the World Health Organization (WHO) [15], selected bacteria of international concern that can exhibit resistance to antibacterial drugs include mainly E. coli, K. pneumonia, and S. aureus, and there are higher rates of extended spectrum cephalosporin-resistant P. aeruginosa and Cbpmn-resistant P. aeruginosa. Moreover, VRE was observed in several studies [28]. Our study concentrates on data from MDRO infections, and the main type of resistance identified was ESBL production. E. coli, following by K. pneumonia, were the main bacteria in both community and hospital environments. Accordingly, a recent study from São Paulo [5] showed E. coli accounted for 27.5% of bacteria resistant to cefepime. ESBL-producing K. pneumoniae accounted for 62.7% of the strains; 18.9% were resistant to carbapenem/meropenem. In another study in South Brazil, the ESBL phenotypic test was positive in all the E. coli and Enterobacter aerogenes isolates and 53.8% of the K. pneumoniae isolates [29]. On the other hand, Prestes-Carneiro et al. [5] reported 55.7% of isolates were MRSA, a percentage much higher than for our centre. One possible explanation for this discrepancy is our study included only nasal and rectal samples, whereas other studies analysed samples from diverse body sites. Even though nasal and rectal samples represent respiratory and gastrointestinal infection, two of the four main nosocomial infection sites [3], we demonstrated a high rate of MDRO infections in the studied ICU. These data reinforce the importance of such research.
Harris et al. [30] described ICU admission cultures from 5,209 patients; 117 were colonised with ESBL-producing E. coli and Klebsiella spp., and 29 (25%) had a subsequent ESBL-positive clinical culture. The analysis showed that age > 60 years and chronic disease score are associated with ESBL-producing bacteria colonisation [15]. Although the present study did not find a difference regarding age, the main MDRO bacterial types were similar.
The widespread use of antibiotics during the past few decades has led bacteria to acquire genes responsible for resistance enzymes. This acquisition had consequently improved their survival rates in the environment and patients. ESBL-producing bacteria can hydrolyse extended-spectrum cephalosporins, but they are usually susceptible to inhibition by clavulanic acid and tazobactam [31]. The genes for ESBL production, first reported in the mid-1980s, are carried on plasmids. These vectors contribute to the spread of these resistant genes in the bacterial population. Currently, ESBL-producing strains are mostly encountered in K. pneumoniae and E.coli infections [32], data that are consistent with our study.
The increasing prevalence of ESBL-producing Enterobacteriaceae (ESBL-PE) carriage upon ICU admission raises important questions on empiric therapy policies in patients that present with an infection. One such policy may include the use of a carbapenem as a first-line therapy. However, carbapenemase-producing Enterobacteriaceae have now emerged (notably among K. pneumoniae) as a group of highly drug-resistant gram-negative bacilli that cause infections associated with significant morbidity and mortality [33].
A. baumannii strains are generally susceptible to carbapenems and aminoglycosides. These antibiotics are usually used in combination to treat infections by this microorganism. However, multidrug-resistant strains have increasingly emerged as a problematic pathogen responsible for hospital-acquired infections worldwide and have resulted in a considerable clinical impact [34]. Outbreaks of OXA-23-producing A. baumannii, one carbapenemase-producing resistant strain, were reported in some Brazilian hospitals [35, 36]. This strain was also present at our ICU centre. Moreover, a new oxacillinase-resistant strain, OXA-143-like, was recently described in this country [35, 36]. This finding demonstrates the increase of resistant A. baumannii in Brazil.
There are some limitations to our study. Not all medical records were available for analysis, and so other risk factors for MDRO, as well as associated comorbidities and causes of ICU hospitalisation, could not be included in the results to provide information that would complement this research. Moreover, there was a lack of information about health conditions from the population in this geographical region and some missing medical record data. However, our work has provided more information to increase knowledge for the studied service, as well as its demands and health services protocols, in order to guide health policies for this population.
Furthermore, the present study evaluated community and hospital-acquired colonisation. Intriguingly, the risk of acquiring a hospital-infection was 2.4-fold higher if the patient was transferred from another hospital. Traditionally, these two types of colonisation are classified according to their origin of acquisition, information that is still used to guide treatment decisions [37].
In this study, previous antibiotic use was also associated with MDRO. These data corroborate that the transmission of infection to patients via health workers and the irrational use of antibiotics are preventable aetiological factors for acquired colonisation [38].
CONCLUSION
Considering our population, we concluded that MDRO ICU colonisation was variable and similar to other centres. Crucial factors, including previous hospital stay and antibiotic use, were closely related to MDRO colonisation. Therefore, control interventions should reduce MDRO colonisation rates, mainly in view of the particularities of each geographic centre. Moreover, the rational use of antibiotics and increased hygiene compliance may reduce mortality in ICU patients, especially in developing countries.
LIST OF ABBREVIATIONS
| MDROs | = Multidrug-Resistant Organisms |
| VRE | = Vancomycin-Resistant Enterococci |
| GNB | = Gram-Negative Bacilli |
| BSIs | = Bloodstream Infections |
| MRSA | = methicillin-resistant Staphylococcus aureus |
| ESBL | = Extended-spectrum Betalactamase producers |
| ICU | = Intensive Care Unit |
| TSB | = Tryptic Soy Broth |
| VRSA | = S. aureus with intermediate resistance to Vancomycin |
| Cbpnm | = Carbapenem |
| CA-MDRO | = Community-Associated MDRO Patients |
| HA-MDRO | = Hospital-Associated MDRO Origin |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the National Ethics Committee - Brazil Platform, Brazil (approval number: 2.155.862).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial, or otherwise.
ACKNOWLEDGEMENTS
Declared none.


