All published articles of this journal are available on ScienceDirect.
Investigating the Resurgence of Malaria Prevalence in South Africa Between 2015 and 2018: A Scoping Review
Abstract
Background:
Malaria remains a serious concern in most African countries, causing nearly one million deaths globally every year. This review aims to examine the extent and nature of the resurgence of malaria transmission in South Africa.
Methods:
Using the Arksey and O'Malley framework, this scoping review includes articles published between the years 2015 and 2018 on the resurgence of malaria occurrence in South Africa. Articles were searched between October 2018 to January 2019 using the following electronic databases: CINAHL, Pubmed, Science Direct and SCOPUS. Grey literature from Google Scholar was also hand searched. Key search terms and subject headings such as climate variables, climate changes, climatic factors, malaria resurgence, malaria reoccurrence and malaria increase over epidemic regions in South Africa were used to identify relevant articles. Three independent reviewers performed the selection and characterization of articles, and the data collected were synthesized qualitatively.
Results:
A total number of 534 studies were identified. Among these, 24 studies met the inclusion criteria. The results were grouped by factors (four main themes) that influenced the malaria resurgence: Climatic, Epidemiological, Socio-economic, and Environmental factors. Climatic factors were found to be the major factor responsible for the resurgence of malaria, as more than 55% of the selected articles were climate-focused. This was followed by epidemiological, socio-economic and environmental factors, in that order. Grey literature from Google Scholar yielded no results.
Conclusion:
This study shows that malaria transmission in South Africa is more associated with climate. Climate-based malaria models could be used as early warning systems for malaria over the epidemic regions in South Africa. Since epidemiological factors also play significant roles in malaria transmission, regular and unrelaxed use of Indoor Residual Spraying (IRS) should be encouraged in these regions. Individuals should also be educated on the importance and the usefulness of these deliveries. While some studies have indicated that the vectors have developed resistance to insecticides, continuous research on developing new insecticides that could alter the resistance are encouraged. Furthermore, all efforts to eradicate malaria in South Africa must also target malaria-endemic neighbouring countries.
1. INTRODUCTION
Malaria continues to be a major threat in most African countries in the south of the Sahara, claiming a significant number of lives every year [1]. Although its burden has declined recently in sub-Saharan countries due to improvement in prevention, diagnosis and treatment [1, 2], the region still carries the largest share of the global malaria burden [1]. In South Africa, malaria-endemic is mainly found in three epidemic provinces, namely; Limpopo, Mpumalanga and KwaZulu-Natal [3, 4]. The vector mainly responsible for malaria transmission in South Africa is the female Anopheles mosquito (An. arabiensis and An. funestus), which transmit Plasmodium falciparum [1-7]. In sub-Saharan Africa, three Anopheles species, Anopheles gambiae, Anopheles arabiensis and Anopheles funestus are the major vectors responsible for malaria transmission. The first two species are considered to be the most effective malaria vectors and are classified under the An. gambiae complex group [1]. Also, An. arabiensis and An. funestus live in sympatry in South Africa living [5].
Malaria transmission over the South African region is seasonal, with the greatest risk between September and May, with peaks in January and April [5]. It is well established that weather fluctuations significantly affect not only the life expectancy or completion of the life-cycle of the mosquito but also the development of the sporogonic stages of the malarial parasite within the mosquito’s body [8, 9]. The biting rate and gonotrophic processes are also temperature-dependent [8-10]. For these reasons, a qualitative relationship between the vector abundance and the climate variables could help identify the peaks of the vector population through meteorological monitoring and forecast [11, 12]. As part of the Malaria Elimination Strategic Plan (MESP), South Africa and a few other African countries are working towards the eradication of malaria since the major outbreak in 2000 across all the epidemic provinces [3, 4].
Several control activities and interventions have been put in place across the endemic provinces. For instance, efforts are currently ongoing to increase the coverage of Indoor Residual Spraying (IRS) to over 85% for successful control of the vectors (An. funestus near zero and low density of An. arabiensis), annually by spray teams [3, 4]. Although these activities significantly reduced malaria transmission from 2004 through 2014, the nation recently experienced a noticeable resurgence between 2015 and 2018 across the three epidemic provinces [5-11].
The recent resurgence spurred several studies to investigate the possible factors influencing malaria transmission over the nation. These studies established that malaria parasites are very sensitive to climatic conditions [12-15], with the resulting epidemics strongly dependent on climate variability. Also, since mosquitoes thrive better in warm moist environments, there is a big concern that the projected global warming may make malaria parasites spread over more regions across the nation, thereby exposing more people to the deadly disease [12-15]. Anopheles arabiensis, one of the main vectors of malaria in South Africa, are more aggressive in the summer and do not lay eggs in winter [16, 17]. Due to climate or other factors, new malaria-transmitting mosquito species are likely to be found. For example, a new mosquito species carrying malaria parasite was recently found in South Africa in the provinces of Mpumalanga and KwaZulu-Natal [18].
Furthermore, other studies have linked malaria transmission in South Africa to factors other than climate. For instance, the transmission has been associated with malaria-drug resistance [19], mosquito resistance to insecticides [20] and socio-economic factors [21]. The current study aims to establish the major factor responsible for the recent malaria resurgence in South Africa between 2015 and 2018.
2. METHODS
In this study, we adapted the Arksey and O’Malley [2] framework for conducting scoping reviews. This framework was considered appropriate for the aim of our study, and has been used in other studies [22, 23]. The framework includes (1) framing of questions for the review; (2) identifying relevant work; (3) assessing the quality of studies; (4) summarizing the evidence; and (5) interpreting the findings. However, steps that we considered irrelevant to this study were omitted.
2.1. Framing of the Question for the Review
The question for the review was framed using PICO (Population- Malaria, Intervention- Factors that are responsible for the resurgence, Comparison- Factors not responsible for the resurgence, and Outcome- Resurgence of malaria) mnemonics [24]. This addressed the factors responsible for the resurgence of malaria prevalence in South Africa between 2015 and 2018.
2.2. Identifying Relevant Studies
The search for relevant studies was conducted by three (3) reviewers using four (4) databases: CINAHL, PubMed, Science Direct and SCOPUS, while grey literature was searched using Google Scholar. Terms that described climate variables combined using the Boolean operator (e.g. factors) with terms that described malaria resurgence (e.g. malaria reoccurrence) were used. An initial search using all identified keywords and index terms were undertaken across all included databases. The text words contained in the title and abstract, and in the index and medical subject heading (MeSH) terms were used to ensure that all relevant materials were captured. Secondly, the reference list of all identified articles was searched for additional studies to identify studies that could not be located through this search strategy.
After key concepts were identified and databases selected, search terms were combined with Bloom operators and searches were run in selected databases. The articles were imported to Endnote reference manager, where titles and abstracts were screened and full-text articles were reviewed for eligibility based on the inclusion criteria (PICO). A grey literature search using Google Scholar with keywords and search terms was also conducted. We used the “sort by relevance ranking” within Google Scholar to bring the most relevant results to the top of the list. We further set the page numbers to be screened to the first ten pages of each search result. This strategy ensures that the most relevant results are captured and also that a reasonable amount is screened. The results were further screened using the inclusion criteria for the review.
2.3. Selection of Studies Included
2.3.1. Inclusion Criteria
- Articles focused on malaria epidemic regions in South Africa between 2015 and 2018.
- Phenomenon of interest/Outcome: This review focused on malaria resurgence, malaria re-occurrence or malaria increase.
- Type of study: This review considered empirical studies, but not limited to designs such as non-experimental observational studies, descriptive studies and case studies conducted in South Africa.
- The search included published articles from 2015-2018 in South Africa on malaria resurgence during this period.
- The search was restricted to English language papers and studies conducted in South Africa.
2.4. Extraction and Charting of Data from Included Studies
We reviewed articles for inclusion, manually extracted data from the selected articles using Microsoft Excel and summarized in a tabular form. Extracted data included: authors, article title, year of publication, study location, study population, aims of the study, methodology/instruments, outcome measure, and importance of the result (Supplementary Table). The table enabled the identification of differences, similarities and common themes in the studies. The extracted data were synthesized, summarized and presented in a narrative summary.
3. RESULTS AND DISCUSSION
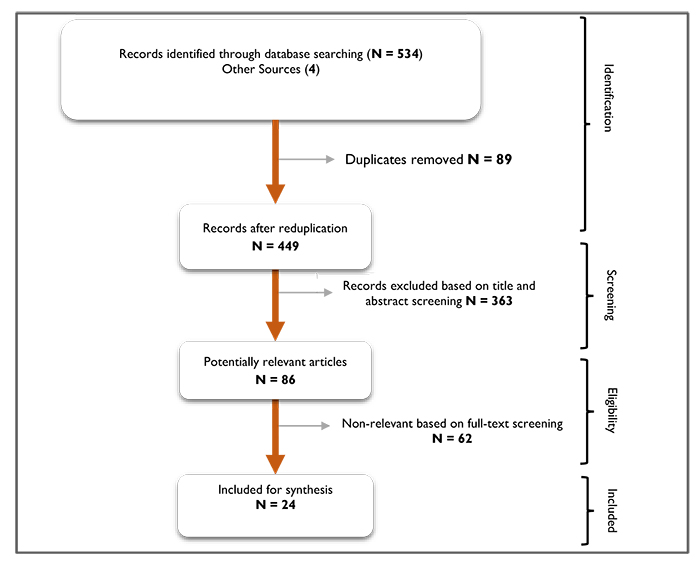
The initial 534 records and 4 from other sources are highlighted (Fig. 1). After duplicates were removed, 449 records were left. A further 363 records (irrelevant) were excluded based on the inclusion criteria discussed above. A total of 86 potentially relevant articles after the title and abstract screening were screened for full-text, of which a further 62 were excluded. Finally, 24 papers were left for review and any further citation search yielded no new articles.
3.1. Climatic Factors
With various approaches, malaria transmission in South Africa has been linked to climatic conditions. For instance, 14 studies (58.3%) out of 24 presented in supplementary table highlighted the significant roles that climate plays on the resurgence. Although some of the 14 studies also mentioned other factors, the emphasis was more on climate. However, climatic factors affecting malaria transmission in South Africa cannot be generalized across the epidemic regions [5]. For instance, the temperature seems to be the major factor in KwaZulu-Natal province, as highlighted in 4 of the 14 studies [25-28]. However, two of the four studies [25, 27] also acknowledge the importance of rainfall on malaria transmission over the province. Rainfall seems more significant on malaria resurgence in Limpopo than temperature as indicated by four other studies [5, 6, 29, 30]. Also, two of the four likewise pointed to the significance of temperature [5, 30]. In addition, Behera et al. [30] attributed malaria resurgence over the province to El Niño/La Niña and sea surface temperature (SST) from the south-western Indian Ocean. We found only three studies [5, 11, 31] linking the resurgence to the climate in Mpumalanga province over the study period. Kapwata and Gebreselassie [31] and Adeola et al. [5] believe that transmission over the province is associated with surface land temperature and other non-climatic factors, while Abiodun et al. [11] found that rainfall and relative humidity are more significant.
Similar findings were established over some South African neighbouring countries such as Zimbabwe [32, 33], Mozambique [34, 35], Botswana [36] and the East African highlands [37-39].
It is established that rainfall contributes to the proliferation of mosquito breeding sites, while temperature contributes to the development of the sporogonic stages of the malaria parasite within the mosquito’s body. The biting rate and gonotrophic processes are also temperature-dependent [8-11].
3.2. Epidemiological Factors
Half of the 24 papers highlight the importance of mosquito abundance on malaria resurgence across the epidemic regions in South Africa [5, 11, 18, 25-28, 40-44]. For example, using climate-based mathematical models, the impact of mosquito (mainly An. arabiensis) population dynamics on malaria transmission was investigated over KwaZulu-Natal [25, 26, 28, 40, 43], Limpopo [28] and Mpumalanga provinces [11]. Other studies also confirm that the vector population correlates with the resurgence [5, 42, 44]. More importantly, the resurgence has been traced to the arrival of new mosquito species across the epidemic regions [18, 45]. Under suitable conditions, mosquitoes bite more aggressively to produce more eggs, and in the process, transmit malaria faster than usual.
Similarly, the importance of mosquito abundance on malaria transmission has been investigated in Zimbabwe [46, 47], Mozambique [48, 49] and Namibia [50].
3.3. Socio-economic Factors
Six (25%) out of the 24 selected studies connected malaria resurgence in South Africa with socio-economic factors [6, 41, 51-54]. Migration from neighbouring countries for socio-economic reason was found to be a major strain on malaria control in Limpopo [5, 6] and KwaZulu-Natal provinces [41]. According to Adeola et al. [55], imported malaria cases account for about 26% of total malaria cases recorded in Limpopo from 1998-2017. This is largely due to the cross-border movement of farmworkers, mainly from Zimbabwe and Mozambique to South Africa, who work on the large commercial farmers in Limpopo and Mpumalanga [5, 56]. Similarly, malaria is highly regarded as a disease of poverty. A recent study by Lowe et al. [56] indicated that there is a significant correlation between malaria and economic status, particularly income and housing conditions. Most researchers and stakeholders working on malaria in South Africa believe that South Africa’s 2018 malaria elimination target was not realistic due to lack of new tools, resources and the capacity to fight malaria; coupled with poor cross-border collaborations; overreliance on partners to implement; poor community involvement; and poor surveillance [51, 52]. Inadequate communication channels on malaria control, such as Sterile Insect Technique (SIT), also play significant roles in malaria transmission [54]. Practicing animal husbandry, residing in household structures that had not been sprayed were found to be associated with malaria infection over KwaZulu-Natal province [41]. It has also been established that the odds of malaria infection are lower in modern, improved housing compared to traditional housing in sub-Sahara Africa [53]. The impacts of socio-economic factors on malaria transmission have also been highlighted in Malawi [57] Gambia [58] and Ghana [59].
3.4. Environmental Factors
Four studies (16.7%) linked malaria transmission to environmental factors. A high correlation was reported between malaria cases and derived environmental metrics such as the Normalized Difference Water Index (NDVI) [5, 31] Normalized Difference Water Index (NDWI) and altitude, water body and irrigated land [5, 60] in Mpumalanga province. Furthermore, Malahlela [42] concluded that malaria resurgence in Limpopo province is traceable to vegetation moisture and vegetation greenness, which are proxy/surrogate for rainfall. The availability of an adequate amount of rainfall for the provision of ideal environmental conditions (such as open water, green vegetation) provides suitable breeding and resting places for the major vector. We found no study in this regard over KwaZulu-Natal within the study period. Environmental factors such as vegetation index, waterbody, among others have been similarly linked to malaria incidence in Kenya [61], Zimbabwe [62, 63], Botswana [12] and Eritrea [64]. The significant correlation of the high values of vegetation index and water body with malaria incidence is strongly associated with rainfall.
In general, climate variables play major roles in malaria transmission across all the epidemic provinces (Limpopo, Mpumalanga and KwaZulu-Natal). For instance, four of the papers suggested that both temperature and rainfall are responsible for the transmission in KwaZulu-Natal, while two suggested similar findings over Limpopo. However, three additional papers concluded that the impact of temperature on the transmission is more significant than that of rainfall on the KwaZulu-Natal and Mpumalanga provinces. This implies that the effect of temperature is more pronounced over the two provinces. Rainfall is seen to be more significant than temperature over Limpopo province.
CONCLUSION
The inclusion criteria above show that climate variables (mainly, rainfall, temperature and relative humidity) are mostly mentioned but not solely responsible for the malaria resurgence in all the epidemic regions in South Africa. Environmental and socio-economic factors were also mentioned as vital elements to be considered in order to understand the dynamics of malaria prevalence. Consequently, some preventive measures and interventions are suggested to minimize and gradually eradicate malaria from these regions.
Therefore, malaria models that integrate all variables could be used to monitor the progression of malaria and assist in intervention and prevention efforts with respect to malaria. These models could be implemented for developing methodologies necessary to detect malaria pathogen, vector and habitat preference. More importantly, climate-based malaria models should be used as a framework for strengthening the institutional capacity of malaria surveillance by providing malaria-risk management based on climate information and early warning system.
Regular and unrelaxed use of Indoor Residual Spraying (IRS) should be encouraged over epidemic regions in South Africa. Sufficient insecticides-impregnated bed nets should also be supplied to the communities in these regions, and more importantly, individuals should be educated on the importance and the usefulness of these deliveries.
Furthermore, since it has been established that most malaria cases found in South Africa are imported from neighbouring countries [6], it is important to strengthen cross-border control of malaria to minimize its spread. All goals to eliminate malaria in South Africa must also target the epidemic neighbouring countries (Zimbabwe, Mozambique, Botswana and Namibia).
AUTHORS' CONTRIBUTIONS
All authors made substantial contributions to study design, including the development of systematic review procedures. GJA, BA and ROA conducted the literature search and data extraction and independently assessed the methodological quality of included studies and conducted the analysis. OO, KEO, AMA and VJS adjudicated in any disagreements in methodological quality assessments and contributed to the analysis. GJA, BA, ROA, OSM and RDD drafted the original manuscript while KOO, KYN, PJW and AA critically reviewed the manuscript for important intellectual content. All authors have given approval of this final version to be published and agree to be accountable for all aspects of the work.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by the University of Pretoria Institute for Sustainable Malaria Control (UP ISMC) and Malaria Research Control (MRC) collaborating centre for malaria research.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the Expert Advisory Panel and Systematic Review Team for their guidance and assistance in the completion of this scoping review.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers website along with the published article.


