All published articles of this journal are available on ScienceDirect.
Significant Association between Physical Performance and Inflammatory Biomarkers in Older Adults with Frailty in Rural Thailand
Abstract
Background:
The etiology of frailty is complex and incompletely understood, and is associated with alterations in the immune system, resulting in chronic low-grade inflammation. However, few studies have explored the inflammatory biomarkers related to physical performance in the elderly.
Methods:
We evaluated the prevalence of frailty with a cross-sectional study among older adults in rural communities in Thailand (n = 457, mean age of 71.4 ± 5.8 years) with Fried’s frailty phenotype including five criteria: weight loss, exhaustion, slowness, weakness, and inactivity. The association between inflammatory biomarkers (serum interleukin-6, IL-6 and C-reactive protein, CRP levels) and physical performance (grip strength, walk times and VO2Max) was examined in frail participants (n=64).
Results:
The prevalence of frailty and pre-frailty in this population was 37.4% (95% CI, 32.9-42.0) and 54% (95% CI, 49.4-58.7). Multiple linear regression analysis found that serum IL-6 level was significantly elevated in frail older adults with low grip strength (beta = -0.348, SE= 0.155, p = 0.029). Serum CRP level was also elevated significantly in frail older adults with low grip strength (beta = -0.049, SE= 0.023, p = 0.04) and low VO2Max (beta = -0.047, SE= 0.019, p = 0.016) after adjustment for sex, age, hypertension, diabetes, osteoporosis, heart disease, and BMI.
Conclusion:
Our findings support a low level of grip strength as predictor of inflammatory biomarkers in older adults with frailty. Primary care practitioners could use frailty indicators and performance combined with serum biomarkers for early health risk detection in older adults.
1. INTRODUCTION
In a report from a survey of older people in 2017, Thailand (NSO) showed that the number of elderly has increased to 11.23 million or 17.13 percent of the total population in 2017, and it will become an aging society in 2021 when the number will rise to 13.1million or 20 percent of the total population [1, 2]. Thailand is a developing country where government support, employer-provided pensions, health care and various support services are not widely available or sufficiently funded. Thai older adults in rural areas have less opportunity to receive health care when compared with urban areas [3]. The main factors that affect the health of older adults in a rural area are low education, low income, and agriculture occupation before retirement [3-5].
Aging-related changes in skeletal muscle result in a decline in physical function, physical performance, physical activity, and an increased risk of adverse health outcomes [6]. Frailty is a geriatric syndrome associated with aging and increased vulnerability to stressors, which result in reduced physiological reserves and deregulation of multiple systems. The adverse health outcomes of frailty are poor functional and cognitive status, falls, institutionalization, and mortality [7-9]. Studies on the prevalence of frailty worldwide have shown a range of frailty in Spain between 3-37% [10]; associated factors include being female and advanced age [11]. China found high frailty prevalence in rural areas with low-level of education and sedentary behavior [12]. Frailty affects long-term care costs, rising health costs and adverse health outcomes [13, 14]. A few studies on the prevalence of frailty in Thailand reported a range of frailty between 8-22% [15, 16]. Other studies showed the cause of frailty to be based on the interplay of genetic, biological, physical, psychological, social, and environmental factors [8, 9, 17, 18]. Elders with other confounding factors such as dependency, chronic disease or complex medical or psycho-social problems are at increased risk of frailty [19].
Frailty has been linked to several pathophysiological factors, including oxidative stress, mitochondrial dysfunction, and cellular senescence. Moreover, dysregulation of inflammatory processes seems to be one of the possible reasons for the development of frailty [20]. According to this inflammation hypothesis, chronic low levels of pro-inflammatory and inflammatory cytokines have been associated with the development of frailty. Pro-inflammatory cytokines may influence frailty either directly by promoting protein degradation, or indirectly by affecting important metabolic pathways. A direct association between frailty and elevated levels of inflammation has been observed in the regulation of muscle protein turnover [21]. Increasing levels of proinflammatory cytokines represent a common feature of several pathophysiological processes leading to muscle loss. Sarcopenia has been proposed as a biological change in frailty. Previous studies highlighted a strong relationship between frailty and inflammation of elevated serum interleukin-6 (IL-6), IL-1, tumor necrosis factor-a (TNF-α), and C-reactive protein (CRP) levels, which are related to impaired function and mobility [16, 22, 23]. Circulating IL-6 levels which are released from circulating macrophages and infiltrating adipose tissue have long been known to increase in older adults and in several pathophysiological processes such as atherosclerosis, osteoporosis and sarcopenia. Raised IL-6 levels are associated with functional decline, disability and mortality in older adults [24]. Moreover, elevated serum CRP levels have been recognized to be associated with increased vulnerability to mortality in frail older individuals [25, 26]. Therefore, inflammatory mediators such as IL-6, CRP and TNF-α might be used as biomarkers and could form a model of the decreased physical functions and physiological processes contributing to frailty.
The etiology of frailty is complex and incompletely understood. Although a number of clinical frailty assessments (e.g., Fried Frailty Phenotype, Rockwood Frailty Index) are widely available and clinically useful, their main limitations are the subjectivity and lack of clinimetric properties as evaluation outcome measures, particularly responsiveness to intervention [27, 28]. To overcome these shortcomings, recent studies have focused on the identification of frailty biomarkers which not only could be used to indicate the efficacy of an intervention but also to identify the frailty mechanisms. Frailty biomarkers are promising to aid diagnosis and intervention. However, few studies have addressed inflammatory biomarkers related to inactivity and a decrease in physical function and performance in an aging population with frailty. Moreover, in Thailand, little information is available regarding the physical frailty phenotype and its determinants, especially in rural areas that have the highest proportion of the aging population. These results would provide early detection of geriatric syndromes and primary prevention could be implemented. This study aimed to determine an association between physical performance (grip strength, walk times and VO2Max) and inflammatory biomarkers (IL-6 and CRP) as the predictor of an inflammatory biomarker in frail older adults and also to determine the prevalence of frailty and associated factors among community-dwelling older adults in rural Thailand.
2. MATERIALS AND METHODS
2.1. Study Design and Participants
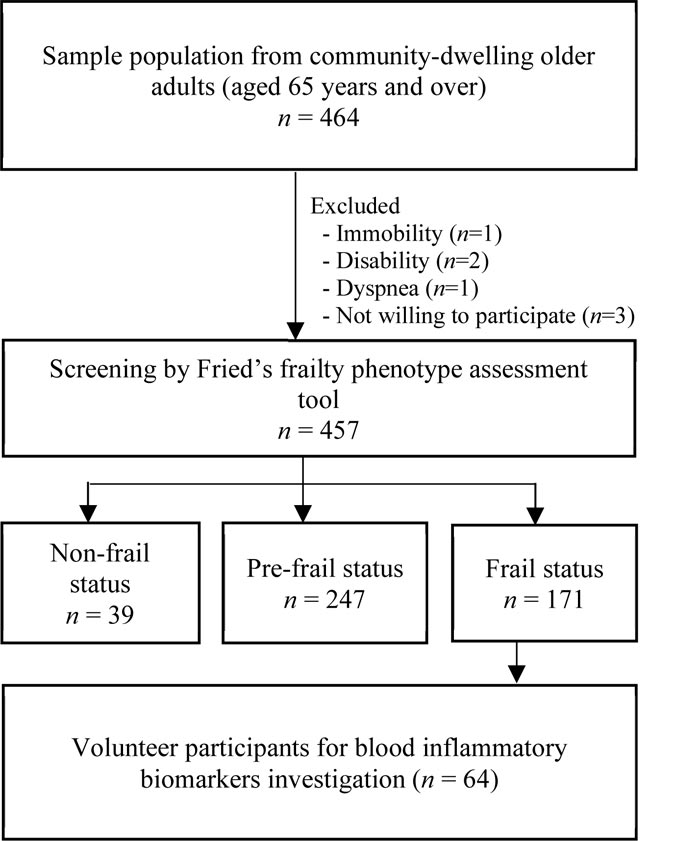
A cross-sectional study was conducted with the recruitment of 755 older people aged 65 years and over who were community-dwelling adults living in Maeka Sub-district, Phayao Province, Northern Thailand. We estimated the sample size using n4studies application to estimate the Infinite population with the standard formula [29]. We used the expected proportion (p) with regard to the frailty prevalence of a previous study in Thailand of 14.0% [30]. A total of 464 participants were recruited to measure frailty with a precision of 5% and an error(d) of 0.02.
In the first step, eight villages out of 18 in the municipality of Maeka were selected by simple random sampling to represent rural communities. We used the list of the older population from the primary care center records for sample selection using simple random sampling. Four hundred and fifty-seven participants were available at the time of data collection. The inclusion criteria were older adults residing in the sampled villages for at least one year who agreed to participate in this study. The exclusion criteria were immobility, disability, dyspnea, psychological and neurological problems, severe illness, and dementia (Mini-Mental State Examination-Thai version-MMST10 less than 10) [31].
We also screened the frailty in the older population with the Fried Frailty Phenotype [7]. There were 171 participants who were defined as frail. They were invited to participate in a serum inflammatory biomarker assessment. Sixty-four were willing to participate. They were selected to investigate associations between physical activity, physical performance, and inflammatory biomarkers. The sample selection diagram is presented in Fig. (1).
2.2. Measurement
The questionnaires were administered in a face-to-face interview by trained staff. The three sections of the interview were as follows: (i) sociodemographic characteristics (gender, age, marital status, education level, income, and living status); (ii) health conditions (comorbidities; number of medications used; reported disease diagnosis such as hypertension, diabetes, osteoporosis, and heart disease; self-health rate, and body mass index-BMI). BMI was compared with weight (in kilograms) to height (in meters squared). The BMI cutoff for Asians is BMI ≥25 as obese, BMI 23-24.9 as overweight, BMI 18.5-22.9 as healthy, and BMI <18.5 as underweight.
2.2.1. Measurement of Frailty
Frailty was measured based on the CHS criteria for five components and was divided into three categories: frail, pre-frail, and non-frail. Participants who met 3 to 5 of the criteria were defined as frail, 1 to 2 criteria defined as pre-frail, and those who did not meet the criteria were defined as non-frail [7]. The components included:
- (1) Weight loss was evaluated by participants’ self-reporting regarding their unintentional weight loss of more than 4.5 kg in the previous year.
- (2) Exhaustion was assessed by the self–report of participants with two questions: “I felt that everything I did required effort” and “I could not get going”. Then, the participants were asked how often in the last week he/she felt this way.
- (3) Slow walking speed was measured by the number of times participants walked 4.5 meters as fast as possible, with or without a walking aid. Then the cutoff was stratified by sex and height. Those participants with a waking time of six seconds or more were classified as having slow walking speed.
- (4) Weakness was measured by grip strength three times on the participant's dominant side using a digital handgrip dynamometer (T.K.K. 5401, Takei Scientific Instruments Corporation, Tokyo, Japan). We used the mean of grip strength (kg) value to determine the weakness based on sex and BMI. Those participants who were male having a grip strength of 30 kg or less were classified as weak and female participants with 18 kg or less were classified as weak.
- (5) Low physical activity was measured using the Global Physical Activity Questionnaire (GPAQ) [32]. Men with a physical activity per week <383 Kcal and women with physical activity per week <270 Kcal were classified as frail.
2.2.2. Measurement of VO2 Max
VO2Max was assessed by the six-minute walk test (indirect calculation method) [33]. In this test, the participants were asked to walk as fast as possible in six minutes throughout the test. A 30-meter distance was prepared (15 meters out and 15 meters back). Blood pressure measurements were taken after walking came to an end and recorded for RPP calculation. Participants were permitted to use a walker or cane if needed, while the observer recorded symptoms. The formula of VO2Max was calculated as follows:
VO2Max(ml.kg-1 .min-1) = [0.02 * distance (m)] – [0.191 * age (yr)] – [0.07 * weight (kg)] +
[0.09 * height (cm)] + [0.26* RPP(* 10-3)] + 2.45
m = distance in meters; y = year; kg = kilogram; cm = centimeter
RPP = rate pressure product (HR * systolic BP in mmHg)
2.2.3. Measurement of Serum Interleukin-6 and C-reactive Protein Levels
Peripheral blood samples were collected by venipuncture and transferred to serum separator vacutainer tubes. Blood samples were allowed to clot for 60 min and then centrifuged for 10 min at 1,500x g. Serums were aliquoted and stored frozen at −80°C until analysis. Serums were not subjected to any freeze-thaw cycle. The assays were performed in duplicate for all cytokine quantifications.
Serum interleukin-6 (IL-6) and C-reactive protein (CRP) levels were determined by the sandwich enzyme-linked immunosorbent assay (ELISA) method, with the human IL-6 ELISA MaxTM Set Deluxe Kits (BioLegend, San Diego, CA, USA) and the human CRP ELISA (Hycult Biotech, Uden, the Netherlands) commercial kits, respectively. The assay protocol was accomplished according to the manufacturers’ instructions. The lower detection limit was 4 pg/ml for IL-6 and 5 ng/ml for CRP. The detection range was 7.8-500 pg/ml for IL-6 and 5-100 ng/ml for CRP.
2.3. Statistical Analysis
SPSS version 26.0 (IBM, New York, NY, USA) was used to perform all data analysis. The descriptive statistics of all variables were calculated and continuous variables were expressed as mean ± SD, while categorical variables were presented as number and percentage. For comparison between variables, the Chi-square test and Fisher’s exact test were used to analyze categorical data. Pearson’s correlation coefficient was used to analyze continuous data. For the adjusted model, Multinomial logistic regression analysis was applied to analyze the associations of risk factors with frailty, taking the non-frail group as the reference category. Preprocessed data for multiple linear regression analysis, variables, i.e., CRP, walking speed, physical activity, were transformed to the common logarithm (log10) to adjust for normality of the distribution, which was verified by means of kurtosis tests. Multiple linear regression analysis was used to identify the associations between physical performance and inflammatory biomarkers. Two adjusted models were created and a stepwise method was used for selected variables. A p-value of less than 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Assessment of Fried’s Frailty in Community-dwelling Older Adults
Of the 457-study population, in the frailty assessment, 37.4% were found to be frail and 54.0% pre-frail. The prevalence of frailty in females was higher than males (42.6%and 30.8%), whereas the prevalence of pre-frailty in males was higher than females (61.2%in males and 48.4% in females). We further analyzed the frailty indicators, and the results showed that the participants had a high prevalence of weakness (73.1%) and low physical activity (71.6%). When compared between males and females, there was a significant difference in weakness, slow walking speed, and low physical activity (p<0.01). Females had a significantly higher prevalence of slow walking speed and low physical activity than males (p<0.001 and p<0.01, respectively). Males had a significantly higher prevalence of weakness than females (p<0.001) (Table 1).
| Indicators | Prevalence %, 95%CI | p-Value | ||
|---|---|---|---|---|
| Overall(n=457) | Male(n=201) | Female(n=256) | ||
| aFrailty status | ||||
| Frailty | 171 (37.4), 32.9-42.0 | 62(30.8), 24.5-33.7 | 109(42.6),36.4-48.9 | 0.05* |
| Pre-frailty | 247(54.0),49.4-58.7 | 123(61.2), 54.1-68.0 | 124(48.4),42.2-54.7 | |
| Non-frailty | 39(8.6), 6.1-11.5 | 16(8.0), 4.6-12.6 | 23(9.0), 5.8-13.2 | |
| aFrailty indicators | ||||
| Weight loss | 38(8.3), 7-10.8 | 16(7.9), 4.1-11.7 | 22(8.6), 5.1-12.0 | 0.81 |
| Exhaustion | 130 (28.4), 24.3-32.6 | 48(23.8), 17.9-29.8 | 82(32.0), 26.3-37.7 | 0.05* |
| Weakness | 334(73.1), 69.0-77.1 | 171(85.1), 80.1-90.0 | 163(63.6),57.7-69.6 | 0.001** |
| Slow walking speed | 141(30.9), 26.6-35.1 | 36 (17.9), 12.5-23.2 | 105(41.0),34.9-47.1 | 0.001** |
| Low physical activity | 327(71.6), 67.4-75.7 | 132(65.6), 59.0-72.3 | 195(76.1),70.9-81.4 | 0.01* |
|
bGrip strength (kg), mean±SD |
19.56±7.11 | 23.63±6.69 | 16.31±5.53 | 0.01* |
|
bWalking speed (sec), mean±SD |
6.59±2.90 | 5.83±2.48 | 7.21±3.06 | 0.001* |
|
bPhysical activity (Kcal), mean±SD |
1,851±4,353.57 | 2,628.94±5,551.86 | 1,241.52±3,030.35 | 0.001** |
3.2. Characteristics of Community-dwelling Older Adults According to Frailty Status
The characteristics of community-dwelling older adults living in the rural area according to frailty status are shown in Table 2. 89.3% of the participants had a companion or spouse staying with them, 64.5% were married and 56% were female. The mean age was 71.4 ± 5.8 years and the educational level was mainly elementary level (74.8%). Most individual incomes were less than 1,000 baht (approx $30US) per month (77.2%). The mean number of comorbidity of participants was 1.1 ± 0.9. The most prevalent comorbidity of participants was hypertension at 42.2%; 65.6% of the participants reported good health. We found that gender, age, marital status, education level, living status, individual income, self-reported medical diagnosis (hypertension and osteoporosis), number of medications used and BMI, were associated with frailty status (p <0.05).
| Characteristics |
Overall (n=457) |
Non-frail (n=39), n (%) |
Pre-frail (n=247), n (%) |
Frail (n=171), n (%) |
p-Value |
|---|---|---|---|---|---|
| aGender | |||||
| Male | 201 (44.0) | 16 (8.0) | 123 (61.2) | 62 (30.8) | 0.05* |
| Female | 256 (56.0) | 23 (9.0) | 124 (48.4) | 109 (42.6) | |
| bAge (y), Mean±SD | 71.4±5.8 | 68.8±4.3 | 70.3±4.7 | 74.0±6.9 | |
| 65-74 | 287 (62.8) | 36 (12.5) | 179 (62.4) | 72 (25.1) | <0.001** |
| 75-84 | 131(28.7) | 3 (2.3) | 58 (44.3) | 70 (53.4) | |
| 85 and above | 39 (8.5) | 2 (5.1) | 8 (20.5) | 29 (74.4) | |
| aMarital Status | |||||
| Single | 69 (15.1) | 3 (4.4) | 33 (47.8) | 33 (47.8) | 0.004** |
| Married | 295 (64.5) | 22 (7.5) | 175 (59.3) | 98 (33.2) | |
| Widow/divorced/separated | 93 (20.3) | 14 (15.1) | 39 (41.9) | 40 (43.0) | |
| aEducation Level | |||||
| No school | 88 (19.2) | 4 (4.5) | 33 (37.5) | 51 (58.0) | <0.001** |
| Elementary school | 342 (74.8) | 33 (9.7) | 195 (57.0) | 114 (33.3) | |
| High school and above | 27 (5.9) | 2 (7.4) | 19 (70.4) | 6 (22.2) | |
| aLiving Status | |||||
| Lives alone | 49 (10.7) | 4 (8.2) | 18 (36.7) | 27 (55.1) | 0.022* |
| Has a companion | 408 (89.3) | 35 (8.6) | 229 (56.1) | 144 (35.3) | |
| aIndividual Income (Baht/month) | |||||
| < 1,000 | 353 (77.2) | 21 (6.0) | 184 (52.1) | 148 (41.9) | <0.001** |
| 1,000 – 5,000 | 79 (17.3) | 13 (16.5) | 49 (62.0) | 17 (21.5) | |
| > 5,000 | 25 (5.5) | 5 (20.0) | 14 (56.0) | 6 (24.0) | |
| bNo. of comorbidities, Mean±SD | 1.1±0.9 | 0.6±0.7 | 1.0±0.9 | 1.3±0.9 | <0.001** |
| aSelf-reported Medical Diagnosis | |||||
| Hypertension | 193 (42.2) | 7 (3.6) | 105 (54.4) | 81 (42.0) | 0.004* |
| Diabetes | 54 (11.8) | 4 (7.4) | 30 (55.6) | 20 (37.0) | 0.942 |
| Osteoporosis | 48 (10.5) | 2 (4.2) | 18 (37.5) | 28 (58.3) | 0.006* |
| Heart disease | 39 (8.5) | 2 (5.1) | 18 (46.2) | 19 (48.7) | 0.283 |
|
bNo. of Medications used Mean±SD |
2.5±3.1 | 1.4±2.9 | 2.4±3.0 | 2.8±3.1 | |
| 0-1 | 229 (50.1) | 29 (12.6) | 127 (55.5) | 73 (31.9) | <0.001** |
| 2-3 | 106 (23.20) | 4 (3.8) | 58 (54.7) | 44 (41.5) | |
| >3 | 122 (26.7) | 6 (5.0) | 62 (50.8) | 54 (44.2) | |
| aSelf-health Rate | |||||
| Good | 300 (65.6) | 30 (10.0) | 169 (56.3) | 101 (33.7) | 0.151 |
| Fair | 33 (7.2) | 1 (3.0) | 15 (45.5) | 17 (51.5) | |
| Poor | 120 (26.2) | 8 (6.7) | 62 (51.6) | 50 (41.7) | |
| bBMI+(kg/m2), Mean±SD | 21.5±4.9 | 22.2±3.4 | 21.9±4.7 | 20.8±5.3 | |
| 18.5 – 22.9 (Normal weight) | 103 (22.5) | 4 (3.9) | 53 (51.5) | 46 (44.6) | 0.040* |
| < 18.5 (Underweight) | 186 (40.7) | 10 (5.4) | 98 (52.6) | 78 (42.0) | |
| 23 – 24.9 (Overweight) | 58 (12.7) | 4 (6.9) | 36 (62.0) | 18 (31.1) | |
| ≥ 25 (Obese) | 106 (23.2) | 12 (11.3) | 66 (62.3) | 28 (26.4) | |
3.3. Association between Risk Factors and Frailty Status among Community-dwelling Older Adults
Multinomial logistic regression was carried out to analyze the risk factors associated with frailty status, with a comparison between non-frail, pre-frail, and frail older adults, as shown in Table 3. In a comparison model between pre-frail and non-frail after adjusting for variables, we found that ages over 85 years (OR = 1.60, 95% CI 1.45-8.91) and low income (less than 1000 bahts/month) (OR = 2.58, 95% CI 1.13-18.58) were associated with pre-frailty in older adults. With regard to frailty and non-frailty comparison, ages over 85 years (OR = 2.77, 95% CI 1.18-6.52), low income (less than 1000 baht/month ($30 US approx.)) (OR = 5.46, 95% CI 1.44-20.70), and number of comorbidities (OR = 3.86, 95% CI 1.33-11.17) were associated with frailty among older adults.
| Characteristics | Pre frail vs. Non-frail | Frail vs. Non-frail | ||
|---|---|---|---|---|
| Crude OR (95%CI) | Adjusted OR (95%CI) | Crude OR (95%CI) | Adjusted OR (95%CI) | |
| Age (y) | ||||
| 65-74 | 1 | 1 | 1 | 1 |
| 75-84 | 4.21 (1.97-8.30)* | 0.30 (0.08-1.07) | 3.85 (3.52-8.35)* | 2.30 (0.74-7.19) |
| >85 | 4.44 (1.95-10.11)* | 1.60 (1.45-8.91)* | 5.30 (2.64-5.34)* | 2.77 (1.18-6.52)* |
| Gender | ||||
| Male | 1 | 1 | 1 | 1 |
| Female | 1.94 (0.79-4.02) | 1.15 (0.53-2.49) | 1.09 (0.46-2.46) | 1.07 (0.37-3.18) |
| Marital Status | ||||
| Married | 1 | 1 | 1 | 1 |
| Single | 0.49 (0.20-1.21) | 0.48(0.17-1.28) | 0.74 (0.29-1.15) | 0.44 (0.15-1.24) |
| Widow/divorced/ separated |
0.43 (0.18-0.99)* | 0.34 (0.12-1.11) | 0.91 (0.39-2.10) | 0.49 (0.18-1.32) |
| Individual Income(Baht/month) | ||||
| > 5,000 | 1 | 1 | 1 | 1 |
| 1,000-5,000 | 1.34 (0.40-4.42) | 1.62 (0.41-6.38) | 1.09 (0.27-4.37) | 1.07 (0.20-5.67) |
| < 1,000 | 3.12 (1.02-9.55)* | 2.58 (1.13-18.58)* | 5.87 (1.64-20.95)* | 5.46 (1.44-20.70)* |
| Living Status | ||||
| Has a companion | 1 | 1 | 1 | 1 |
| Lives alone | 0.694 (0.22-2.17) | 0.37 (0.09-1.50) | 1.66 (0.54-5.08) | 0.69 (0.16-2.86) |
| Number of Comorbidities | 2.14 (1.60-2.87)* | 2.25 (0.79 –6.37) | 3.13 (2.31-4.24)* | 3.86 (1.33-11.17)* |
| Hypertension≠ | 3.40 (1.25-9.24)* | 2.10 (0.78-5.66) | 3.85 (1.40-10.59)* | 1.06 (0.38-2.92) |
| Osteoporosis ≠ | 1.02 (0.22-4.65) | 0.83 (0.12-5.56) | 2.36 (0.53-10.52) | 1.04 (0.15-7.08) |
| Number of medications used | ||||
| 0-1 | 1 | 1 | 1 | 1 |
| 2-3 | 0.42 (0.16-1.07) | 0.42 (0.24-7.3) | 1.28 (0.11-0.72)* | 0.53 (0.19-1.49) |
| > 3 | 1.40 (0.37-5.22) | 1.53 (0.68-3.45) | 0.22 (0.32-4.60) | 1.04 (0.45-2.42) |
| BMI+ (kg/m2) | ||||
| Normal weight | 1 | 1 | 1 | 1 |
| Underweight | 1.26 (0.42-3.76) | 1.24 (0.41-3.78) | 1.72 (0.57-5.19) | 1.43 (0.45-4.55) |
| Overweight | 0.55 (0.19-1.61) | 0.57 (0.18-1.74) | 0.67 (0.23-1.99) | 0.62 (0.19-2.04) |
| Obese | 1.54 (0.52-4.59) | 1.84 (0.59-5.68) | 1.21 (0.39-3.72) | 1.24 (0.38-4.07) |
3.4. Characteristics of Community-dwelling Older Adults with Frailty According to Inflammatory Biomarkers
The inflammatory biomarkers assessment (serum IL-6 and CRP levels) of 64 frailty participants is shown in Table 4. Most of the frail older adults were female (60.9%), the mean age was 77.78 (SD = 7.24), and the mean BMI was 21.32 (SD = 3.95). The most common disease was hypertension at 48.4%. The mean IL-6 level was 11.29 (SD ± 6.36)and CRP was 3.94 (SD ± 5.29).
3.5. Association between Physical Performance and Inflammatory Biomarkers in Community-dwelling Older Adults with Frailty
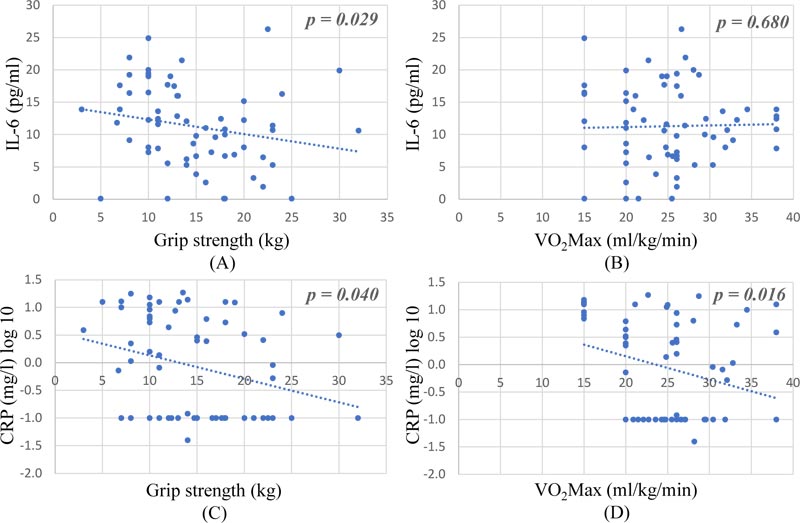
The relationship between physical performance and inflammatory biomarkers (serum IL-6 and CRP levels) among older adults with frailty is shown in Fig. (2). We found that the grip strength was significantly correlated with serum IL-6 level (p = 0.029) and CRP (log10) level (p = 0.040) while the VO2Max was significantly correlated with serum CRP level (p = 0.016) using Linear regressions.
The association between demographics and health characteristics, physical activity, physical performance and inflammatory biomarkers (serum IL-6 and CRP levels) among frail older adults using multiple linear regression is shown in Table 5. We found that grip strength and VO2Max were inversely associated with IL-6 and CRP, respectively. Low grip strength was also associated with a high level of IL-6 and CRP. Moreover, low VO2Max was also associated with a higher level of CRP. When the analyses were further adjusted for BMI (Table 5, Model 2), the magnitude of the coefficients for grip strength and VO2Max was only slightly reduced, and both grip strength and VO2Max remained significantly associated with higher levels of inflammatory biomarkers.
|
Characteristics (n = 64) |
n (%) | Inflammatory Biomarkers | ||
|---|---|---|---|---|
| IL-6 (pg/mL) | CRP (mg/L) | |||
| Overall | 64 (100) | 11.29± 6.36 | 3.94± 5.29 | |
| Age (y) | Mean ± SD | 77.78± 7.24 | ||
| 65-74 | 21 (32.8) | 10.64 ± 6.60 | 2.72 ± 4.27 | |
| 75-84 | 32 (50.0) | 12.46 ± 6.05 | 4.74 ± 5.92 | |
| >85 | 11 (17.2) | 9.12 ± 6.59 | 3.95 ± 5.11 | |
| Gender | Male | 25 (39.1) | 12.30 ± 7.83 | 3.76 ± 5.51 |
| Female | 39 (60.9) | 10.64 ± 5.21 | 4.06 ± 5.22 | |
| Hypertension | Yes | 31 (48.4) | 10.50 ± 6.76 | 3.82 ± 5.31 |
| No | 33 (51.6) | 12.03 ± 5.96 | 4.06 ± 5.35 | |
| Diabetes | Yes | 9 (14.1) | 11.14 ± 4.76 | 2.81 ± 4.27 |
| No | 55 (85.9) | 11.31 ± 6.62 | 4.13 ± 5.45 | |
| Osteoporosis | Yes | 7 (10.9) | 10.65 ± 4.44 | 2.66 ± 4.55 |
| No | 57 (89.1) | 11.37 ± 6.58 | 4.10 ± 5.39 | |
| Heart disease | Yes | 8 (12.5) | 8.12 ± 8.26 | 2.85 ±4.59 |
| No | 56 (87.5) | 11.74 ± 5.99 | 4.10 ± 5.41 | |
| BMI + (kg/m2) | Mean ± SD | 21.32 ± 3.95 | ||
| Normal weight | 32 (51.6) | 13.06 ± 6.24 | 3.61 ± 4.96 | |
| Underweight | 15 (24.2) | 10.95 ± 6.71 | 3.90 ± 6.03 | |
| Overweight | 6 (9.7) | 8.38 ± 4.84 | 2.14 ± 2.24 | |
| Obese | 9 (14.5) | 9.11 ± 5.64 | 5.89 ± 6.33 | |
| Variables (n = 64) | IL-6(pg/mL) | CRPlog10 (mg/L) | ||||
|---|---|---|---|---|---|---|
| B | SE | p-Value | B | SE | p-Value | |
| Model 1 | ||||||
| Grip strength (kg) | -0.382 | 0.156 | 0.018* | -0.045 | 0.023 | 0.057 |
| Walking speed log10 (sec) | -0.969 | 4.480 | 0.830 | -0.033 | 0.669 | 0.961 |
| VO2Max (ml/kg/min) | -0.048 | 0.128 | 0.710 | -0.048 | 0.019 | 0.015* |
| Physical activity log10 (kcal) | -4.663 | 2.313 | 0.049* | -0.072 | 0.345 | 0.836 |
| Exhaustion | 1.837 | 1.724 | 0.292 | 0.085 | 0.257 | 0.743 |
| Model 2 | ||||||
| Grip strength (kg) | -0.348 | 0.155 | 0.029* | -0.049 | 0.023 | 0.040* |
| Walking speed log10 (sec) | -0.076 | 4.448 | 0.986 | -0.139 | 0.670 | 0.836 |
| VO2Max (ml/kg/min) | -0.052 | 0.126 | 0.680 | -0.047 | 0.019 | 0.016* |
| Physical activity log10 (kcal) | -3.940 | 2.323 | 0.096 | -0.158 | 0.350 | 0.653 |
| Weight loss | 2.285 | 2.079 | 0.277 | -0.076 | 0.313 | 0.810 |
| Exhaustion | 1.802 | 1.699 | 0.294 | 0.089 | 0.256 | 0.729 |
4. DISCUSSION
The purpose of this study was to examine the prevalence of frailty and the association of inflammatory biomarkers with physical performance in frail older adults in rural communities. To our knowledge, this study is the first to assess the relationship between physical performance and blood inflammatory biomarkers in frail community-dwelling older adults in the rural area of Thailand.
Our study used Fried’s phenotype criteria to measure frailty [7] in a rural area. The phenotype includes the observable physical and biochemical characteristics of the older adults. The health characteristics of the older population in rural areas are rarely diagnosed with underlining diseases. Therefore, we were unable to use the accumulation of disease as an assessment [17]. To collect data at the health care center, trained interviewers and health care volunteers in the community were used to help facilitate access to participants. Because of the northern Thai dialect used in these villages, a local native speaker was trained to interview to ensure participants’ understanding. Participants who were very old and could not come to the health care center were visited and interviewed in their home.This assessment strategy improved the cooperation and response rate of participants. It also improved the completeness of the participant data.
We have shown that the prevalence of frailty ranges from 32.9%to 42.0%, as defined by Fried [7], among Thai community-dwelling older adults aged 65 years and over, which is a high proportion when compared with other studies [34, 35]. The prevalence in this study was higher than the previous studies in Northern Thailand (15% and 13.9%) [16, 30]. The reasons may be that our study areas were rural communities, and the mean age of participants was higher. Also, the risk factors of the frailty of this study were advanced age, low income, and more comorbidities, while in previous studies in other counties, the risk factors were related to frailty, female gender, age, low income, low education, being unmarried, separated, divorced or widowed, poor social support, and lower cognitive function [34, 36-38]. Another study in Northern Thailand found that the risk factors of frailty were age, lower education, having no spouse, more comorbidities, and osteoporosis [30].
The association between demographics and health characteristics and inflammatory biomarkers in frail older adults found the highest mean interleukin-6 (IL-6) level, agreeing with the previous study, which was associated with frailty status in the older institutionalized men [23]. Our study found that grip strength was negatively associated with IL-6 level that was supported by the previous study which showed higher grip strength to be associated with lower levels of inflammation at 8 year follow up [39]. IL-6, also known as “aging cytokines “, are the most frequently cited in literature [25, 40, 41]. There is consistent scientific evidence showing that IL-6 is associated with sarcopenia and inflammation in the elderly and their association with limited mobility, disability, falls, morbidity and mortality [21, 42, 43]. These results suggest that inflammatory mediators, for example, IL-6 might be biomarkers for frailty and decreased physical function in older adults, which was consistent with other studies that evaluated inflammatory factors related to frailty and used to predict frailty as IL-6, TNF-, C-Reactive Protein [44-46]. The serum concentration of IL-6, a cytokine that plays a central role in inflammation, increases with age and higher circulating levels of IL-6 predict disability onset in older persons [47]. Also, chronically elevated blood levels of inflammatory cells and proteins lead to rapid deterioration in the cardiovascular system in older adults and also advancing chronic cardiovascular diseases of old age [48, 49].
C-reactive Protein (CRP), an acute-phase protein, is a well-known biomarker of cardiovascular disease [50]. It relates to arterial stiffness, atherosclerosis progression, and cardiovascular events. Our study found a negative association between CRP level and physical performance as grip strength and VO2Max. This is consistent with previous literature that mild increases in CRP levels are associated with an increased risk of sarcopenia, cardiovascular diseases, and disability in older adults [51]. Grip strength as a marker of health is a component of the five physical frailty criteria [7, 52, 53] and is also considered a biomarker of aging and a predictor of disability, morbidity and mortality. In addition, CRP is also associated with poor physical performance [54].
Several limitations of this study are (a) cross-sectional design based on frailty screening among community-dwelling older adults in rural area that showed biased findings and could not infer causality; (b) the sample size was small for assessing blood biomarkers which could not evaluate the effect of gender, age, and other chronic diseases; (c) there was no comparison between other groups, such as non-frail group and (d) data of our study involved a self-reported questionnaire that may distort some information. However, the strength of this study is that the aging biomarkers appear to be predictors of physical performance and help in early detection of frailty in older adults.
CONCLUSION
This study showed that the prevalence of frailty in older adults in rural Thailand was high when compared with other countries. These findings also indicate that frailty is more prevalent with respect to advanced age, low income and more comorbidity. This finding confirms the low level of grip strength as predictor of inflammatory biomarkers in older adults with frailty. However, the validation of these associations between physical performance and inflammatory biomarkers should be confirmed through further studies. Primary care practitioners could use frailty phenotype indicators and physical performance combined with serum inflammatory biomarkers for early detection of health risks in the older population. Healthcare policies should consider the importance of detection of frailty in the elderly population.
AUTHORS’ CONTRIBUTIONS
All authors approved the final manuscript and agreed to submit to the Open Public Health Journal for publication.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand (No: 273/2560).
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration.
CONSENT FOR PUBLICATION
Written informed consent was obtained. The investigators explained the details of the study to the participants. After agreeing to participate and giving informed consent, the research team started the interviews and assessments.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [P.S] upon request.
FUNDING
The study was funded by the Faculty of Medicine, Chiang Mai University, Thailand (Grant No. 030/ 2018) and the National Research Council of Thailand (Grant No. 2561NRCT32295).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to gratefully acknowledge the Faculty of Medicine, Chiang Mai University, Thailand, and National Research Council of Thailand, for funding this study and all participants who contributed to this study.


