All published articles of this journal are available on ScienceDirect.
Prevalence and Associated Factors of Cognitive Impairment and Poor Sleep Quality among Community-Dwelling Older Adults in Northern Thailand
Abstract
Background:
Early detection of cognitive impairment and poor sleep quality are necessary to prevent dementia and the improve the quality of life further. This study aimed to investigate the cognitive impairment and poor sleep quality in the community-dwelling older adults and its association with socio-demographic and health characteristics.
Methods:
A cross-sectional study of 1,180 people in Northern Thailand aged 60 years and above was conducted in 2017. Mental State Examination-Thai version (MSET10) was used to measure cognitive function while the Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality. Multiple logistic regression was used to analyze associations.
Results:
The prevalence of CI in older adults was 52.45% (95% CI: 49.64 - 55.42) which increased with age. The prevalence of poor sleep quality was 44.15% (95% CI: 1.29-47.03). Age, illiteracy, hypertension, comorbidities of hypertension and diabetes, alcohol consumption, lack of exercise, and depression were significantly associated with increased risk of CI, while being single, comorbidities of hypertension and diabetes, and depression were significantly associated with poor sleep quality.
Conclusion:
The rate of CI and poor sleep quality in older adults was relatively high in Thailand. Early detection of CI and poor sleep quality and screening for all risk factors are important to improve in access to service, optimization of medical management, reduction in risk factors, and increased quality of life in older adults.
1. INTRODUCTION
In 2019, the United Nations reported that 703 million people globally were 65 years and older and that this number would double to 1.5 billion in 2050 [1]. Improvements in health care have contributed to healthier lives and people living longer. Longer life spans result in increasing numbers of people with non-communicable diseases and health problems, namely, heart disease, insomnia, and dementia [2].
Functional decline associated with increasing age or not with diseases may cause an impact on the physical functions necessary to maintain independence, normal life function, and self-care. Causes of cognitive deficit among the elderly are dementia. Cognitive impairment (CI) and dementia are increasing globally [2], however, the cognitive deficit is less severe than in dementia. Cognition is a combination of skills that include attention, visuospatial skills, learning, memory, language, and executive function, such as goal setting, decision making, planning, and judgment. It is a chronic condition that is a precursor to dementia in up to one-third of cases [3, 4]. Therefore, pre-dementia syndromes comprising of different types of CI are very worthwhile to assess the prevalence and incidence in populations, out of which the Mini-Mental State Examination (MMSE) is one of the oldest and most widely used to study cognitive measures. The populations who are at risk for CI are older adults [4, 5]. Some research on the prevalence of CI in the elderly showed considerable variability with estimates ranging from 4 to 5% to 40% or more. Furthermore, the prevalence of CI has been inconsistent in different regions and subjects, with a range of 15.8% to 25.7% [6-8]. This variability depends on different diagnostic criteria used, the degree of severity of clinical manifestations, and the age range used in the study, among other factors [9, 10].
The reported prevalence of Mild Cognitive Impairment (MCI) in Thailand ranged from 16.7% to 71.4%, depending on the study methodology [11-13], and previous research showed that CI was associated with older age, heart problems, blood pressure, diabetes, female sex, low education and central obesity [14-16]. However, there are few studies investigating the prevalence and factors associated with CI in Thailand, and it is essential that future studies use a fixed methodology to estimate changes in CI prevalence.
Sleep is a fundamental activity that is vital to life. During sleep, the body has various physiological changes [17, 18]. Chronic insomnia will affect various systems in the body, including cognition level and mood. Insomnia affects older adults more than any other group, perhaps due to changes in sleep-related hormone levels that affect the sleep-wake cycle, such as the advanced circadian tendency and sleep architecture [19, 20]. Sleep quality is a useful indicator that assists in screening for sleep problems [21]. In Thailand, the estimated prevalence of insomnia in older adults aged 50 and older was 60% [22]. Other factors associated with sleep quality were sex, education level, family, disease, cognition, and mental health [23-27].
Recognizing CI and poor sleep quality among older adults will allow health professionals to design better public health programs and screening for CI and poor sleep quality may improve the quality of life in this population. Many sociodemographic and health variables can influence cognitive impairment and sleep quality in the older adults, with different impacts depending on age, gender, education, marital status, illness, and health risk behavior. This study aimed to investigate the cognitive impairment and poor sleep quality in the community-dwelling older adults in Northern Thailand and their association with socio-demographic and health characteristics.
2. MATERIALS AND METHODS
2.1. Study Design and Study Population
This cross-sectional study included older adults residing in Northern, Thailand; the study was conducted in 2017. The inclusion criteria were: 1) Thais aged 60 years old and above who live in Chiang Mai and 2) willingness to participate in the study. Exclusion criteria included severe head injuries, a history of psychological, mental or neurological disorders, taking anti-depressive agents, antihistamines, or steroids, hearing loss, and refusal to participate. Sampling was performed at the district level and then at the sub-district level. The sample size was calculated based on Yamane's formula [28] with an error of 2% and with a confidence coefficient of 98%. Based on a total population of 2000, the initial sample size was 1,111. Taking into account a 5% non-response rate, the final sample size was determined to be 1,167 and up to 1,180 participants. The recruitment of participants used simple random sampling from 2,000 people.
2.2. Measurements
The data collection used face-to-face interviews, which were conducted by trained interviewers. All interviewers participated in a half-day training on questionnaire administration. The questionnaire was of two parts. The first part collected data about socio-demographic characteristics (such as age, gender, marital status, and education), and heath characteristics such as comorbidity diagnosed, smoking, alcohol consumption and exercise. The second part included the Mental State Examination-Thai 10 (MSET10), the Patient Health Questionnaire (PHQ-9), and the Pittsburgh Sleep Quality Index (PSQI).
Participant cognition was assessed by Mental State Examination Thai 10 (MSET10) that includes 10 questions to asses 7 areas including orientation, registration, attention and calculation, and recall, namely the ability to follow complex commands. This study used the total score to assess cognition, and total scores can range from 0 to 29. Scores of 18-22 indicate MCI, while scores of 12-17 show moderate CI and scores of 12 and lower indicate severe CI. Any score higher than 22 indicate an absence of CI [29 - 31].
The depression was assessed using the nine-item Patient Health Questionnaire (PHQ-9). Any score over 7 indicates depression [32]. Background information on the participants was collected and included: sex, age, marital status, education, medical history of hypertension, diabetes, depression, and poor sleep quality. Additional data related to health behavior was also collected on smoking, alcohol consumption, and exercise.
The Pittsburgh Sleep Quality Index (PSQI), used to assess sleep quality, is a self-reported measure of sleep quality and sleep patterns. The PSQI has seven components, including sleep latency, sleep duration, subjective sleep quality, sleep efficiency, sleep disturbance, daytime dysfunction, and use of medication for sleep. Scoring for each item ranged from 0 to 3, with 3 reflecting the most negative option. The total score was the sum of the seven components and ranged from 0 to 21. A total PSQI score of ≥5 indicated poor sleep quality, whereas <5 reflected good sleep quality [33].
2.3. Data Analysis
All data were analyzed using parametric normal distribution test. Descriptive statistics, which included frequency and percentage were estimated. The statistics were reported with a 95% confidence interval. Multiple logistic regression models were used to assess the associations between various factors of CI and poor sleep quality such as age, gender, marital status, education, hypertension, diabetes, depression, smoking, alcohol consumption, and exercise.
3. RESULTS
3.1. Socio-demographic Characteristics of Participants
A total of 1,180 participants were interviewed and all of them completed the MSET10 test and PSQI in a single visit. Overall, the mean age of participants was 69.03 years (SD ± 7.30). Almost two-thirds were female (64.23%). More than half were married (57.28%) and 94.57% were literate. In terms of health, 45.25% had hypertension, 6.50% had diabetes, 16.94% had hypertension with diabetes, and nearly half had poor sleep quality (44.15%). Almost three-quarters of the participants exercised (74.96%), while 37.11% smoked and 41.51% consumed alcohol (Table 1).
3.2. Prevalence of Cognitive Impairment in Older Adults
The prevalence of CI among older adults was 52.45%, 95% (CI:49.64-54.40), which increased with age. The proportion of CI in participants aged 60-64 was 46.30%, 95% (CI: 41.44-51.20), however, participants 80 and older had a higher prevalence (78.29%; 95% CI: 70.18-85.06). Rates of CI were slightly higher in females. The prevalence of CI was calculated as 49.50%; 95% CI: 44.65-54.40 for males and 54.20%, 95% CI: 50.59-57.81 for females. We found that the prevalence of CI in older adults who were single (56.94%, 95% CI: 52.49-61.31) was higher than in participants who married. Illiterate participants (89.06%, 95% CI: 78.75-95.48) had higher rates than those who were literate (Table 1).
The prevalence of CI in older adults with chronic conditions such as hypertension (56.92%, 95% CI: 57.60-61.17), comorbidities of hypertension and diabetes (55.50%, 95% CI: 48.32-62.50), depression (68.67% 95% CI: 57.55-78.41), and poor sleep quality (54.28%, 95% CI: 49.91-56.60), had higher rates of CI than those without these chronic conditions, and prevalence of CI in older adults who had diabetes (46.75%, 95%CI: 35.28-58.47) (Table 1).
Furthermore, the prevalence of CI in older adults with poor health habits such as smoking (51.03%, 95%CI: 46.22-55.83), lack of exercise (62.71%, 95% CI: 56.91-68.24) and alcohol consumption (49.80%, 95%CI: 45.37-54.42) was higher than those who maintained healthly lifestyles (Table 1). Besides, our study showed in Fig. (1a) that the percentages of cognitive impairment level, i.e. severe CI, moderate CI, and mild CI were 35.50%, 1.70%, 15.30%, respectively.
| Characteristics |
N (%) (n =1,180) |
Cognitive Impairment (CI) | Poor Sleep Quality | ||||
|---|---|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | ||||
| Prevalence | 1,180 | 620 (52.45) | 49.64 - 55.42 | 521(44.15) | 41.29 - 47.03 | ||
|
Gender Male Female |
422 (35.76) 758 (64.23) |
209 (49.50) 411 (54.20) |
44.65 - 54.40 50.59 - 57.81 |
177 (41.90) 344 (45.38) |
37.18 - 46.81 41.79 - 49.00 |
||
|
Age (y) Mean ± SD |
69.03 ± 7.30 | ||||||
|
Age group (y) 60-64 65-69 70-74 75-79 80 and above |
419 (35.50) 302 (25.59) 176 (14.91) 154 (13.05) 129 (10.93) |
194 (46.30) 139 (46.02) 91 (51.70) 95 (61.68) 101 (78.29) |
41.44 - 51.20 8.20 - 15.74 44.06 - 59.28 53.52 - 69.39 70.18 - 85.06 |
190 (45.34) 130 (43.04) 70 (39.77) 86 (55.84) 45 (34.88) |
40.50 - 50.25 37.78 - 48.84 32.48 - 47.40 47.62 - 63.83 26.70 - 43.00 |
||
|
Marital status Married Single/Widow/ Divorced/separated |
676 (57.28) 504 (42.71) |
333 (49.20) 287 (56.94) |
45.42 - 53.09 52.49 - 61.31 |
279 (41.27) 242 (48.01) |
37.53 - 45.04 43.57 - 52.47 |
||
|
Education Literate Illiterate |
1116 (94.57) 64 (5.42) |
563 (50.44) 57 (89.06) |
47.47 - 53.42 78.75 - 95.48 |
497 (44.53) 24 (37.50) |
41.59 - 47.50 25.70 - 50.49 |
||
| Hypertension | 534 (45.25) | 304 (56.92) | 56.60 - 61.17 | 237 (44.38) | 40.11 - 48.71 | ||
| Diabetes | 77 (6.50) | 36 (46.75) | 35.28 - 58.47 | 28 (36.36) | 25.69 - 48.11 | ||
| hypertension with diabetes | 200 (16.94) | 111 (55.50) | 48.32 - 62.50 | 101 (50.50) | 43.35 - 57.62 | ||
| Depression | 83 (7.05) | 57 (68.67) | 57.55 - 78.41 | 47 (56.62) | 45.29 - 67.47 | ||
| Poor sleep quality | 521 (44.15) | 285 (54.28) | 49.91 - 56.60 | - | - | ||
| Cognitive impairment | 204 (17.28) | - | - | 88 (43.13) | 36.24 - 50.23 | ||
| Smoking | 435 (37.11) | 222 (51.03) | 46.22 - 55.83 | 203 (46.66) | 41.90 - 51.47 | ||
| Alcohol consumption | 489 (41.51) | 244 (49.89) | 45.37 - 54.42 | 229 (46.83) | 42.33 - 51.36 | ||
| Lack of exercise | 295 (25.04) | 185 (62.71) | 56.91 - 68.24 | 135 (45.76) | 39.97 - 51.63 | ||
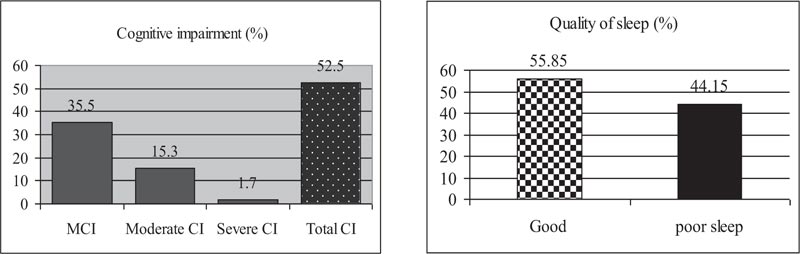
3.3. Prevalence of Poor Sleep Quality in Older Adults
The prevalence of poor sleep quality in older adults was 44.15%, 95% CI: 41.29-47.03. In males and females, the prevalence of poor sleep quality was 41.90%; 95% CI: 37.18- 46.81 and 45.38%; 95% CI: 41.79-49.00, respectively. We found that the prevalence of poor sleep quality in older adults, who were single (48.01%, 95% CI: 43.57-52.47) was higher than in those who were married. Literate participants (44.53%, 95% CI: 41.59-47.50) had a higher chance of having poor sleep quality than those who were illiterate (Table 1).
Older adults who had comorbidities of hypertension and diabetes (50.50%, 95% CI: 43.35-57.62) had a higher prevalence than those without these diseases or who had only hypertension or diabetes, and the prevalence of poor sleep quality in older adults who had hypertension, and diabetes were 44.38%, 95% CI:40.11-48.71, 36.36%, 95% CI: 25.69-48.11, respectively. Also, the prevalence of poor sleep quality was higher in those experiencing depression (56.62%, 95% CI: 45.29-67.47) than those without this disease (Table 1).
Moreover, the prevalence of poor sleep quality in older adults with poor health habits such as smoking (46.66%, 95% CI: 41.90-51.47), alcohol consumption (49.80%, 95% CI: 45.37-54.42), and lack of exercise (45.76%, 95% CI: 39.97-51.63) was higher than who had adopted healthy lifestyle (Table 1). Furthermore, our study showed in (Figs. 1a and 1b) that percentage of the characteristic of sleep quality in older adults was 55.85%, 44.15% of older adults who had good sleep quality and poor sleep quality, respectively.
3.4. Factors Associated with Cognitive Impairment in Older Adults
Our study showed that being older, illiterate, having hypertension or hypertension with diabetes, lack exercising, consuming alcohol, and having depression were significantly associated with CI (p <0.001, 0.001, 0.002, 0.03, 0.003, 0.001 and 0.008, respectively). Being older had an AOR = 1.05, 95% CI: 1.03-1.07. Illiterate older adults were 5.87 times (AOR = 5.87, 95% CI: 2.60-13.25) more likely to have CI than those who were literate. Older adults with hypertension and having hypertension with diabetes were 1.45 times (AOR = 1.45, 95% CI: 1.10-1.92) and 1.47 times (AOR = 1.47, 95% CI: 1.02-2.10) more likely to have cognitive impairment compared to participants who did not have these conditions. Older adults who had no physical activity and consumed alcohol were 1.66 times (AOR = 1.66, 95% CI: 1.24-2.20) and 1.70 times (AOR = 1.70, 95% CI: 1.19-2.42) more likely to have CI compared to older adults who exercised regularly and did not drink alcohol. Older adults with depression were 1.77 times (AOR = 1.77, 95% CI: 1.07-2.94) more likely to have CI compared to those who did not (Table 2).
| Associated Factors | Cognitive Impairment (CI) | Poor Sleep Quality | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p-value | AOR | 95% CI | p-value | |
| Age, y | 1.05 | 1.03-1.07 | 0.001*** | 0.99 | 0.97-1.01 | 0.45 |
| Gender | - | - | - | - | - | - |
| Male | 1 | - | - | 1 | - | - |
| Female | 1.22 | 0.91-1.63 | 0.16 | 1.11 | 0.84-1.47 | 0.44 |
| Marital status | - | - | - | - | - | - |
| Married | 1 | - | - | 1 | - | - |
| Single/Widow/ Divorced/Separated |
1.39 | 0.87-1.47 | 0.32 | 1.38 | 1.07-1.78 | 0.01** |
| Education | - | - | - | - | - | - |
| Literate | 1 | - | - | 1 | - | - |
| Illiterate | 5.87 | 2.60-13.25 | 0.001*** | 0.67 | 0.39-1.16 | 0.16 |
| Hypertension | 1.45 | 1.10-1.92 | 0.002** | 1.20 | 0.91-1.58 | 0.17 |
| Diabetes | 1.08 | 0.66-1.78 | 0.74 | 0.81 | 0.49-1.33 | 0.41 |
| hypertension with diabetes | 1.47 | 1.02-2.10 | 0.03* | 1.48 | 1.05-2.10 | 0.02* |
| Depression | 1.77 | 1.07-2.94 | 0.008** | 1.65 | 1.04-2.61 | 0.033* |
| Poor Sleep quality | 1.12 | 0.88-1.43 | 0.34 | - | - | - |
| Cognitive impairment | - | - | - | 0.97 | 0.70-1.33 | 0.81 |
| Smoking | 0.93 | 0.62-1.40 | 0.73 | 1.37 | 0.92-2.04 | 0.11 |
| Alcohol consumption | 1.70 | 1.19-2.42 | 0.003** | 1.22 | 0.86-1.72 | 0.26 |
| Lack of exercise | 1.66 | 1.24-2.20 | 0.001*** | 0.99 | 0.75-1.31 | 0.99 |
3.5. Factors Associated with Poor Sleep Quality in Older Adults
The multivariate logistic regression analysis showed that being single, having hypertension with diabetes, and depression were significantly associated with poor sleep quality (p = 0.01, 0.02, and 0.03, respectively). Single older adults were 1.38 times (AOR = 1.38, 95% CI: 1.07-1.78) more likely to have poor sleep quality than those married. Older adults who had hypertension with diabetes were 1.48 times (AOR = 1.48, 95% CI: 1.05-2.10) more likely to have poor sleep quality compared to those without these conditions. Older adult’s depression was 1.65 times (AOR = 1.65, 95% CI: 1.04-2.61) more likely to have poor sleep quality compared to those without depression (Table 2).
4. DISCUSSION
We assessed the prevalence and factors associated with Cognitive Impairment (CI) in older adults in Northern Thailand. More than half of the participants in our study had CI. The prevalence of CI in our study (52.45%) was higher than in other studies which had rates of 46.4% and 39.5%, respectively [34, 35]. These variations may be due to the differences in geography, study population, and the duration of data collection. The difference could also be due to the definitions used by each study. Multivariate logistic regression analysis showed seven factors that were associated with CI, including older age, illiteracy, having hypertension, having hypertension with diabetes, had depressive symptoms, alcohol consumption, and lack of exercise.
Our study showed that the prevalence of CI increased with age, which is consistent with studies from Vietnam in 2019 [35], Zhejiang China in 2014 [36], and Sub-Saharan Africa in 2019 [37], which showed that the prevalence of dementia increased with age and was significantly associated with cognitive impairment. This could be explained by the biological mechanisms of aging that lead to reduced cognitive function. The progression of CI speeds up and is more severe as the body ages over time [35]. In this study, the prevalence of CI in females was higher than in males. This result is partially consistent with the finding, which showed that females were more likely to have CI compared to males [37] and the prevalence of CI in females was higher than males, especially when older than 75 years [38]. This may be explained by the fact that women on an average live longer than men and Alzheimer’s disease in women is linked to lower levels of estrogen after menopause [39].
Moreover, our study showed that 42.71% of participants were single. This statistic included older adults who were divorced or widowed. The older adults who were single were more likely to have CI than older adults in relationships. Similarly, previous studies found that CI was higher in older adults who had never married [40, 41]. There is no robust estimate of the impact of dementia on loneliness [42]. However, living as part of a couple might expose individuals to social challenges that have a protective effect against CI [43]. Our results also showed a difference in CI prevalence based on literacy, with literate older adults having a lower prevalence than those who could not read. This result was consistent with previous studies, which showed that illiterate older adults had a higher probability of CI, while higher levels of education were associated with a lower prevalence of dementia [44, 45]. This could mean that continuous exposure to an intellectually challenging environment will have direct beneficial effects on brain structure and function, resulting in greater neurological development, the lack of which may increase the risk of developing CI in later life [46, 47].
This study also showed how certain factors were significantly associated with the prevalence of CI in older adults: hypertension, hypertension and diabetes, depression, and poor sleep quality. This finding agrees with previous studies that CI was associated with high systolic blood pressure, and that hypertension and diabetes mellitus were associated with increased risk of dementia. They also found that rates of depressive disorder were higher in older adults [37, 48, 49]. Higher blood pressure may precipitate dysfunction in the blood-brain barrier, resulting in the interaction of blood-borne substances with neuronal or synaptic function, which in turn, results in CI and subsequently, dementia [50]. Moreover, depression is a risk factor in part that causes dementia, and depression in earlier adulthood appears to increase the risk of dementia later in life [51]. Our study found a higher prevalence of CI in older adults who do not exercise than in those who exercised, and multivariate analysis showed that not exercising was significantly associated with CI. This finding supported by the previous studies from the Alzheimer’s Society and London School of Economics and Political Science which reported that physical inactivity had a direct effect on the structure and function of the brain and lack of activity decreases cognitive function [42].
Our study showed that the prevalence of poor sleep quality in older adults was 44.15% (95% CI: 41.29 - 47.03). The prevalence of poor sleep quality in older adults varied in other studies. A cross-sectional survey with 2,195 participants in Northern China, a cross-sectional in Ethiopia with 422 participants, a central Thai study with 378 participants, and an urban mainland China with 1,063 participants had a prevalence of 33.8%, 65.4%, 52.12%, and 41.5%, respectively [25, 26, 52-54]. This variation may be due to differences in the study areas, sample size, duration of data collection, and socioeconomic status of participants. Moreover, our study showed that participants who were single/ widow/ divorced/ separated, had hypertension and diabetes, and depression were significantly found to be associated with poor sleep quality. This finding is consistent with the previous studies from China which found a higher prevalence of poor sleep quality in people who were single. A study in northern Taiwan found that living with family was associated with a decreased risk of insomnia [25, 26]. Being single may lead to a feeling of isolation, which is compounded by possible grief if the single state is due to death of partner or divorce. This may intensify insomnia in older adults.
The present study showed a connection between poor sleep quality and depression in older adults. The multivariate analysis showed that depression had a significant association with poor sleep quality. This result was consistent with a previous Thai study, which found that poor sleep was associated with depression [55, 56], and a meta-analysis showed that major depression was associated with sleep complaints [57]. Sleep problems could be one indicator of depression that may also increase the risk of developing depression. This may also be indicative of underlying neurobiological mechanisms linking sleep disturbance to a psychiatric disorder, presenting a bidirectional relationship [58].
Furthermore, our study found the prevalence of poor sleep quality was very high in older adults who had both hypertension and diabetes and having these conditions was significantly associated with poor sleep quality. This is similar to the previous studies, which showed a high prevalence of poor sleep in older adults with medical conditions including co-morbidity and that these factors were significantly associated with poor sleep quality [55]. Meanwhile, the other study found that hypertension was correlated with sleep quality [58]. This may explain how chronic illness results in sleep problems that can be described by a bidirectional relationship as well [57].
The limitations of this study are that first, the research was only conducted in older adults in Northern Thailand and thus,the representation and generalizability are poor. Second, the present study only screened for cognitive impairment with MSET 10 scores and did not use other instruments for the evaluation of cognitive function. Third, we only evaluated the above risk factors for cognition and did not consider the effects of medication history, family history, or social interaction on cognitive function. Finally, this was a cross-sectional study and so causation could not be identified.
CONCLUSION
The prevalence of CI and poor sleep quality in older adults is high. We found risk factors of scores 18-22 that include age, illiteracy, hypertension, co-morbidity of hypertension with diabetes, lack of exercise, alcohol consumption, and depression. Moreover, being single, being hypertension and diabetes, and having depression were significantly associated with poor sleep quality. Early detection of CI and poor sleep quality and screening for all risk factors are important to improve access to service, optimization of medical management, reduction in risk factors, and increased quality of life in older adults. Further studies should include older adults from another region of Thailand also, for good representation and generalizability. Moreover, other instruments of cognitive function should be used for investigation and screening for cognitive impairment with MSET10 scores are needed.
AUTHORS’ CONTRIBUTIONS
All authors approved the final manuscript and agreed to its submission to the Open Public Health Journal for publication.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University, Thailand (No: 538/2015).
HUAMN AND ANIMAL RIGHTS
No animals were used in this study. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The legal protection of all participants was ensured and confirmed by written consent.
STANDARDS OF REPORTING
STROBE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available on request from the corresponding author [P.S].
FUNDING
This study was supported by the Faculty of Medicine, Chiang Mai University, Thailand (Grant number 050/2016).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thankful to the subjects who participated in this study and are grateful to Dr. Johan Van Rooyen of Webster University (Thailand) for editing the English language of the manuscript.


