All published articles of this journal are available on ScienceDirect.
Cost-Effectiveness of Bedaquiline in Multidrug Resistant Tuberculosis: A Review
Abstract
Background:
Multidrug Resistant Tuberculosis (MDR-TB) remains a burden on the healthcare system and public health. Evidence on cost and cost-effectiveness of MDR-TB treatment option is necessary in order to provide evidence-based recommendation for policymakers. The main therapy for MDR-TB consists of a combination of at least five types of anti-tuberculosis drugs, including second-line injections that have proven to be effective. Bedaquiline is a relatively new drug recommended by the World Health Organization (WHO) and European Medicines Agency (EMA) for the treatment of MDR-TB.
Aims and Objectives:
This study examines the cost-effectiveness of using regimens containing bedaquiline compared to those containing second-line injections.
Methods:
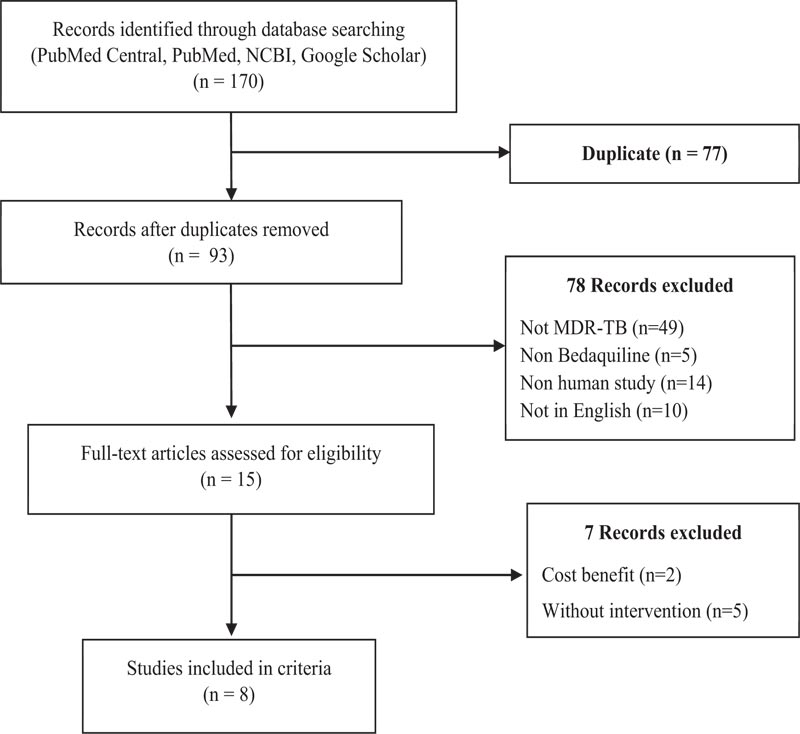
The design of this study is a literature review study. The following keywords used for the search were: “MDR-TB,” “cost effectiveness analysis of MDR-TB,” “cost effectiveness analysis of MDR-TB patients,” “WHO guideline for MDR-TB,” “Bedaquiline cost effectiveness,” and “kanamycin cost effectiveness.” The relevant references were derived from several databases, including PubMed, NCBI, and the Journal of Indonesian Health Economics. A total of 170 articles were obtained during the initial search, then extracted with inclusion criteria, namely articles assessing cost effectiveness, QALY, DALY, articles in English and Indonesian, and publications within the last 10 years.
Results:
The addition of bedaquiline in standard therapy showed favourable effect and safety due to faster culture conversion time and less incidence of side effects, based on the results of studies. The faster the culture conversion occurs and the less patients experiencing side effects, the faster their health improvement, which prospectively will reduce treatment costs and productivity loss.
Conclusion:
This is demonstrated by the results of cost-effectiveness analysis which shows that the replacement of the second-line injection regimen to bedaquiline, and the addition of bedaquiline to the standard regimen of therapy was assessed to be a more cost-effective option.
I. INTRODUCTION
Multidrug Resistant Tuberculosis (MDR-TB) is defined as simultaneous resistance to isoniazid and rifampin, regardless of whether it is resistant to other anti-tuberculosis drugs [1]. MDR-TB remains a major public health challenge worldwide in the last few years and is still a difficult health problem to overcome [2]. The detection rate of Tuberculosis (TB) cases in the world in 2019 was 206,030 cases; this shows an increase of 10% compared to the previous year, which only had 186,883 cases in 2018 and these cases are expected to continue to increase [1-3]. Treatment for MDR-TB requires a long duration of treatment, which is around 18-24 months. Anti-TB agents used in the treatment are second-line injection drugs with high levels of toxicity and higher costs compared to first-line antituberculosis drugs [4]. Recent research states that the average patient care cost for MDR-TB in 87 countries is US$6430 (I$6430). Patients with MDR-TB must spend 5-20 times more than those with sensitive TB [5].
For its effectiveness, studies in Armenia showed that the mean time to culture conversion in the bedaquiline group was 2.7 months earlier than the standard group, namely 5.7 months. In addition, patient culture conversion occurred faster in the bedaquiline group at 73% (P <0.0001) [6]. Another study conducted by Mbuagbaw et al. (2019) showed that the total treatment success results in the bedaquiline group were 65.8% greater than the standard group, which was only 54-58% and the total mortality rate in the bedaquiline group was 11.7% lower than the standard group namely 13.8-15% [7]. In the cost effectiveness side, there is one study in Africa that showed that the average cost of treatment per patient in the bedaquiline group was 4.3% higher by US$4647 (I$4647) than the standard group of US$4439 (I$4439). However, the bedaquiline group was able to prevent 0.17 DALY compared to the standard group who received an additional fee of US$1242 (I$1242) per DALY [8]. Another comparable study in Africa showed that the average cost of treating the bedaquiline group was US$49.07 (I$49.07) lower than the standard group, which was US$332.78 (I$332.78) per patient. The total QALY for the bedaquiline group was lower and provided a cost-effectiveness saving of US$982 (I$982) per DALY averted [9].
After going through the last few decades without any new anti-TB agents, bedaquiline was finally discovered as a new TB agent that can potentially be used in the treatment of MDR-TB. Nowadays, WHO has recommended bedaquiline into the therapy guide as one of the MDR-TB treatment regimens [10]. Bedaquiline is considered effective and a replacement for the previous injection regimen in the form of second-line injection drugs such as Capreomycin (Cm), Kanamycin (Km), and Streptomycin (S) [11]. Besides being effective, bedaquiline is also considered safer than second-line injections because it only has an 8.8% lower incidence of side effects compared to kanamycin [10-12]. This review was conducted to assess the potential cost and therapeutic effectiveness of adding bedaqui- line to the MDR-TB treatment regimen.
2. METHODS
This review contains references from various sources with keywords in Indonesian and English. The following keywords were used for the search: “MDR-TB,” “cost effectiveness analysis of MDR-TB,” “cost effectiveness analysis of MDR-TB patients,” (Table 1) “WHO guideline for MDR-TB,” “Bedaquiline cost effectiveness,” and “kanamycin cost effectiveness.” The relevant references were derived from several databases, including PubMed, NCBI, and the Journal of Indonesian Health Economics. In addition, this review also considered references from relevant data available on the official WHO website. A total of 170 articles were obtained during the initial search, then extracted with inclusion criteria, namely articles assessing cost effectiveness, QALY, DALY, articles in English and Indonesian, and publications within the last 10 years. Reviews, non-English studies, and irrelevant studies, such as non-human studies, were excluded (Fig. 1). QALY, or it can be called the Adjusted Quality Number of Years of Life, is an expected outcome/outcome from an intervention that is closely related to the quality of life. These health interventions are efforts to improve health, such as pharmacological and non-pharmacological therapies.
Then, after calculating QALY and Cost, the incremental effectiveness cost ratio (ICER) is determined using the following formula:
 to find out how much it will cost to have a good quality of life in 1 year. ACER is determined from the cost versus the effectiveness/outcome value of a drug in percent (%).
to find out how much it will cost to have a good quality of life in 1 year. ACER is determined from the cost versus the effectiveness/outcome value of a drug in percent (%).
The inclusion criteria of literature used for this study were a literature that only analyses cost-effectiveness or cost-utility effectiveness, using sample of patients whose primary diagno- sis was MDR-TB, using samples of patients who received different bedaquiline alloy therapy and standard alloys without bedaquiline, using patients undergoing MDR-TB treatment program in early and advanced phases, containing complete details of the total cost of treatment. While the exclusion criteria were literature with patients undergoing switching to MDR-TB antibiotic therapy, patients with comorbid other infectious diseases, patients whose primary diagnosis was not MDR-TB, and patients who did not receive health inter- ventions.
2.1. Quality of Reporting
Of the 8 studies obtained, an evaluation of the quality of reporting was assessed by the statement of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [13]. With the aim of obtaining an assessment of the overall reporting quality. However, the results of the reporting quality assessment are not a measure of the quality of the study. To say that some points were not reported does not mean that the quality of the study was low. The CHEERS assessment checklist was conducted to provide additional information and not to generate additional factors of interest in the study. With the intention of obtaining over all reporting quality assess- ments, studies were assigned 1 point per item if the requir- ement from the checklist was fulfilled, 0.5 each when partially fulfilled and 0 point when no or insufficient information was reported. Even though the CHEERS checklist is not a designed asscoring instrument, the application of a scoring method for CHEERS checklist has been used and published elsewhere. Twenty-four checklist items were divided into six main categories (title and abstract, introduction, methods, results, discussion, and other). These items were subsequently calculated as a percentage score with the under- lying assumption that all criteria were weighted equally and criteria, which were not applicable, were excluded from the estimation. Studies with a score higher than 75% were categorized as good, studies in the range 50-74% were categorized moderate and studies with score slower than 50% were categorized as low (Table 2).
3. RESULTS
Bedaquiline is an MDR-TB treatment regimen with an oral route that was first recommended by WHO in 2018 in Rapid Communication, “Key changes to the treatment of multidrug- and rifampicin-resistant tuberculosis.” These recommendations were included in the guidelines of early 2019 [10-12]. Bedaquiline is a group diarylquinoline with specific activity against M. tuberculosis [14]. Systematically, bedaquiline affe- cts only ATP synthase activity in dormant and active (repli- cating) microbacteria [15, 16]. Pharmacoeconomic reviews that will be carried out are cost effectiveness analysis and cost utility analysis. Cost-effectiveness analysis is an analysis used to compare costs where the effect of one intervention is higher; treatment outcomes are measured in health indicators, valuations/costs are in international dollar (I$). Whereas, cost utility analysis is an analysis used to compare costs where the effect of one intervention is higher, treatment outcome is measured in Quality-Adjusted Life Years (QALY) in the international dollar (I$). The international dollar is used in this review so it can be comparable with other studies.
3.1. Effectiveness of Bedaquiline Therapy
Studies in Armenia showed that the mean time to culture conversion in the bedaquiline group was 2.7 months earlier than the standard group, namely 5.7 months. In addition, patient culture conversion occurred faster in the bedaquiline group at 73% (P <0.0001) [6]. Another study conducted by Mbuagbaw et al. (2019) showed that the total treatment success results in the bedaquiline group were 65.8% greater than the standard group, which was only 54-58% and the total mortality rate in the bedaquiline group was 11.7% lower than the standard group namely 13.8-15% [7]. A study in South Africa showed that the treatment success rate of the bedaquiline group was greater at 49.3% than the standard group, 42.8%. In addition, this study showed the mortality rate in the standard group was greater at 9.7% compared to the 6.5% difference in the bedaquiline group [17]. In line with this study, an African study showed that the bedaquiline group reduced treatment failure rates by 11.5%. The bedaquiline group had a failure rate of 5.9%, while the standard group was 17.4% [18]. In the UK, the culture conversion yield of the bedaquiline group was greater than 50%, while the standard group was less than 30% [19].
Studies in several countries show that bedaquiline has the lowest risk of serious side effects, namely (2%), linezolid has the highest risk (17%), followed by thioacetazone (14.6%), PAS (14.3%), kanamycin. (10.8%), and amikacin (10.3%) [4]. The study shows that bedaquiline has a lower risk of side effects compared to other drugs. An interim analysis of the endTB trials shows that linezolid has the highest risk of side effects, with 11% of patients receiving linezolid experiencing at least one side effect such as peripheral neuropathy and optic neuritis [20]. Studies in India show that safely, bedaquiline has a higher degree of safety than other TB drugs [21].
3.2. Cost Effectiveness Analysis
Overall, 7 of the 8 studies showed regimens containing bedaquiline were more cost-effective (Table 1). There is one study in Africa that showed that the average cost of treatment per patient in the bedaquiline group was 4.3% higher by US$4647 (I$4647) than the standard group of US$4439 (I$4439). However, the bedaquiline group was able to prevent 0.17 DALY compared to the standard group who received an additional fee of US$1242 (I$1242) per DALY [8]. Another comparable study in Africa, showed that the average cost of treating the bedaquiline group was US$49.07 (I$49.07) lower than the standard group, which was US$332.78 (I$332.78) per patient. The total QALY for the bedaquiline group was lower and provided a cost-effective saving of US$982 (I$982) per DALY averted [9].
A study in Korea showed that the bedaquiline group received an additional 1.2 QALY or 1.29 years longer than the standard group. The total cost of treatment for the bedaquiline group was 86,043,831 KRW (I$ 78.203,38) with a total QALY of 5 per patient, while the total cost of the standard group was 72,082,172 KRW (I$ 65.613,93) with a total QALY of 3.80 per patient [22]. The cost of treatment for the bedaquiline group in Germany was €28,652 (I$ 34.988,68) lower than the standard group at €30,270 (I$ 36.967,84). The bedaquiline group resulted in a lower average additional cost of €22,238 (I$ 27.158,60) per QALY [23]. The study in Italy showed that the total cost of QALY in the bedaquiline group was lower at €21,650 (I$ 26.440,50) compared to the standard group at €34,261 (I$ 41.841,93) [24]. In a study in Hong Kong, treatment with standard therapy was the lowest cost, about US$47.396 (I$47.396) with the lowest QALY of 6,347. However, compared to the standard group and the delamanid group, the bedaquiline group gave a greater additional value to the QALY, around 0.731 QALY at an additional cost of US$9 (I$9). Overall the bedaquiline group was more cost effective than the other interventions, as it reduced costs by 99.98% [25]. Studies in the UK show the bedaquiline group was more cost-effective than the standard group. The bedaquiline group saved £11,434 (I$ 13.964,00) and an additional 1.14 QALY [19]. Another African study showed that the bedaquiline group could reduce the cost per successful treatment of the Long Course Regimen (LCR) and Short Course Regimen (SCR) tested in India, Russia, and South Africa. Conversion of total treatment with the bedaquiline group resulted in a reduction in ICER of US$122.878 (I$122.878) in Russia, US$7.721 (I$7.721) in India, and US$10.341 (I$10.341) in South Africa [26]. The study characteristics of both cost-effectiveness and cost-utility study of Bedaquiline are provided in Table (1).
| No. | Author/Country | Type of Study | Type of Intervention | Effectivity Parameter | Type of Cost | Results | ||
|---|---|---|---|---|---|---|---|---|
| Medical Direct Cost | Non-Medical Direct Cost | Indirect Cost | ||||||
| 1. | Schnippel et al. [8] (2017) South Africa |
CEA and CUA, Retrospective Cohort Study |
- Bedaquiline + Standard TB Therapya - TB Therapy Standarda |
DALY | Drug costs, hospitalization, outpatient care, and laboratory examinations | - | - | - The mean total cost of the bedaquiline group was US$4647 (I$4647) (4.3% higher) than the non-bedaquiline group US$4439 (I$4439). - The treatment success rate for the bedaquiline group was higher at 60.6% than the 56.3% non-bedaquiline group. - The bedaquiline group decreased the mortality rate by 26.2%. - The ICER value is US$1242 (I$1242)/ DALY in the bedaquiline group, more cost-effective because it is below the GDP / capita of South Africa (US$5718) (I$5718). |
| 2. | Agnarson et al. [9] (2020) South Africa |
CEA and CUA, Markov Model, Experimental Study |
- Bedaquiline + Standard TB Therapya - TB Therapy Standarda (Short Course Regimen (SCR)b) |
DALY | Hospitalization, outpatient care, monitoring, and incidence of side effects | - | Productivity loss | - The total cost of treatment for the bedaquiline group was lower at US$597 (I$597) compared to US$657 (I$657) for non-bedaquiline. - Total QALY for the bedaquiline group was US$1.922.13 (I$1.922.13) lower than the non-bedaquiline group US$2.348.168 (I$2.348.168). - US$982 (I$982) per DALY, cost effective because it is below the GDP / capita of South Africa (US$5718) (I$5718). |
| 3. | Ionescu et al. [26] (2018) South Africa |
CEA, experimental study | - Bedaquiline + Standard TB Therapya - TB Therapy Standarda (Short Course Regimen (SCR)b) (Long Course Regimen (LCR)c) |
-Time to achieve culture conversion. -Adverse event |
- Costs associated with drugs hospitalization - side effects - nutritional supplements - laboratory testing - end of life care) |
- | - | - In the SCR regimen, the bedaquiline group was more cost effective than the non-bedaquiline group. - The bedaquiline group saved a total of US$3.1477 (I$3.1477) in total medical costs in India, US$5.361 (I$5.361) in Africa. - In the LCR regimen, the bedaquiline group was more cost effective than the non-bedaquiline group. The bedaquiline group saved a total of US$147.129 (I$147.129) in total medical costs in Russia, US$14.214 (I$14.214) in India, US$20.211 (I$20.211) Africa. - LCR costs reduced to US$7915 and SCR costs US$5361 (I$5361), cost-effective because it is below the GDP / capita of South Africa US$5718 (I$5718) and save up to 50% of costs. |
| 4. | Park et al. [22] (2016) South Korea |
CEA and CUA, Cohort-Based Decision Analytic Model |
- Bedaquiline + Standard TB Therapya - TB Therapy Standarda |
QALY | Drug costs, monitoring, hospitalization, outpatient care, and care | Transportation costs for hospitalization and BMHP costs during inpatient | loss of productivity | - The bedaquiline group received an additional 1.2 QALY or 1.29 years longer than standard group. - The average cost of treatment for the bedaquiline group was 13,961,659 KRW (I$ 78.203,38) per QALY, cost-effective because it was below the GDP / capita of South Korea I$ 31362.75. (continue) |
| 5. | Wirth et al. [23] (2017) Germany |
CEA and CUA, Cohort Study |
- Bedaquiline + Standard TB Therapya - Delamanid / linezolid + Standard TB Therapya - TB Therapy Standarda |
QALY | Cost of drugs, hospitalization, outpatient care, monitoring, and adverse events | - | - | - The bedaquiline group had better effectiveness than the standard group, the mean time to culture conversion of the bedaquiline group was 83 days while the standard group was 125 days. - €22,238 (I$ 27.158,60) per QALY, cost effective because it is below German GDP / capita (I$47.603.03). |
| 6. | Codecasa et al. [24] (2017) Italy |
CEA dan CUA, Markov Model, Experimental Study |
- Bedaquiline + Standard TB Therapya - TB Therapy Standardsa |
LYG and QALY | Cost of drugs, outpatient care, hospitalization, and laboratory tests | - | Loss of productivity | - The bedaquiline group was more cost effective than the standard group. - The total QALY of the bedaquiline group was 4.36 greater than the standard 3.29 QALY group. - Cost effective because it is below the GDP / capita of Italy (I$34483.20). |
| 7. | Wolfson et al. [19] (2015) United Kingdom |
CEA and CUA, cohort-based Markov model | - Bedaquiline + Standard TB Therapya - TB Therapy Standarda |
QALY and DALY | Costs of hospitalization, outpatient care, medicines, monitoring, surgery | - | - | - The bedaquiline group saved £11,434 in medical expenses (I$ 13,9673) and an additional 1.14 QALY. - The total cost of treatment for the bedaquiline group was £2,170,394 (I$2.651.266,49) and the standard group was £2,403,442 (I$2.935.948,61). (continue) |
| 8. | Fan Q, et al. [25] (2019) Hongkong |
Decision-analytic model | - Bedaquiline + Standard TB Therapya - Delamanid + Standard TB Therapya - TB Therapy Standarda |
QALY | - Drug costs - Hospitalization costs - Cost of long stay (Lengthofstay) |
- Clinic visit fee - costs follow-up Clinicover time. - Number of clinic visits |
- | - Overall regimens with bedaquiline can save costs by 99.98%. - The average cost of treatment for the standard group was US$47396 (I$47369), the bedaquiline group was US$47405 (I$47405) and the delamanid group was US$67650 (I$67650). - The bedaquiline group received an additional QALY of 0.731 while the delamanid group only received 0.012 QALY. |
3.3. Sensitivity Analysis
In conducting a pharmacoeconomic analysis such as cost-effectiveness analysis (CEA) and cost utility analysis (CUA), each data generated is commonly based on a number of assumptions, some of which may not represent reality and could lead to uncertainty. For this uncertainty to be properly accounted for, it must be appropriately identified, assessed and interpreted. To analyze this uncertainty, a sensitivity analysis must be performed [27, 28]. While 3 of 8 studies perform a sensitivity analysis with different values of addition and subtraction. The addition and subtraction of these values depend on the perspective. Studies in Germany, conducted a sensitivity analysis by adding and subtracting 20% of each parameter to the value of cost effectiveness. The results of the addition and subtraction are then entered into the ICER calculation. The calculation result will show the lower limit, namely ICER-20% and the upper limit, namely ICER + 20%. The farther the distance between the lower limit and upper limit, the more influence will be on the ICER value. The results of the sensitivity test showed that the sequence from the longest to the shortest range, namely the longest one was the effect of bedaquiline on sputum culture conversion with ICER €17,711 (I$ 21.653,47), while the shortest was the cost of Adverse Effect (AE) [23].
A study in Africa shows that the most influential analysis was the proportion of patients who successfully experienced culture conversion while receiving bedaquiline therapy, with a 25% increase resulting in a 2.3-fold increase in ICER, thus saving US$3908 (I$3908) in medical costs per DALY. This analysis also affects the cost of bedaquiline therapy with an increase of 25%, where the cost of therapy incurred has increased the ICER by 90%, so it can save costs of US$2242 (I$2242) per DALY [8]. In addition, the UK study showed the most influential analysis was the length of time spent on MDR-TB treatment, in which the average time required for MDR-TB treatment was 2 years. Additional costs per QALY earned is £35,174 or I$42.992,48 for a cost savings of £2,079 or I$2.541,12 per DALY earned [19].
3.4. Quality of Reporting
Based on reporting quality assessment from the CHEERS checklist, four studies were ranked as good, and four as moderate. Table 2 shows the proportion of each item in the CHEERS checklist that is reported sufficiently, partially, or not at all by all included studies in the review. On average, the studies that stopped had good and moderate reporting quality. Most studies failed to materialize to report on effectiveness measures (synthesis-based estimation). There is one study that did not report details on how resources and costs were collected and estimated, along with poor reporting of the discount rate. Additionally, four out of nine studies also did not report the method that charges fees and converts fees into common currency fees based on exchange rates. Results relating to study parameters are only part of the studies that are fully reported. Only half of the studies reported sources of funding and potential conflicts of interest.
| CHEERS Section/Item |
Item No. |
References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| [8] | [9] | [26] | [22] | [23] | [24] | [19] | [25] | ||
| Title and Abstract | |||||||||
| Title | 1 | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | 2 | Y | Y | Y | Y | Y | Y | Y | Y |
| Introduction | |||||||||
| Background and objective | 3 | Y | Y | Y | Y | Y | Y | Y | Y |
| Methods | |||||||||
| Target population and subgroups | 4 | Y | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | 5 | Y | Y | Y | Y | Y | Y | Y | Y |
| Study perspective | 6 | Y | Y | P | P | Y | Y | Y | Y |
| Comparators | 7 | P | Y | Y | Y | P | Y | Y | Y |
| Time horizon | 8 | P | P | P | Y | Y | Y | Y | Y |
| Discount rate | 9 | P | P | NA | Y | Y | Y | Y | Y |
| Choice of health outcomes | 10 | Y | Y | Y | Y | Y | P | Y | Y |
| Measurement of effectiveness (single study-based estimated) | 11a | NA | NA | NA | NA | NA | NA | NA | NA |
| Measurement of effectiveness (synthesis-based estimated) | 11b | Y | P | Y | Y | P | P | P | P |
| Measurement and valuation of preference based outcomes | 12 | Y | P | Y | Y | Y | Y | P | N |
| Estimating resources and costs (single study-based economic evaluation) | 13a | NA | NA | NA | NA | NA | NA | NA | NA |
| Estimating resources and costs (model-based economic evaluation) | 13b | Y | N | Y | P | Y | P | N | N |
| Currency, price date, and conversion | 14 | N | Y | Y | N | N | N | Y | Y |
| Choice of model | 15 | Y | Y | P | Y | Y | Y | P | Y |
| Assumptions | 16 | Y | Y | Y | Y | P | Y | Y | Y |
| Analytical methods | 17 | P | P | P | Y | P | P | Y | P |
| Results | - | - | - | - | - | - | - | - | - |
| Study parameters | 18 | P | Y | P | Y | Y | P | P | Y |
| Incremental costs and outcomes | 19 | Y | Y | Y | Y | Y | Y | Y | Y |
| Characterizing uncertainly (single study-based economic evaluation) | 20a | NA | NA | NA | NA | NA | NA | NA | NA |
| Characterizing uncertainly (model-based economic evaluation) | 20b | P | N | NA | P | P | N | P | NA |
| Characterising heterogeneity | 21 | NA | NA | Y | P | P | NA | N | P |
| Discussions | - | - | - | - | - | - | - | - | - |
| Study findings, limitations, generalizability, and current knowledge | 22 | Y | Y | Y | Y | Y | Y | Y | Y |
| Other | - | - | - | - | - | - | - | - | - |
| Source of funding | 23 | NA | NA | Y | NA | Y | Y | Y | NA |
| Conflict of interest | 24 | NA | NA | Y | Y | Y | NA | NA | NA |
| Reporting quality based on % score* | - | Moderate | Moderate | Good | Good | Good | Moderate | Good | Moderate |
4. DISCUSSION
The results of the cost effectiveness analysis are expected to assist in the decision-making process about these choices. If the new strategy is more effective and cheaper than the previous one, then the strategy is worth implementing. How- ever, if the new strategy is less effective and more expensive, no change in strategy is necessary. A cost-effectiveness analysis needs to be performed to assess the benefits of the costs incurred [29]. Supporting data such as data on costs in MDR-TB treatment are still not widely found; these costs are needed to provide input to policymakers who have the authority to plan the treatment costs for MDR-TB patients [30]. In a cost-effectiveness and cost-utility analysis, the most frequently used measures are Disability-Adjusted Life Years (DALY) and Quality-Adjusted Life Years (QALY). One DALY can be thought of as one lost year of a ”healthy” life. The total DALY across population, called disease burden, can be considered as a measure of the gap between current health status and an ideal health situation in which the entire population lives to old age, free from disease and disability [31]. Meanwhile, QALY is an expected outcome of a health intervention that is closely related to the quality of life [27].
Depends on the perspective, cost-effectiveness analyses can incorporate both direct and indirect costs of the health interventions. Direct costs are costs that incurred directly e.g. medical, treatment and service cost, as well as costs in supporting units, such as costs related to radiology and laboratories. Moreover, indirect costs are defined as the costs incurred from either reduction or discontinuation of work productivity (or productivity loss) due to morbidity and mortality associated with the disease [30].
Cost and effectiveness are factors that affect the value of the cost effectiveness and that make MDR-TB treatment more effective MDR-TB [20, 32]. People living with MDR-TB have to spend more and more time at tasks because of the side effect including relapse, permanent hearing, that makes them unable to work or return to their daily activities [33]. Successful MDR-TB treatment cannot be accomplished with just one new antibiotic. Rather, it requires a combination of three to four new antibiotic classes simultaneously. This is a huge financial and technical challenge and requires great cooperation. The average cost of developing a new drug is over US$1.5 (I$1.5) billion and the average time for drug discovery and develo- pment from target identification to approval is 10 to 14 years [34].
CONCLUSION
The addition of bedaquiline in standard therapy showed favorable effect and safety due to faster culture conversion time and less incidence of side effects, based on the results of studies conducted in England, South Korea, South Africa, Italy, Russia, and Germany. The faster the culture conversion occurs and the less patients experiencing side effects, the faster their health improvement, which prospectively will reduce treatment costs and productivity loss. This is demonstrated by the results of cost-effectiveness analysis which shows that the repla- cement of the second-line injection regimen to bedaquiline, and the addition of bedaquiline to the standard regimen of therapy was assessed to be more cost-effective option.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


